
Morphological Investigation of Bacterial Cellulose/Cassava Starch
Nanocomposites Produced by In-situ Process in Agitated Culture
C. F. Zuhra
1*
, Yugia Muis
1
, S. Gea
1
, S. A. Amaturrahim
1
, K. M. Pasaribu
1
, and S. U. Rahayu
2
1
Departement of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus Padang Bulan USU Medan 20155, Indonesia
2
Departement of Physics, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus Padang Bulan USU Medan 20155, Indonesia
Keywords: Bacterial cellulose, Cassava starch, Nanocomposites, In-situ process, Agitated culture
Abstract: The existence of starch in the formation of bacterial cellulose was found to have the capability to enhance
the viscosity of culture medium, thus it can affect the agitation process. The aim of this study was to
investigate the morphological structure of bacterial cellulose/cassava starch (BC/CS) nanocomposites
produced by in-situ process in agitated culture. The fabrication of BC/CS was carried out at 28°C for 7 days
under agitated condition at 100 rpm with five different masses of CS. The crystallinity of BC/CS
nanocomposites was studied by using X-ray Difraction (XRD) pattern, whereas the morphological structure
was discovered from the digital photograph, optical microscope (OM) picture and scanning electron
microscope (SEM) pictures. From the SEM analysis, it was observed that the cassava starch layer had
occupied the pores of the fibre network of bacterial cellulose and increased the average size of the fibres.
Also, it was well dispersed in the network of bacterial cellulose. The high content of starch on the culture
caused the changes in orientation of the nanocomposites’ surface morphology and reduced the shaking
effect of the culture. The study found that the BC/CS nanocomposites with variation of 5 g CS showed the
best morphological properties.
1 INTRODUCTION
Bacterial cellulose (BC), which produced by
Acetobacter xylinum, was one of environmentally
friendly products that has grown the researchers’
attention nowadays due to its outstanding features,
such as its higher tensile strength, crystallinity, and
capacity of water absorption; besides, it has ultra-
fine fibre network structure, good transparency,
good chemical stability, considerable fibre binding
ability, appropriate biocompatibility,
biodegradability, and moldability [Ishihara et al.
2002; Klemm et al. 2001; Shezad et al. 2009;
Vandamme et al. 1998] . One of its applications is as
a material to solve the problem of the dependence on
the petroleum products and the environmental
damage [Averous, 2004; Lu et al. 2006]. As a
biodegradable material, BC can be employed as a
matrix of nanocomposites material.
Regarding to the above explanation, starch,
which has a proper biodegradability, low cost
production, and a wide availability, could be a good
filler for the matrix of bacterial cellulose to form
BC/cassava starch nanocomposites [Angles and
Dufresne, 2000]. The integration of those two
biodegradable materials was employed since the
starch itself has some drawbacks, namely, lower
mechanical properties, high hygroscopicity, and high
permeability to gases [Vandamme et al. 1998].
Therefore, by combining these two materials, the
new biodegradable materials with enhanced
properties could be yielded.
As reported by Haigler et al. (1982) the starch
which is added to the culture medium of
Acetobacter xylinum does not affect the reaction of
cellulose formation even though different carbon
sources, such as glucose, fructose, and gluconate,
can be used to synthesize the cellulose. In other
word, the starch is not consumed by the bacteria. It
can be proved by the appearance of blue color on the
nanocomposites after being added by iodine, which
shows the existence of starch on the nanocomposites
[Zhang et al. 2006; Kuipers et al.1994]. The
existence of starch on the nanocomposites of
1042
Zuhra, C., Muis, Y., Gea, S., Amaturrahim, S., Pasaribu, K. and Rahayu, S.
Morphological Investigation of Bacterial Cellulose/Cassava Starch Nanocomposites Produced by In-situ Process in Agitated Culture.
DOI: 10.5220/0010096710421046
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1042-1046
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

BC/starch, furthermore, could strengthen the
interaction between cellulose membrane and the
wall inside the culture vessel during the inoculation
process because of the high viscosity of the medium
after gelatinization process of starch. That
interaction, which was called as wall effect, can
compress the production of cellulose by limiting the
increase of cellulose membrane thickness [Homung
et al. 2006]. Besides, the decrease of fluidity and the
increase of viscosity due to the existence of
gelatinized starch could limit the diffusion process
of the glucose substrate on the cellulose matrix and
the motion of Acetobacter xylinum in the culture
medium [Yang et al. 2014].
There are two methods that are commonly used
to produce the BC, namely static method and
agitation method. The use of the static method on
the production of BC has been proved to have some
disadvantages over the agitation method. The
agitation method can provide enough oxygen for the
bacteria, while the static method failed to do so;
thus, the agitation method can increase the
production of cellulose. Also, the agitation method
can reduce the crystal size and form the more stable
crystal. However, based on several studies, the
agitation methods can cause the stretching among
the woven of cellulose fibre, thus forming the larger
pores. This makes the layers of formed cellulose
separated each other, so that the degree of
crystallinity reduced [13–1Watanabe et al. 1998;
Yamanaka et al. 2000; Yamamoto et al. 1996].
Based on the above issues, the production of
BC/starch nanocomposites using agitation method
should be investigated. The agitation method was
chosen because it can provide enough oxygen, while
the existence of starch can reduce the shaking effect
of agitation process. To date, there is no study
discussed about the production of BC/starch
nanocomposites using agitation method. Although
there have been several research studied about the
production of BC/starch nanocomposites [Yang et
al. 2014; Grande et al. 2009; Martin et al. 2009;
Woehl et al. 2010], those studies still used the
conventional static method. This study aimed to
investigate the morphological properties of
BC/cassava starch (BC/CS) nanocomposites
produced by in-situ process in agitated culture
because it can present the effects of starch on BC
visually. Thus, it can provide better understanding in
the effects of starch on the formation of bacterial
cellulose nanocomposites.
2 MATERIALS AND METHODS
2.1 Materials
The materials used in this study were glucose, bacto-
peptone, urea, NaOH, NaOCl, CH
3
COOH, and
distilled water, which were purchased from Merck,
without having further treatments. Coconut water
was supplied from traditional markets in Medan,
Indonesia and the bacteria, Acetobacter xylinum,
was supplied by the Microbiology Laboratory of
Universitas Sumatera Utara.
2.2 Isolation of Cassava Starch
The isolation of cassava (Manihot esculanta) starch
was done by using conventional method. Briefly, the
cassava was peeled. After that, it was washed using
water, then shredded. The resulted cassava was
added with enough water, then blended. The result
was then precipitated after filtered using the gauze.
The precipitation was washed frequently until the
washed waste was transparent. This precipitation
was called as starch. After that, it was dried in the
oven at 45
o
C for 24 hours. The dried starch was
ground and sifted in order to obtain the final starch.
2.3 Preparation of BC/CS
Nanocomposite Film
BC/CS nanocomposites were produced by the
Acetobacter xylinum bacterial strain in culture
medium that containing 100 ml coconut water, 0.5%
(w/v) urea, 1.0 % (w/v) glucose, 1.5% (w/v) bacto-
peptone. The pH of culture medium was adjusted to
4.5 by acetic acid. CS with variations of 1 g, 2 g, 3
g, 4 g, and 5 g was added to culture medium,
followed by autoclave for 30 min at 121°C. The
solutions were magnetically stirred for 15 minutes.
Main cultivation were carried out at 28°C for 7 days
under agitated condition at 100 rpm. The BC/CS
nanocomposites were washed under running tap
water and it was immersed overnight in 2.5% NaOH
and also in 2.5% NaOCL. Then, it was rinsed again
under running tap water to remove any solvent until
it reached neutral pH. The BC/CS nanocomposites
were finally pressed using hot-press with wire-mesh
at 115°C for 10 min.
2.4 Characterization
The BC/CS nanocomposites were characterized by
X-ray diffraction (XRD), scanning electron
Morphological Investigation of Bacterial Cellulose/Cassava Starch Nanocomposites Produced by In-situ Process in Agitated Culture
1043
microscope (SEM), and optical microscope (OM).
The XRD pattern were taken by Shimadzu XRD-
6100 diffractometer using Cu-Kα radiation (λ =
0.154 nm) at scanning rate of 2°/min, a voltage of
40kV and a current of 200mA. The diffraction angle
(2θ) range from 5° to 30° with a step size of 0.02°.
The degree of crystallinity (Crystallinity Index, CrI)
was calculated from diffracted intensity data using
the method described by previous researcher (Seagel
et al., 1959) as shown on the formula (1)
CrI (%) = (1-(I
AM
-I
200
)) x 100% (1)
Where the I
AM
and I
200
represented the intensity of
diffraction in the same units at approximately 2θ =
18° and maximum intensity of (002) lattice
diffraction at approximately 2θ = 22.7°, respectively.
The scanning electron microscope was done
using SEM EDX EVO MA 10 Carl Zeiss Bruker.
All samples were sputter coated with gold-palladium
and observed using an accelerating voltage 20 kV.
Samples were viewed at magnification between
1000 and 10000 times from their original sizes.
The optical microscope pictures were taken using
American Optical Microscope with the
magnification of 100 times
3 RESULT AND DISCUSSION
3.1 XRD Analysis
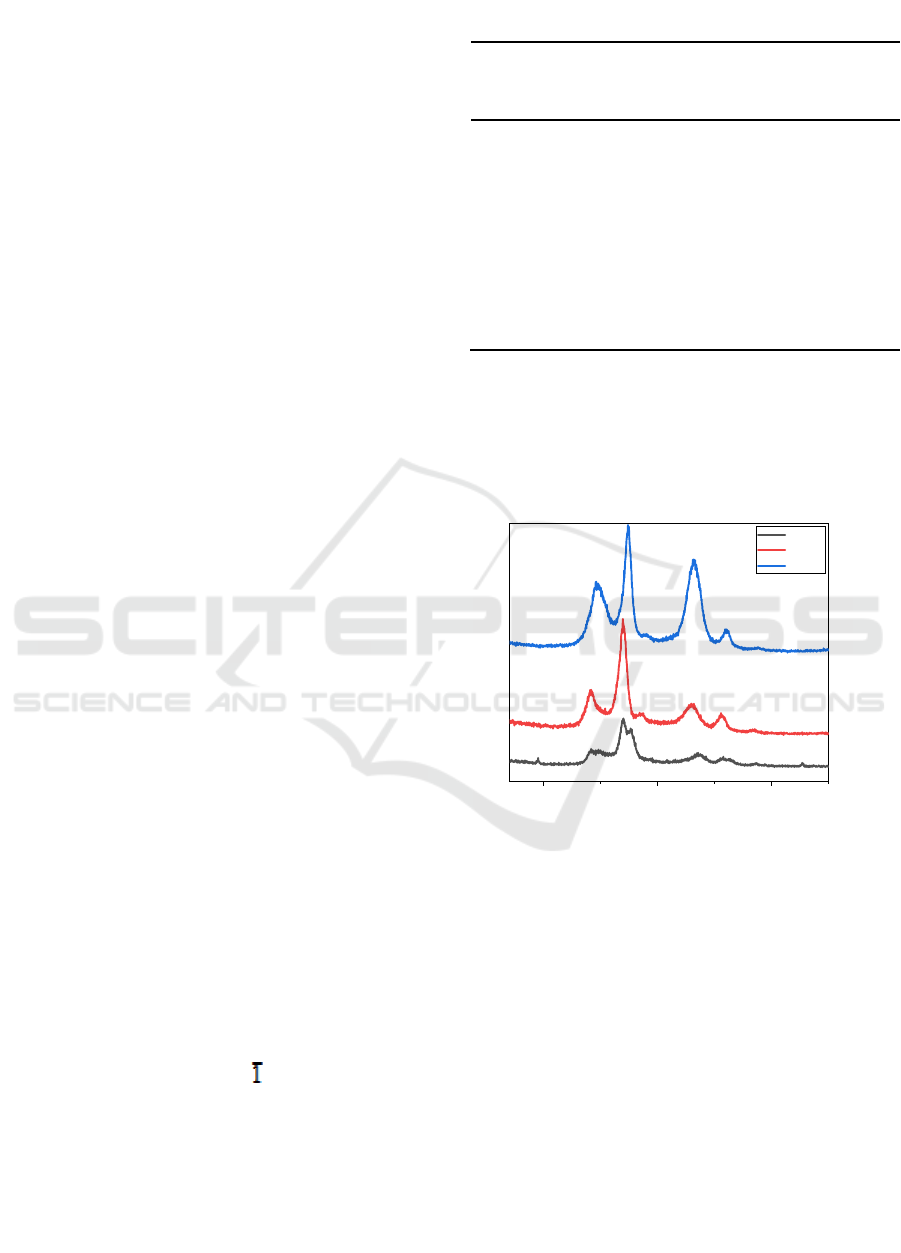
As shown in Figure 1, the diffraction peak of starch
was found at 2θ of 14,780°, 16,924°, and 22,040°.
This is in agreement with the results reported by
Grande et al. [Grande et al. 2009]. The starch
showed lower crystallinity value. This was caused
by two conditions from the gelatinization process
that occurred during the formation of
nanocomposites. As gelatinized CS, the crystal from
starch granules was damaged and the intensity
related to the peak of diffraction will decrease, or
even disappear. The crystallinity of starch was equal
to zero because there are no crystal peaks observed
on the spectra.
The diffraction peaks for BC was discovered at
2θ of 14.208°, 16.930°, and 22.924°, which showed
the diffraction lattice of (1ȋ0), (110), and (002),
respectively, at the polymorph of cellulose I. This
result is in accordance with the result reported by
Table 1. Diffraction peaks, d-spacing, and degree of
crystalinity of CS, BC, and CS/BC nanocomposites.
Sample 2θ (°) d (nm) Degree of
crystallinity
(%)
CS 15.030° 5.889 ~0%
17.880° 4.956
23.173° 3.835
BC 14.827° 5.969 92%
17.362° 5.103
23.168° 3.836
BC/CS 14.208° 6.228 53%
16.930° 5.233
22.924° 3.876
Yang et al. 2014. As also can be seen from
figure 1, the BC has sharp peaks because of the high
crystallinity degree of it (92%, shown in table 1). It
can be explained as a result of the intermolecular
hydrogen bonding in the cellulose structure.
.
10 20 30
2θ (degree)
Intensity (-)
CS
BC
BC/CS
Figure 1. X-ray diffraction spectra of CS, BC, and CS/BC
nanocomposites.
For the BC/CS nanocomposites, the diffraction
peaks was placed at 2θ of 14.827°, 17.362°, and
23.168°. This result is also similar to the results
obtained by Yang et al. (2014). As previously
mentioned that the BC itself has a high degree of
crystallinity, the existence of cassava starch,
furthermore, reduce its degree of crystallinity to
53% as shown in table 1. The decrease in degree of
crystallinity of BC/CS nanocomposites occurred due
to two possible reasons: 1.The migration of
Acetobacter xylinum was blocked by the poor
fluidity of medium due to the existence of
gelatinized starch. In other word, the motion of
bacteria was limited; 2. There was a high steric
obstruction in amylopectin branching on that prevent
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1044
the formation of cellulose bands since the
amylopectin stick in the cellulose microfibrils [Yang
et al. 2014]
3.2 The Morphology of BC/CS
Nanocomposites Analysis
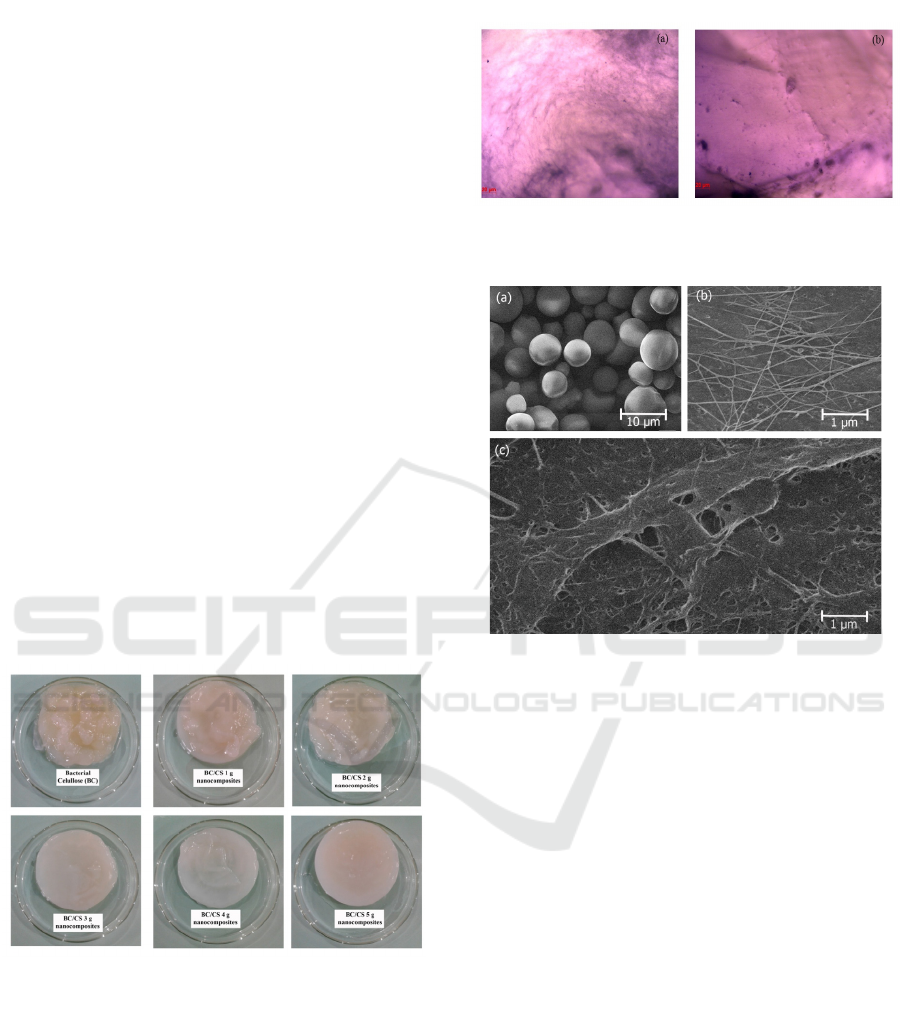
Figure 2 shows the digital photograph of the BC
membrane and the BC/CS membrane with various
mass of CS after 7-day agitation process. It can be
seen that the mass of CS affects the layer yielded,
the more CS added, the more organized the BC/CS
nanocomposites obtained. The BC/CS
nanocomposites with the variation of 5 g CS show
the most organized one. It is similar to the BC/CS
nanocomposites produced by static method. This can
be occurred because the starch can increase the
viscosity of culture medium, thus it can reduce the
shaking effect during the agitation process.
The optical microscope pictures from BC and
BC/CS nanocomposites with the variation of 5
grams of CS in never-dried state were given in
figure 3. As seen in figure 3(a), the surface of BC is
more transparent than that of BC/CS
nanocomposites. The surface of BC reveals the fibre
network of the BC, while the surface of BC/CS
nanocomposites looks solid without having any fibre
(figure 3(b)). This happened because the pores of
BC have been filled by the swollen starch granules.
Figure 2. Digital photos of pure BC and BC/CS
nanocomposites with various mass of starch with
magnitude (a) 100x in never-dried state.
The surface morphology of CS, BC and BC/CS
nanocomposites is also further investigated through
SEM with 10,000 times of magnification and is
shown in Figure 4. Figure 4(a) gives the shape of
starch granules clearly with a perfect oval shape.
Based on our analysis, the average size of them is
around 12.755 µm. The large size of the granules
indicates the high capability of capping the water
during the gelatinization process.
Figure 3. Optical microscope images of BC and BC/CS
nanocomposites with magnitude (a) 100x in never-dried
state
Figure 4. SEM images of (a) CS, (b) BC, and (c) BC/CS
nanocomposites with magnitude 10,000x after hot-
pressing.
Figure 4(b) indicates the irregularity of BC
network obtained by agitation method. This can be
occurred because during the process, the bacteria
move to the oxygen-rich area. The agitation method
will cause the stretching happened among the woven
of cellulose fibre and result in larger pores among
them. Still, this method can reduce the size of fibre
or the size of crystal [Watanabe et al.1998].
The two above results formed another structure
when they combined as BC/CS nanocomposites due
to the interaction between them. This is shown in
Figure 4(c). The gelatinized CS was dispersed
evenly in the matrix of BC. The high content of
starch caused the integration of BC network and
gelatinized starch; the starch did not only stick in the
BC fibre, but also resulted in the changes in the
orientation of nanocomposites’ surface morphology.
Based on the SEM analysis, it was discovered that
the BC has average size of fibre of 67.907 nm, while
BC/CS nanocomposites have the average size of
fibre of 73.470 nm. The increase of fibre size
occurred because the shaking effect decreased after
the addition of starch.
Morphological Investigation of Bacterial Cellulose/Cassava Starch Nanocomposites Produced by In-situ Process in Agitated Culture
1045

4 CONCLUSION
In this study, BC/CS nanocomposites were
successfully obtained by in-situ process in agitated
culture. The XRD pattern shows that the degree of
crystallinity of BC/CS nanocomposites was lower
than that of the BC. From the digital photograph,
OM and SEM pictures, it was proven that the
existence of starch could rectify the irregularity of
cellulose layer produced by agitation method. The
gelatinized CS is well dispersed in the network of
BC and filled the pores of the BC fibre, thus
increases the average size of the nanocomposite
fibres. Also, it causes the changes in the orientation
of nanocomposites’ surface morphology. The more
CS added, the more organized layer yielded. This
can be occurred because the increase gelatinized
starch can raise the viscosity of culture medium and
reduce the shaking effect. Furthermore, it was found
that the BC/CS nanocomposites with the variation of
5 g CS performed the best result.
ACKNOWLEDGEMENT
The authors would like to thank to the Rector of
University of Sumatera Utara for financial support
from the project of PUU-Talenta 2018.
REFERENCES
Anglès, M. Neus, and Alain Dufresne. 2000. “Plasticized
Starch/Tunicin Whiskers Nanocomposites. 1.
Structural Analysis.” Macromolecules.
Avérous L, Boquillon N. 2004. “Biocomposites Based on
Plasticized Starch: Thermal and Mechanical
Behaviour.” Carbohydrate Polymers.
Czaja, Wojciech, Dwight Romanovicz, and R. malcolm
Brown,. 2004. “Structural Investigations of Microbial
Cellulose Produced in Stationary and Agitated
Culture.” Cellulose.
Grande, Cristian J. et al. 2009. “Development of Self-
Assembled Bacterial Cellulose-Starch
Nanocomposites.” Materials Science and Engineering
C.
Hornung, M., M. Ludwig, A. M. Gerrard, and H. P.
Schmauder. 2006. “Optimizing the Production of
Bacterial Cellulose in Surface Culture: Evaluation of
Substrate Mass Transfer Influences on the Bioreaction
(Part 1).” Engineering in Life Sciences.
Ishihara, M, M Matsunaga, N Hayashi, and V Tisler.
2002. “Utilization of D-Xylose as Carbon Source for
Production of Bacterial Cellulose.” Enzyme Microb
Technol.
Klemm, Dieter, Dieter Schumann, Ulrike Udhardt, and
Silvia Marsch. 2001. “Bacterial Synthesized Cellulose
- Artificial Blood Vessels for Microsurgery.” Progress
in Polymer Science (Oxford).
Kuipers, AGJ., E. Jacobsen, and RGF. Visser. 1994.
“Formation and Deposition of Amylose in the Potato
Tuber Starch Granule Are Affected by the Reduction
of Granule-Bound Starch Synthase Gene Expression.”
The Plant cell.
Lu, Yongshang, Lihui Weng, and Xiaodong Cao. 2006.
“Morphological, Thermal and Mechanical Properties
of Ramie Crystallites—reinforced Plasticized Starch
Biocomposites.” Carbohydrate Polymers.
Martins, Ivo M.G. et al. 2009. “New Biocomposites Based
on Thermoplastic Starch and Bacterial Cellulose.”
Composites Science and Technology.
Shezad, Omer, Salman Khan, Taous Khan, and Joong Kon
Park. 2009. “Production of Bacterial Cellulose in
Static Conditions by a Simple Fed-Batch Cultivation
Strategy.” Korean Journal of Chemical Engineering.
Vandamme, E.J. et al. 1998. “Improved Production of
Bacterial Cellulose and Its Application Potential.”
Polymer Degradation and Stability.
Watanabe, Kunihiko, Mari Tabuchi, Yasushi Morinaga,
and Fumihiro Yoshinaga. 1998. “Structural Features
and Properties of Bacterial Cellulose Produced in
Agitated Culture.” Cellulose.
Woehl, Marco Aurélio et al. 2010. “Bionanocomposites of
Thermoplastic Starch Reinforced with Bacterial
Cellulose Nanofibres: Effect of Enzymatic Treatment
on Mechanical Properties.” Carbohydrate Polymers.
Yamamoto, Hiroyuki, Fumitaka Horii, and Asako Hirai.
1996. “In Situ Crystallization of Bacterial Cellulose II.
Influences of Different Polymeric Additives on the
Formation of Celluloses Iα and Iβ at the Early Stage of
Incubation.” Cellulose.
Yamanaka, S, M Ishihara, and J Sugiyama. 2000.
“Structural Modification of Bacterial Cellulose.”
Cellulose.
Yang, Jingxuan et al. 2014. “In Situ Fabrication of a
Microporous Bacterial Cellulose/Potato Starch
Composite Scaffold with Enhanced Cell
Compatibility.”
Cellulose.
Zhang, Qingmin et al. 2006. “Direct Detection of the
Formation of V-Amylose Helix by Single Molecule
Force Spectroscopy.” Journal of the American
Chemical Society.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1046
