
Vitamin E Extraction from Red Palm Biodiesel by using
K
2
CO
3
based Deep Eutectic Solvent with 1,2-Propanediol
as Hydrogen Bond Donor
Renita Manurung
1
, Gilang Ramadhan
1
, Aulia Arief
1
and Halimatussa’diah Siregar
1
1
Chemical Engineering Department, Universitas Sumatera Utara, Jl. Dr. Mansur, No. 9, Medan, Indonesia
Keywords: Biodiesel, DES, Extraction, Vitamin E, 1,2-Propanediol.
Abstract: Deep Eutectic Solvent (DES) is the latest advancement in separation technology to extract vitamin E from its
source. In this research K
2
CO
3
based DES with hydrogen bond donor (HBD) of 1,2-propanediol was used to
extract vitamin E from red palm biodiesel. The DES was synthesized at temperature of 80
o
C for 1 hour by
stirring rate at 300 rpm and K
2
CO
3
to 1.,2-propanediol molar ratio variation of 1:7, 1:8 and 1:9. Red palm oil
was synthesized by degumming of CPO with 85% phosphate acid to obtain Degummed Palm Oil (DPO). The
extraction was performed by mixing between biodiesel and DES at 400 rpm for 3 hours in mass ratio of 1:2,
1:2,5. 1:3, 1:3,5 and 1:4. Based on molar ratio variation of of K
2
CO
3
:1,2-Propanediol and mass ratio of
biodiesel:DES, which effect to extracted vitamin E and biodiesel purity. The HPLC analysis was used to
showed concentration of extracted vitamin E while the GC analysis was used to showed that extraction using
K
2
CO
3
:1,2-propanediol based DES indeed can increase the purity. DES at K
2
CO
3
to 1,2-propanediol molar
ratio of 1:8 and biodiesel to DES mass ratio of 1:3.5 was the most effective in extracting vitamin E up to
392.16 ppm.
1 INTRODUCTION
Biodiesel is generally produced by transesterification
reaction. The palm oil transesterification is a gradual
reaction between palm oil and alcohol (methanol or
ethanol) producing methyl esters or ethyl seters
(Manurung, 2018a). Raw material requirements for
biodiesel production is the main consideration to
increase the economic value of biodiesel production
where waste cooking oil, crude palm oil (CPO) can
be an alternative choice that can replace the usage
palm oil. In Indonesia, palm oil is a raw material that
is quite potential to be used for biodiesel production
because it is available throughout the year in large
quantities (Manurung, 2018b). Crude palm oil is
converted to methyl ester that called biodiesel and
side product in the form of glycerol was obtained
from the reaction between raw materials in the form
of triglycerides using short chain alcohols such as
methanol and ethanol assisted by the presence of
alkaline catalysts. Biodiesel is ecofriendly diesel and
good performance (Manurung, 2018a).
One of the most prospective raw materials in
biodiesel production is obtained from palm oil. This
is due to the availability of palm oil is in large amount
in Indonesia, from September to October 2016, the
average production of palm oil reached 35 million
tons, and exports reached 26 million tons and
domestic consumption of 9.1 million tons in October
2016 (World Market and Trade, 2016). Indonesia as
one of the largest palm oil producer country in the
world has the potential to develop palm oil-based
biodiesel compared to fossil fuels.
One of minor component in CPO-based biodiesel
which can also be classified as an impurity is
carotenoids, and several other micronutrients such as
vitamin E, tocotrienol and sitosterol (β-sitosterol)
which are useful. Crude palm oil is a vegetable oil
containing minor components such as carotenoids
and vitamin E where the vitamin E content in the CPO
is approximately (600-1000 μg/ml) (Sinaga and
Donald, 2015). Among tocopherols, α-tocopherol is
the most important source of vitamin E in human
foods. The ability of antioxidants and their function
as anti-free radicals makes tocopherols become an
attractive molecule for the health and food industry.
Due to the function of tocopherol which is very
important for living things, it can be encourages the
338
Manurung, R., Ramadhan, G., Arief, A. and Siregar, H.
Vitamin E Extraction from Red Palm Biodiesel by using K2CO3 based Deep Eutectic Solvent with 1,2-Propanediol as Hydrogen Bond Donor.
DOI: 10.5220/0010094503380345
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
338-345
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

development of the separation of these compounds
from the sources. Several studies have been carried
out such as adsorption, membrane separation
technology, and the use of solvents extraction
(Bezold, 2017) (Dai, 2015).
To obtain these components from methyl esters, it
is necessary to maintain the components in the palm
oil methyl esters with certain techniques, it can be
performed using Deep Eutectic Solvent (DES). DES
is produced by mixing two components in certain
ratio to produce a mixture which has significantly
lower melting points than each of the components
singularly. Moreover DES has lower toxicity level
and most of them are biodegradable (Manurung,
2018b). As well as the usage of ChCl, in 2013,
potassium carbonate (K
2
CO
3
) and glycerol were used
in different molar ratio to create a new DES system
(Manurung, 2018b).
The development of the DES system is
increasingly in demand because it has been widely
applied as co-solven, liquid-liquid extraction and in
catalysis which can be used economically and
ecofriendly properties (Dai, 2015) (Manurung,
2018b). The use of 1,2-Propanediol as HBD was
carried out by (Manurung, 2018a) in the process of
purifying biodiesel in which biodiesel was purified up
to 99.88% (Manurung, 2018a). This research tried to
synthesize DES based on K
2
CO
3
with 1,2-
Propanediol as HBD to purifing red palm biodiesel
which expected that DES have ability to act in
extracting the oil components in the form of
tocopherols as vitamin E within CPO.
2 MATERIAL AND METHODS
2.1 Materials
Crude Palm Oil (CPO) was obtained from the palm
oil mill (PKS) PTPN IV. Phosphoric acid (85%) was
used to CPO pretreatment to obtained degummed
palm oil (DPO). Biodiesel based red palm oil was
conducted by ethanol (C
2
H
5
OH) as alcohol,
potassium hydroxide (KOH) as catalyst, and aquadest
used to biodiesel washing process. The DES synthesis
was used potassium carbonate (K
2
CO
3
) based 1,2-
Propanediol as HBD. The extraction process used n-
hexane to disperse biodiesel and used methanol to
disperse DES thus the liquid-liquid extraction could
create biphasic system. Recovery of vitamin E used
water-hexane method in 4:1 ratio, then concentrate
was obtained by using rotary evaporator.
2.2 Methods
The synthesis of deep eutectic solvent (DES) was
conducted at temperature of 80
o
C, stirring rate of 300
rpm for 1 hour (Manurung, 2018a), and molar ratio
variation of K
2
CO
3
to 1,2-Propanediol 1:7, 1:8 and
1:9. The transesterification reaction to synthesis
biodiesel was performed at temperature of 70
o
C for
1.5 hours by molar ratio of ethanol : DPO = 9:1, and
KOH concentration of 1.2 wt%. The extraction
process adopts the procedure by is based on a method
performed by (Manurung, 2018) (Manurung, 2018c),
n-hexane was used to disperse biodiesel and methanol
was used to disperse DES which then performed
liquid-liquid extraction by mixing biphasic system
between prepared CPO and DES. Mass ratio of
Biodiesel to DES was varied at 1:2, 1:2.5, 1:3, 1:3.5
and 1:4. Recovery of vitamin E was carried out by of
water-hexane method at ratio of 4:1 v/v. The
concentrate was obtained by using rotary evaporator
the vitamin E rich hexane product from water-hexane
recovery process
3 EXPERIMENTAL PROCEDURE
3.1 Synthesis of Deep Eutectic Solvent
(DES)
DES was performed by K
2
CO
3
and 1,2-Propanediol
with a certain molar ratio was introduced into the
erlenmeyer and sealed it with stopple. The mixture is
heated over hot plate until it reaches the reaction
temperature of 80 °C and then stirring at 300 rpm for
1 hour to obtained homogenous mixture (Manurung,
2018c).
3.2 Pretreatment of Crude Palm Oil
Crude palm oil in certain amount was put into beaker
glass. Palm oil was heated to 80 °C. 85% phosphoric
acid was added as much as 0.15% of the used palm
oil weight. The oil was then stirred at 300 rpm for 15
minutes. CPO was filtered using filter paper. The
filtrate was taken and called by Degummed Palm Oil
(DPO).
3.3 Synthesis of Biodiesel
DPO, ethanol, and KOH catalyst are prepared in a
certain weight. The equipped Three-neck flask with
magnetic stirrer, a thermometer, and condensation
system are heated by hot plate to temperature of 75
Vitamin E Extraction from Red Palm Biodiesel by using K2CO3 based Deep Eutectic Solvent with 1,2-Propanediol as Hydrogen Bond
Donor
339
o
C to remove water content. DPO in certain weight
was added to the three-neck flask and heated to
temperature of 70
o
C. Ethanol and KOH catalyst were
added to the three-neck flask and turned on the
magnetic stirrer, the stopwatch was run to calculate
the reaction time. After 1.5 hours, the hot plate was
turned off and the mixture cooled. The reaction
mixture was then introduced into the separating
funnel and waited for a few minutes to form 2 layers
so that the separation of the mixture can be
performed. The bottom layer which is a mixture of
glycerol, ethanol, and KOH was separated from the
top layer containing crude palm biodiesel which still
contained some impurities. The top layer then washed
using hot water until the bottom layer is clear to
remove any remaining impurities (Manurung,
2018d).
3.4 Extraction of Vitamin E
Prepared 10 grams of biodiesel was mixed with 100
ml n-hexane. DES with a certain mass ratio to
biodiesel was then mixed with 100 ml methanol.
Mixed both of those two mixtures and stirred at 400
rpm for 3 hours and then let it for 2 hours in the
separation funnel. The mixture will form two layers,
the bottom layer which is a mixture of DES,
methanol and vitamin E was then separated from the
top layer.
The bottom layer mixture was then mixed with
water-hexane by volume ratio of 4:1 v/v in separation
funnel and let it to form 2 layers. The lower layer
which is a mixture of DES, methanol and water was
separated from the top layer. The top layer was
condensed with rotary evaporator till there was no
more hexane remaining to obtained vitamin E, so that
the biodiesel purity can be analyzed (Hadi, 2014).
4 RESULTS AND DISCUSSION
4.1 Characteristic of DES
DES is produced by mixing two components in the
certain ratio to produce a mixture which has
significantly lower melting points than each of the
components singularly (Craveiro, 2016). In this study
used Potassium carbonate (K
2
CO
3
) and 1,2-
Propanediol in different molar ratios to create a new
DES system. The need development for green solvent
which can be used economically and applicable not
only just limited in gas absorption, liquid-liquid
extraction and catalysis (Setyawan, 2011).
4.1.1 Form and Appearance of DES
The goal in this part is to obtain DES with the best
characteristics as a solvent. The used DES was a
mixture of K
2
CO
3
and 1,2-propanediol as HBD with
various molar ratios can be explained in Table 1
below.
Table 1: Code and from of K
2
CO
3
based DES with 1.2
propanediol as HBD.
Ratio Molar of
K
2
CO
3
:1.2
Propanediol
Code
Form of DES
(RoomTemperature)
1 : 2 DES 1
White color, partial
Soli
d
1 : 3 DES 2
White color, partial
Soli
d
1 : 4 DES 3
Turbid, still there
soli
d
1 : 5 DES 4
Turbid, still there
soli
d
1 : 6 DES 5
Transparent, still
there soli
d
1 : 7 DES 6 Trans
p
arent, Li
q
ui
d
1 : 8 DES 7 Transparent, Liquid
1 : 9 DES 8 Transparent, Liquid
Based on Table 1 shows that DES at molar ratio
of 1:2, 1:3, 1:4, 1:5 and 1:6 (DES 1, DES 2, DES 3,
DES 4 and DES 5) visually were still produced poor
DES. The produced product was turbid, white and
freezing at room temperature. The phase of solid,
semi-solid, crystal, or liquid will be formed along and
after the preparation of DES synthesis with different
forming phase.
Important physical properties Characterization of
DES are viscosity, density, refraction index, pH, and
surface tension, (Dai, 2015). In this research, the
obtained DES Characterization were freezing point,
density, and viscosity.
4.1.2 Freezing Point of DES
DES is usually consisted of two or three cheap and
safe components, which can associate with each other
through hydrogen bond donor, to produce eutectic
mixture in the liquid phase when freezing point of
DES is much lower than it’s pure constituents. The
effect of molar ratio to freezing point of DES can be
explained in Figure 1 below.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
340
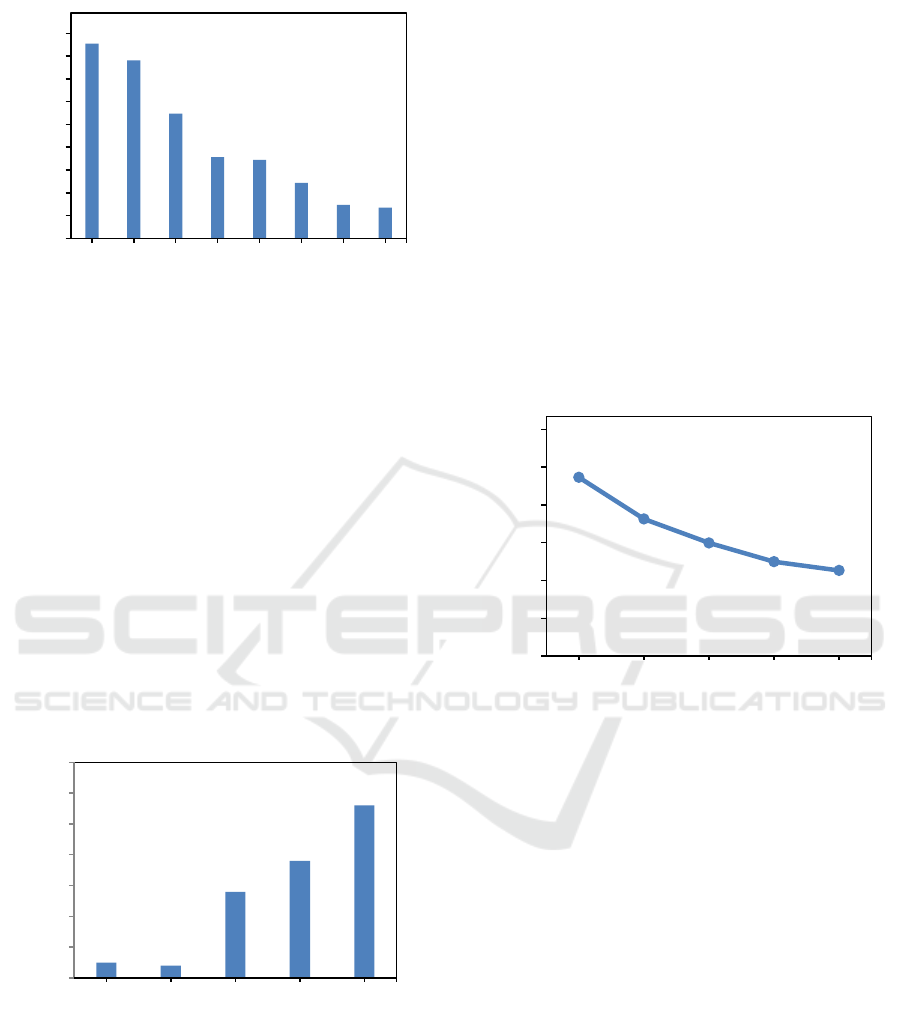
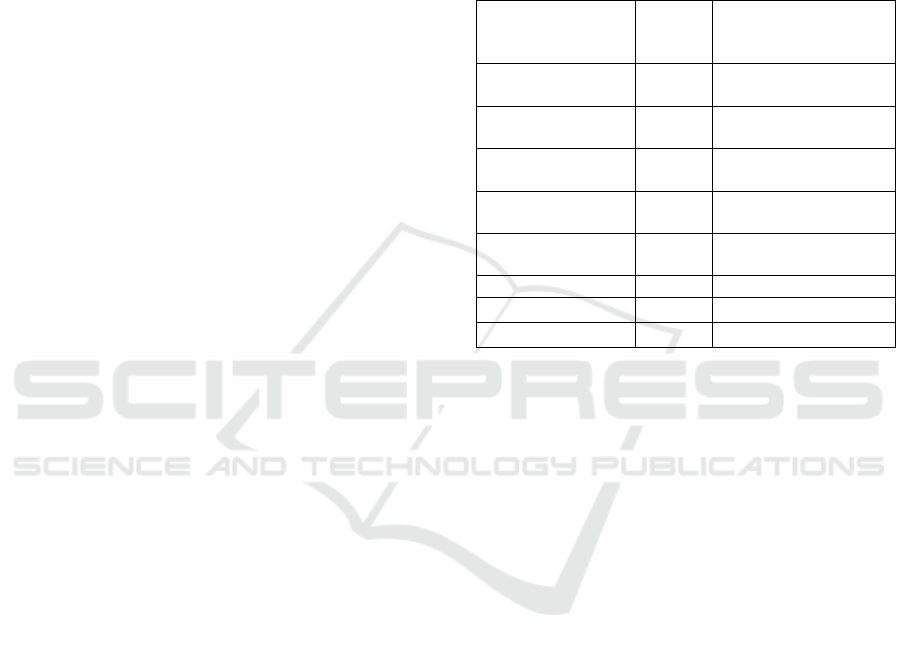
Figure 1: Molar ratio effect to DES freezing point.
Freezing point value is decreased by the increasing of
DES molar ratio. Freezing point value from DES 1
until DES 8 are 86.5, 79.1, 55.8, 36.7, 35.4, 25.4, 15.7
and 14.5
o
C. Mixing ammonium quarterner salt
(K
2
CO
3
) with hydrogen bond donor at a certain molar
ratio to form eutectic mixture forms the liquid phase
of DES. HBD forms a simple complex with anion
salts which leads to a reduction of the lattice energy
in the system and decrease of freezing point
(Manurung, 2018d). So it can be stated that the
suitable DES molar ratio to be used as a solvent are
1:6. 1:7 and 1:8 because these molar ratio produced
DES with the lowest freezing point.
4.1.3 Freezing Point of DES
Figure 2: Molar ratio effect to DES density.
Density is one of DES important physical properties.
The produced DES density also affected by different
molar ratios. It was found that by the increasing of
HBD mole fraction will also increase the DES density
(Manurung, 2018a). Figure 2 shows that the effect of
molar ratio of K
2
CO
3
: 1,2-propanediol to the DES
density value.
All of the DES density values were above 1.04
g/cm
3
. This value lies between the density of K
2
CO
3
and the density of 1,2-propanediol, where their
density are 2.43 g/cm
3
and 1.040 g/cm
3
respectively.
The density value of DES is higher than the density
of water and density of HBD constituent.
4.1.4 Viscosity of DES
Viscosity describes the internal friction forces which
occur in the moving fluid or in other words viscosity
is fluid resistance to each flow. Most reports about
DES show that DES has high viscosity value, usually
above 100 cP. Viscosity is an important characteristic
of DES (Manurung, 2018b,d). Figure 3 shows that the
effect of molar ratio of K
2
CO
3
: 1,2-propanediol to the
DES viscosity value.
Figure 3: Molar ratio effect to DES viscosity.
Based on Figure 3 shows that the viscosity values
for each DES4, DES5, DES6, DES7 and DES8
respectively are 15.18, 11.88, 9.98, 8.49 and 7.80 cSt,
where obtained DES viscosity is below 100 cP at
room temperature (Manurung, 2018b,d). Solid phase
can be seen at molar ratio of K
2
CO
3
to 1,2-
Propanediol of 1:2 and 1:3. In this case can not be
measured and not good choice to applicated as a green
solvent.
The highest of DES viscosity is believed due to
the presence of hydrogen-bonding excess between
combinations that limit the movement of free bonds
within the DES. Other interactions, such as Van Der
Waals and electrostatic interactions, can also affect
the highest viscosity in DES (Zainal, 2017).
4.2 Concentration of Vitamin E
In this part, the goal to be achieved is to know the
potential and effectiveness of K
2
CO
3
-based DES with
86.5
79.1
55.8
36.7
35.4
25.4
15.7
14.5
1
11
21
31
41
51
61
71
81
91
1 : 2 1 : 3 1 : 4 1 : 5 1 : 6 1 : 7 1 : 8 1 : 9
Freezing Point (
o
C)
Molar Ratio of K
2
CO
3
: 1,2-propanediol
1.05
1.04
1.28
1.38
1.56
1
1,1
1,2
1,3
1,4
1,5
1,6
1,7
1 : 5 1 : 6 1 : 7 1 : 8 1 : 9
Density (g/ml)
Molar Ratio of K
2
CO
3
: 1,2-propanediol
15.18
11.88
9.98
8.49
7.8
1
4
7
10
13
16
19
1 : 5 1 : 6 1 : 7 1 : 8 1 : 9
Viscosity (cSt)
Molar Ratio of K
2
CO
3
: 1,2-propanediol
Vitamin E Extraction from Red Palm Biodiesel by using K2CO3 based Deep Eutectic Solvent with 1,2-Propanediol as Hydrogen Bond
Donor
341
1,2-propanediol as HBD usage as solvent in vitamin
E extraction and biodiesel purification.
4.2.1 Pretreatment of Crude Palm Oil
The purpose of this part is to know the quality of the
used raw material in transesterification process to
produce biodiesel. The used raw material was Crude
Palm Oil (CPO), the compositions of used CPO fatty
acid are known from Gas Chromatography analysis
(Type 122-5711, Durabond-5HT; Length = 15 m,
inner diameter = 0.250 mm, film = 0.10 μm) with a
molecular weight of FFA is 271.8016 g/mol, while
the molecular weight of CPO (in the form of
triglycerides) is 853.4571 g/mol. From the analysis of
Gas Chromatography instrument, the composition of
CPO saturated fatty acids is 42.12%, while the
unsaturated fatty acid is 57.88% as shown in Table 2.
Biodiesel production that uses CPO as raw
materials is generally carried out by degumming
process at first. This step is the process of separating
unwanted gum which can usually interfere with the
stability of the oil at the next step. Degumming can
be done by treating CPO with phosphoric acid or
citric acid at certain concentrations (Manurung,
2018b), while in this research using phosphoric acid
85%.
Degumming acid with phosphoric acid is intended
to separate phosphatida which is a source of
unwanted taste and color. Gum contained in CPO
may block the activity of catalysts to accelerate
reaction equilibrium. Used DPO as raw material can
expected the purity and yield come to be higher
Manrung, 2018a,b).
4.2.2 Free Fatty Acid
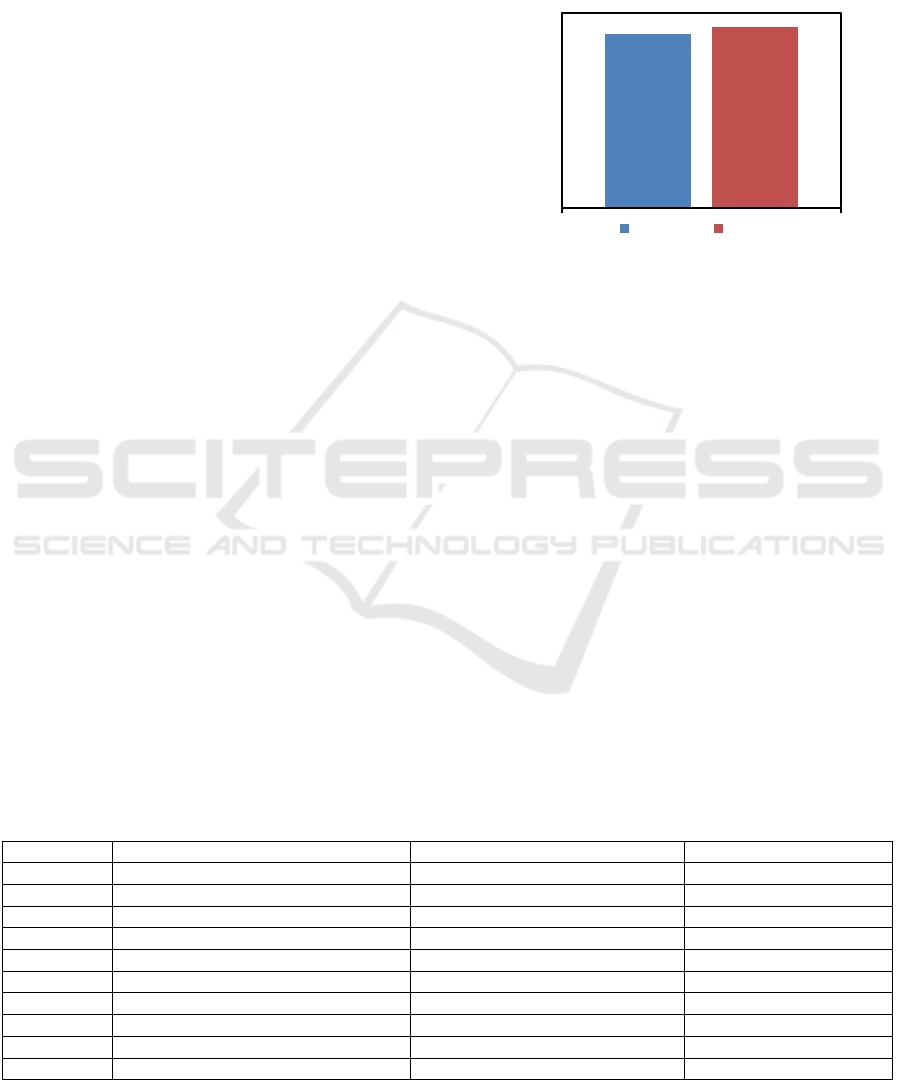
Comparison of free fatty acid (FFA) levels of crude
palm oil (CPO) and degummed palm oil (DPO) is
presented in Figure 4. From Figure 4 it can be seen
that the FFA level in DPO is higher than FFA level in
CPO. FFA level in CPO is 4.52%, while the FFA
level in DPO is 4.77%. The increase of FFA level in
CPO after degumming process is 0.19%. This
increase in FFA level indicates a decrease in most
gum and impurities which can inhibit catalyst activity
and affects the produced product.
Figure 4: Analysis of FFA content in CPO and DPO.
Based on Figure 4 it can be seen that there is an
increase in FFA levels after degumming although not
significant. It is caused by the use of phosphoric acid
capable of hydrolyzing oil or trigliserida in the
presence of heat application (Manurung, 2018a, b).
To produce biodiesel, it is important to keep FFA
levels less than 6% to prevent unwanted
saponification reactions due to the presence of free
fatty acids that react with alkaline. In the presence of
highest FFA levels can reduce the yield of biodiesel.
4.3 Extraction of Vitamin E
The determination of extracted vitamin E content
from red palm biodiesel using instrument analysis.
The analytical instrument used to test the
Table 2: Compositions of fatty acid in CPO (Crude Palm Oil).
No. Retention Time
(
minute
)
Com
p
ounds Com
p
osition
(
%
)
(
w/w
)
1 10.357 Lau
r
ic Aci
d
(
C
12:0
)
0.08
2 12.794 Miristic Aci
d
(C
14:0
)0.61
3 15.213 Palmitic Aci
d
(C
16:0
) 36.37
4 15.464 Palmitoleic Aci
d
(C
16:1
)0.11
5 17.568 Stearic Aci
d
(
C
18:0
)
4.78
6 17.764 Oleic Aci
d
(
C
18:1
)
43.01
7 18.194 Linoleic Acid
(
C
18:2
)
14.49
8 18.760 Linolenic Aci
d
(C
18:3
)0.19
9 19.826 Arachidic Aci
d
(C
20:0
)0.28
10 20.023 Eikoseneic Acid
(
C
20:1
)
0.08
0
1
2
3
4
5
FFA (%)
CPO DPO
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
342
concentration of extracted vitamin E is High
Performance Liquid Chromatography (HPLC).
Figure 5: Concentration of extracted vitamin E vs ratio of
biodiesel : DES.
Figure 5 shows an increased in extracted vitamin E
concentration by the DES6, DES7 and DES8 until
mass ratio of 1:3.5 and continued to decrease by
usage of DES with biodiesel to DES mass ratio of 1:4
and at that point DES6, DES7 and DES8 reached the
lowest concentration of vitamin E which can be
extracted by K2CO3 based DES with 1,2-propanediol
as HBD.
Vitamin E concentration which can be extracted
by DES6 with biodiesel to DES mass ratio of 1:2,
1:2.5, 1:3, 1:3.5 and 1:4 respectively are 251.30,
266.62, 274.44, 296.23 and 282.87 ppm, by DES 7
with biodiesel to DES mass ratio of 1:2, 1:2.5, 1:3,
1:3.5 and 1:4 respectively are 232.62, 334.87, 358.80,
392.16 and 276.87 ppm and by DES8 with biodiesel
to DES mass ratio of 1:2, 1:2.5, 1:3, 1:3.5 and 1:4
respectively are 305.47, 315.75, 369.19, 337.19 and
248.61 ppm.
The polarity of the DES is an important factor in
determining separation efficiency which is influenced
by the interactions between the solute (vitamin E) and
the DES. In this research used water to get vitamin E
concentrate from DES-methanol by using water-
hexane method in the volume ratio of 4:1 v/v in the
funnel separation. The result in this research is fit
with the conducted study by (Dai, 2015). DES
containing high percentage of water will result in high
yield at polar bond, while DES containing low
percentage of water will result in low yield at non-
polar bond in the water-hexane system to recover
vitamin E. So the usage of water is to bind DES and
release Vitamin E from DES into n-hexane (Setyawa,
2011) (Tang, 2015).
The highest concentration of extracted vitamin E
from biodiesel using K
2
CO
3
based DES with 1,2-
Propanediol as HBD is resulted by DES 7. Extraction
using DES 6 and DES 7 resulted the highest
concentration of extracted vitamin E by biodiesel to
DES mass ratio of :3.5 at 296.23 ppm and 392.16
ppm, While on DES 8, the concentration of vitamin E
at biodiesel to DES mass ratio of 1:3 is 369.19 ppm.
This shows that the extraction process using K
2
CO
3
-
based DES with 1,2-propanediol as HBD can ended
extract vitamin E from palm biodiesel under certain
conditions.
4.4 Purity of Biodiesel
Purity of biodiesel is determined by the ester content
in produced biodiesel. Determination of ester content
from red palm biodiesel used Gas Chromatography
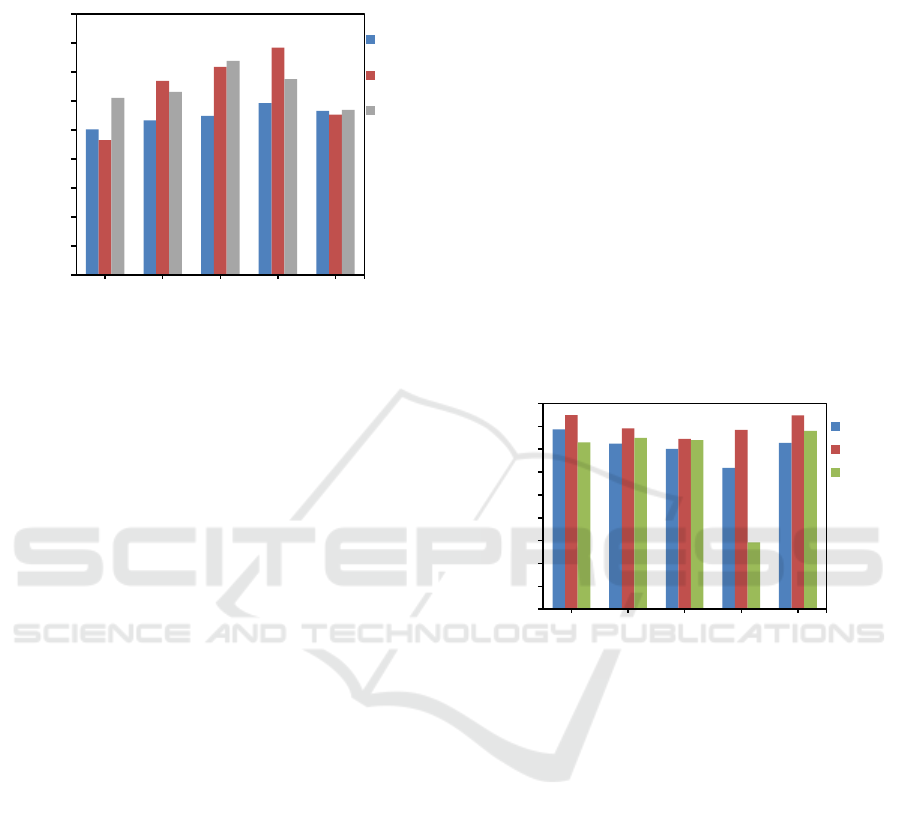
(GC) instrument. Figure 6 shows the effect of
Biodiesel : DES mass ratio to ester content.
Figure 6: Ester content (%) vs. mass ratio of biodiesel: DES.
The resulted ester content by DES6 at biodiesel : DES
mass ratio of 1:2, 1:2.5, 1:3, 1:3.5 and 1:4
respectively are 97.74%, 96.5%, 96.02%, 94.38% and
96.55% , by DES7 at biodiesel to DES mass ratio of
1:2, 1:2.5, 1:3, 1:3.5 and 1:4 respectively are 99.01%,
97.83%, 96.91%, 97.69% and 98.97% and by DES 8
at biodiesel to DES mass ratio of 1:2, 1:2.5, 1:3, 1:3.5
and 1:4 respectively are 96.60%, 97.01%, 96.79%,
87.85% and 97.61%. The higher ester content in
biodiesel means the higher purity of biodiesel. The
purity of biodiesel before extraction and after
extraction can be compared in Figure 7 below.
0
50
100
150
200
250
300
350
400
450
1:2 1:2.5 1:3 1:3.5 1:4
Concentration (ppm)
Mass Ratio of Ester : DES
DES 6
DES 7
DES 8
82
84
86
88
90
92
94
96
98
100
1:2 1:2.5 1:3 1:3.5 1:4
Ester Content (%)
Mass Ratio of Biodiesel : DES
DES 6
DES 7
DES 8
Vitamin E Extraction from Red Palm Biodiesel by using K2CO3 based Deep Eutectic Solvent with 1,2-Propanediol as Hydrogen Bond
Donor
343
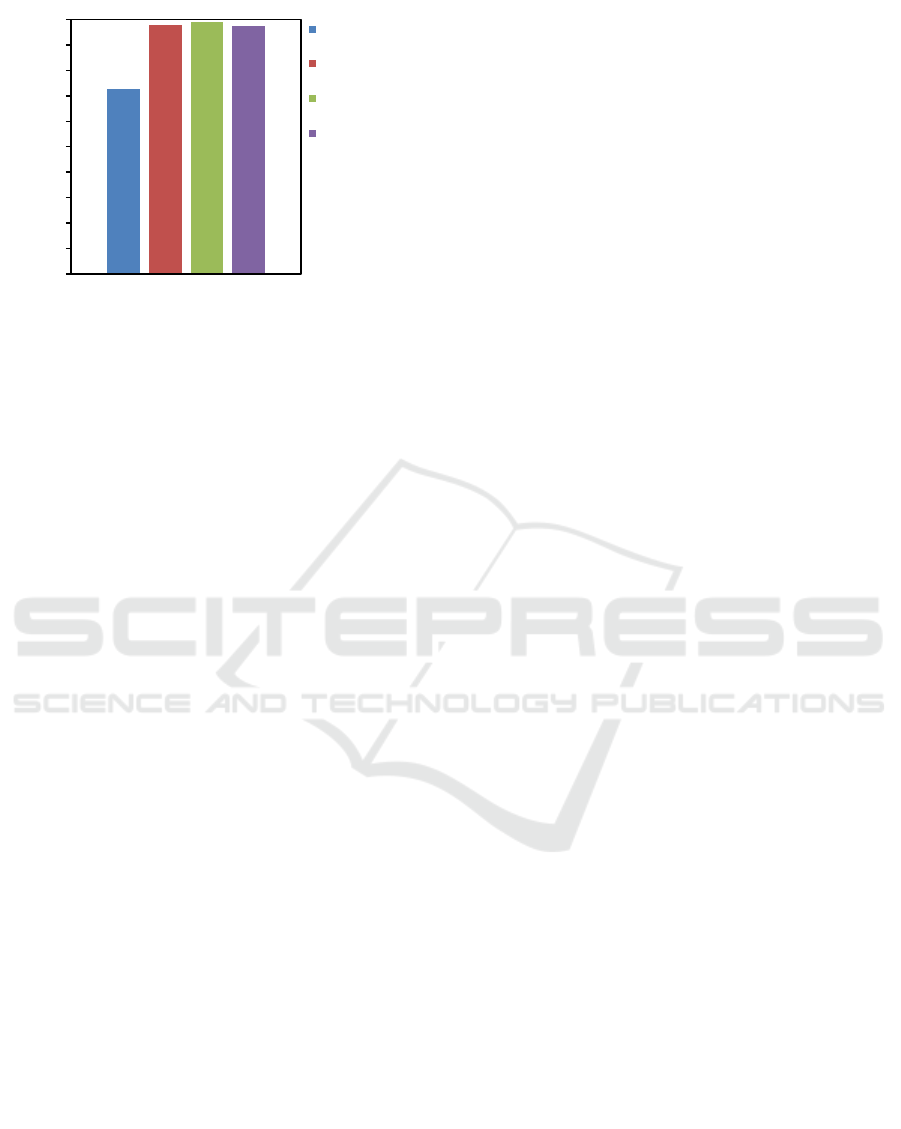
Figure 7: Comparison of biodiesel purity before and after
extraction.
Biodiesel has presence of emulsions or major
impurities such as glycerol, catalyst, alcohol,
triglycerides, diglycerides and monoglyaccharides
and minor impuritives such as carotenoids, vitamin E,
tootrienol and sitosterol (β-sitosterol) (Hamilton,
1995) will affect the purity of the esters produced.
One of minor component in biodiesel that is the
trapped vitamin E, because Vitamin E (tocopherol) is
an antioxidant that is non-polar (hydrophobic)
(Khanum and Thevanayagam, 2017). Besides that
ethyl ester is a compound that has a long carbon chain
that is also hydrophobic (Hadi, 2014), then large
amounts of vitamin E will be bound to ethyl esters
which are both non-polar and stable. In the process of
refining biodiesel with washing method using water
is not enough to increase the purity of palm biodiesel.
So it takes a solvent like DES in the purification
process to bind vitamin E and increase the purity of
palm biodiesel.
It can be seen that the purity of biodiesel before it
was extracted by potassium carbonate based DES
with glycerol as HBD, the obtained ester content was
72.53%, the low purity of produced ethyl ester is due
to contaminant component which is not efficiently
washed by water. Compared to the biodiesel after
extraction using K
2
CO
3
based DES with glycerol as
HBD where the purity of produced biodiesel
increased untill above 96.5% averagely, although
there were some results which have under the avarage
purity value but Figure 7 shows that K
2
CO
3
based
DES with glycerol as HBD not only able to extract
vitamin E but also can improve purify of the palm
ethyl ester into high purity as a biodiesel. The
addition of DES in amounts less than 5% (w/w) was
able to increase the yield of biodiesel produced
(Manurung, 2018c).
5 CONCLUSIONS
K
2
CO
3
based DES with 1.2-propanediol as HBD
which has god characteristics as solvent was obtained
by K
2
CO
3
to glycerol molar ratio of 1:7, 1:8 and 1:9
based on the characteristics of freezing point, density,
and viscosity. The used CPO had 4.52%
concentration of free fatty acid (FFA) and after
pretreatment process the concentration of FFA
increased to 4.77%. DES8 with K
2
CO
3
to 1.2-
propanediol molar ratio of 1:8 was the most effective
DES in extracting vitamin E from palm biodiesel with
biodiesel to DES mass ratio of 1:3.5 by vitamin E
concentration of 392.16 ppm. The highest palm
biodiesel purification ability was performed by using
DES8 with K
2
CO
3
to 1.2-propanediol molar ratio of
1:8 at biodiesel to DES mass ratio of 1: 2 resulted
biodiesel purity of 99.01%.
ACKNOWLEDGEMENTS
The authors are grateful to DRPM DIKTI which has
accomodated the authors in completing this paper.
REFERENCES
Bezold, F., Weinberger, M. E. and Minceva, M. 2017
Computational Solvent System Screening for The
Separation of Tocopherols with Centrifugal Partition
Chromatography Using Deep Eutectic Solvent-Based
Biphasic System. In Journal of Chromatography A,
Volume 1491, 153-158.
Craveiro, R., Aroso, I., Flammia, V., Carvalho, T., Viciosa,
M. T., Dionisio, M., Barreiros, S., Reis, R. L, Duarte,
A. R. C. and Paiva, A. 2016. Properties and Thermal
Behavior of Natural Deep Eutectic Solvents. In Journal
of Molecular Liquids, Elsevier, Volume 215, 0167-
7322.
Dai, Y., Witkamp, G. J., Verpoorte, R. and Choi, Y. H.
2015. Tailoring Properties of Natural Deep Eutectic
Solvent With Water to Facilitate Their Applications. In
Food Chemistry, Volume 187, 14-19.
Hadi, N.A., Ng, M. H., Choo, Y. M., Hashim, M. A. and
Jayakumarz, N. M. 2014. Performance of Choline‑
Based Deep Eutectic Solvents in the Extraction of
Tocols from Crude Palm OiL. In Journal of the
American Oil Chemists' Society, Volume 92(11), 1709-
1716.
Hamilton, R. J. 1995. Developments In Oils and Fats.
Chapman & Hall. London, 1
st
edition.
Khanum, R. and Thevanayagam, H. Lipid Peroxidation: Its
Effects on Formulation and Use of Pharmaceutical
Emulsions. In Asian Journal of Pharmaceutical
Sciences, Volume 12(5), 401–411.
72.3
97.74
99.01
97.61
0
10
20
30
40
50
60
70
80
90
100
BIODIESEL
Ester Content (%)
Before Extraction
Extraction Using
DES 6 ( 1 : 2 )
Extraction Using
DES 7 ( 1 : 2 )
Extraction Using
DES 8 ( 1 : 4 )
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
344

Manurung, R., Syahputra, A. and Alhamdi, M. A. 2018a.
Purification of Palm Biodisel Using Deep Eutectic
Solvent (DES) Based Choline Chloride (ChCl) and 1,2-
Propanediol (C
3
H
8
O
2
). In IOP Conference Series,
Journal of Physics: Conference Series, Volume
1028(1), 1-8.
Manurung, R., Arief, A. and G. R Hutahuruk. 2018b.
Purification of Red Palm Biodiesel by Using K
2
CO
3
Based Deep Eutectic Solvent (DES) with Glycerol as
Hydrogen Bond Donor (HBD). In AIP Conference
Proceedings, Volume 1977(1), 1-8.
Manurung, R., Hutauruk, G. R. and Arief, A. 2018c.
Vitamin E Extraction from Red Palm Biodiesel by
Using K
2
CO
3
Based Deep Eutectic Solvent with
Glycerol as Hydrogen Bond Donor. In AIP Conference
Proceedings, Volume 1977(1), 1-8.
Manurung, R., Alhamdi, M. A. and Syahputra, A. 2018d.
Palm Ethyl Ester Purification By Using Choline
Chloride-1,2 Propanediol as Deep Eutectic Solvent. In
IOP Conference Series: Materials Science and
Engineering, Volume 309, 1-8.
Sinaga, A. G. S. and Siahaan, D. 2015. Characterization and
Antioxidant Activity of Non Polar Extract from Crude
Palm Oil and Palm Methyl Ester. In International
Journal of ChemTech Research, Volume 8(4), 1810-
1816.
Setyawan, H. Y., Hambali, E., Suryani, A. and
Setyaningsih, D. 2011. Separation of Tocopherol from
Crude Palm Oil Biodiesel. In Journal of Basic and
Applied Scientific Research, TextRoad Publication,
Volume 1(9), 1169-1172.
Tang, B., Zhang, H. and Row, K. H. 2015. Application Of
Deep Eutectic Solvents In the Extraction and
Separation of Target Compounds From Various
Sample. In Journal of Separation Science, Volume
38(6), 1053-1064.
United States Department of Agriculture (2016) Oilseeds.
World Markets and Trade.
Abidin, M. H. Z., Hayyan, M., Hayyan, A. and Jayakumar,
S. M. 2017. New Horizon in The Extraction of
Bioactive Compounds Using Deep Eutectic Solvents: A
Review. In Analytica Chimica Acta, Elsevier, Volume
979, 1-23.
Vitamin E Extraction from Red Palm Biodiesel by using K2CO3 based Deep Eutectic Solvent with 1,2-Propanediol as Hydrogen Bond
Donor
345
