
Corn Stalk (Zea Mays L.) Ability on Copper Removal in Continuous
Column (Down Flow)
B. Haryanto
1
, D. A. Fithry
1
, Anita M. H.
1
, Puteri K.
1
, Azhari B. G.
1
, Ashabi S.
1
, Walid A. A.
1
and
Michael J. B.
1
1
Department of Chemical Engineering, University of Sumatera Utara, Medan 20155, Indonesia
Keywords: Corn-stalk, Continus-column, removal-ability.
Abstract:
Corn stalk was used as an adsorbent to observe removal metal ions (Cu
2+
). The loading time and channeling
effect in continuous adsorption column with down flow direction was investigated in concentration 50 ppm
of Cu with variation influent flow rates (5, 10, 15 mL/min) and shape/size adsorbent (1/4 round shape, 50
mesh, and 70 mesh). Kinetic of corn stalk adsorption ability has been observed at influent flow rate 5
mL/min for adsorbent size 70 mesh. The adsorption was applied in the column and down flow direction.
The effluent samples were collected in every interval 28 mL. The results showed that the % removal
efficiency was obtained 98,30; 62,78; 34,74 (%) for with sampling volume 84 mL was reach equilibrium.
The highest removal efficiency obtained 34,74 % at flow rate 5 mL/min with adsorbent size 70 mesh.The
shortest loading time obtained at 15 mL/min with corn stalk adsorbent shape at 1/4 round. Phenomenon of
channeling effect was clearly exist in adsorbent shape at 1/4 round.
1 INTRODUCTION
Copper (Cu
2+
) is known as dangerous heavy metals
which are usually found in wastewater in industrial
activities such as mining, plating, smelting, and on
agriculture such as fertilization, pesticides and so on.
Source of drinking water contaminated with excess
copper can cause various diseases in the body of the
organism (Hui, 2015) (Wang, 2016) (Rehab, 2016).
Recommendation from WHO (World Health
Organization) for safe amount of Cu
2+
ions is 2
mg/L in drinking water and 3 mg/L in industrial
waste disposal (Rifaqat and Umra, 2017).The
adsorption method is a commonly used method
because it is effective and also economical to
remove various metals from waste water. The key to
the success of the adsorption method is the selection
of adsorbents. Adsorbents used can be derived from
agricultural waste and industrial solid waste (Malihe,
2015).
The adsorption process can be operated with two
systems: batch system and continuous system
(column) (Martin, 2016). The column system is an
effective and economical method for large volume
capacity, simple design and scale up of system
(Shahram, 2016). In the column system can be done
with two-way flow of the flow from top to bottom
(down flow) and flow from bottom to top (up flow)
(Maksudur, 2015).
Corn stalks have good potential to be used as
bioadsorbents, due to their presence in abundant and
untapped environments. Corn stalk has been
investigated to remove copper metal by using batch
method in solution with concentration 50 ppm and
pH 4,5 (Haryanto, 2017). The utilization of corn
stalk as an adsorbent to absorb Cu2+ metal ions has
been done by previous researchers (Haryanto, 2017).
The study was conducted in a batch system by
varying the form of adsorbent, contact time and
stirring speed.
This research is a continuation of the above
research is to know the ability of corn stalks
adsorbent on Cu2+metal in the adsorption column is
continuous (down flow). Down flow flow direction
is used because it provides ease of operation and
also ability as a filter simultaneously. This stream
Haryanto, B., Fithry, D., M. H., A., K., P., B. G., A., S., A., A. A., W. and J. B., M.
Corn Stalk (Zea Mays L.) Ability on Copper Removal in Continuous Column (Down Flow).
DOI: 10.5220/0010093903230327
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
323-327
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
323
can be operated with the help of gravity (Sunil and
Jayant, 2015).
2 METHODOLOGY
The material used in this research is corn stalk
obtained from corn garden in Padang Bulan Village-
Medan Selayang Sub-district Medan, Indonesia.
Corn stalk used in this study is ¼ round shape with a
thickness of ± 0.5 cm, then the size of 50 mesh and
70 mesh. The solution used is CuSO
4
. 5H
2
O,
hydrochloric acid (HCl) was purchased from
Mallinckrodt Baker, Inc., Paris, sodium hydroxide
(NaOH) was purchased from Merck KgaA,
Darmstadt, Germany, as a pH and water regulator of
the Aquadestilator model: SMN BIO, as a solvent.
The equipment used in this study includes
adsorption columns (diameter 1.5 cm and 7.5 cm
high) and peristaltic pumps. Analyzes used AAS,
FTIR and SEM.
After the tools and materials are prepared, it is
determined first loading time on each variation and
observed the channeling effect that is formed. The
adsorption ability was obtained from the analysis of
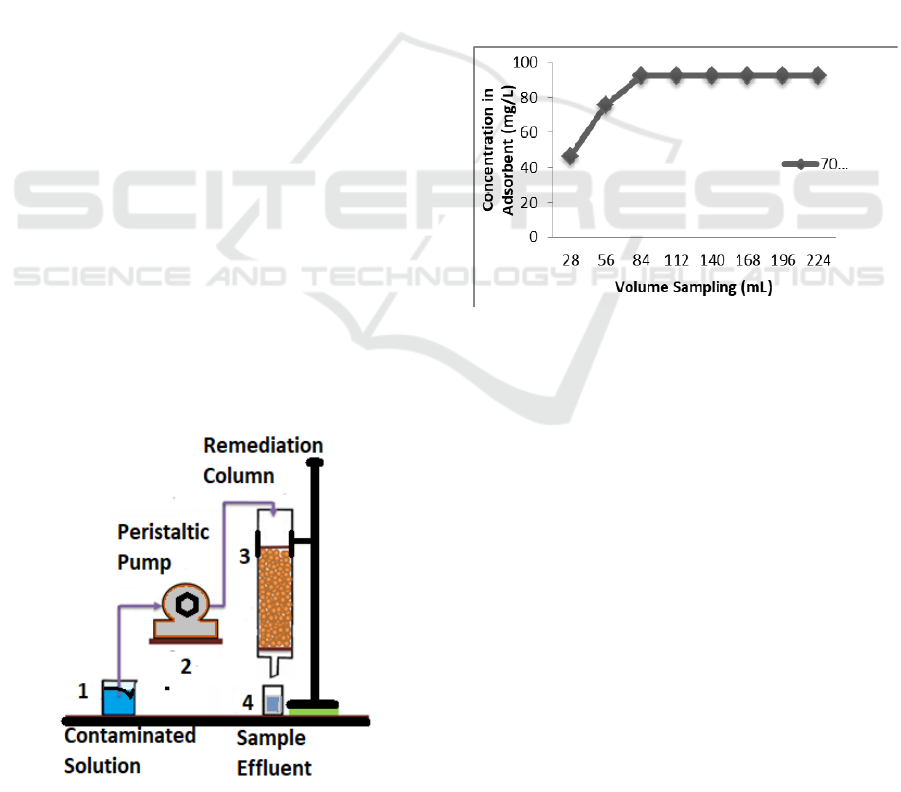
metal solutions at 28 mL intervals. Figure 1 shows a
series of adsorption equipment.
Process description: A 50 ppm metal solution in
beaker glass (1) is pumped with a peristaltic pump
(2) with an X mL / min flowrate to a column
containing corn stalk (3). Then the effluent of the
contamination results is accommodated on a
measuring cup (4) and is determined as a sampling
point which is then analyzed by AAS to examine
how much of the absorbed Cu
2+
metal and the ability
of the corn stalk to absorb Cu
2+
metals.
Figure 1: Adsorption equipment set (Dalia, 2015).
3 RESULTS AND DISCUSSION
3.1 Corn Stalk Removal Efficiency
Kinetic Adsorption (Size 70 mesh)
The highest corn stalk adsorbent capability in
absorbing copper metal ions occurs at the beginning
of the adsorption process, but with increasing
absorption time decreases until equilibrium
conditions are achieved. This condition is caused,
the adsorbent active site which absorbs metal ions
has saturated (Guyo, 2015).
In Figure 2 shows kinetics removal efficiency
based on the accumulation of sampling volume.
Removal efficiency increases with the accumulated
volume of sampling. From the data obtained it can
be concluded that 1 gr of corn bar adsorbent able to
absorb Cu2+ metal ions of 92.2634 ppm.
Figure 2: Removal efficiency kinetics on corn stalk
adsorption volume accumulation.
3.2 Effect of Flow Rate and Adsorbent
Shape/Size on Removal Efficiency
The result data of the influence of the flow rate and
the shape/size of the adsorbent on removal
efficiency on corn stalk adsorption are presented in
Figure 3.
In Figure 3 removal efficiency increases with
increasing surface area of corn stalk adorbent. At a
flow rate of 5 mL with the shape and size of a 1/4
round; 50 mesh; 70 mesh obtained removal
efficiency 9.77; 25,47; 34.74 (%). At a 10 mL flow
rate with the shape and size of a 1/4 round; 50 mesh;
70 mesh obtained removal efficiency 2,46; 17,36;
22.50 (%). At a flow rate of 15 mL with the shape
and size of a 1/4 round; 50 mesh; 70 mesh obtained
0.84 removal efficiency; 7.40; 9.53 (%). On the
adsorbent size of 70 mesh obtained higher removal
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
324
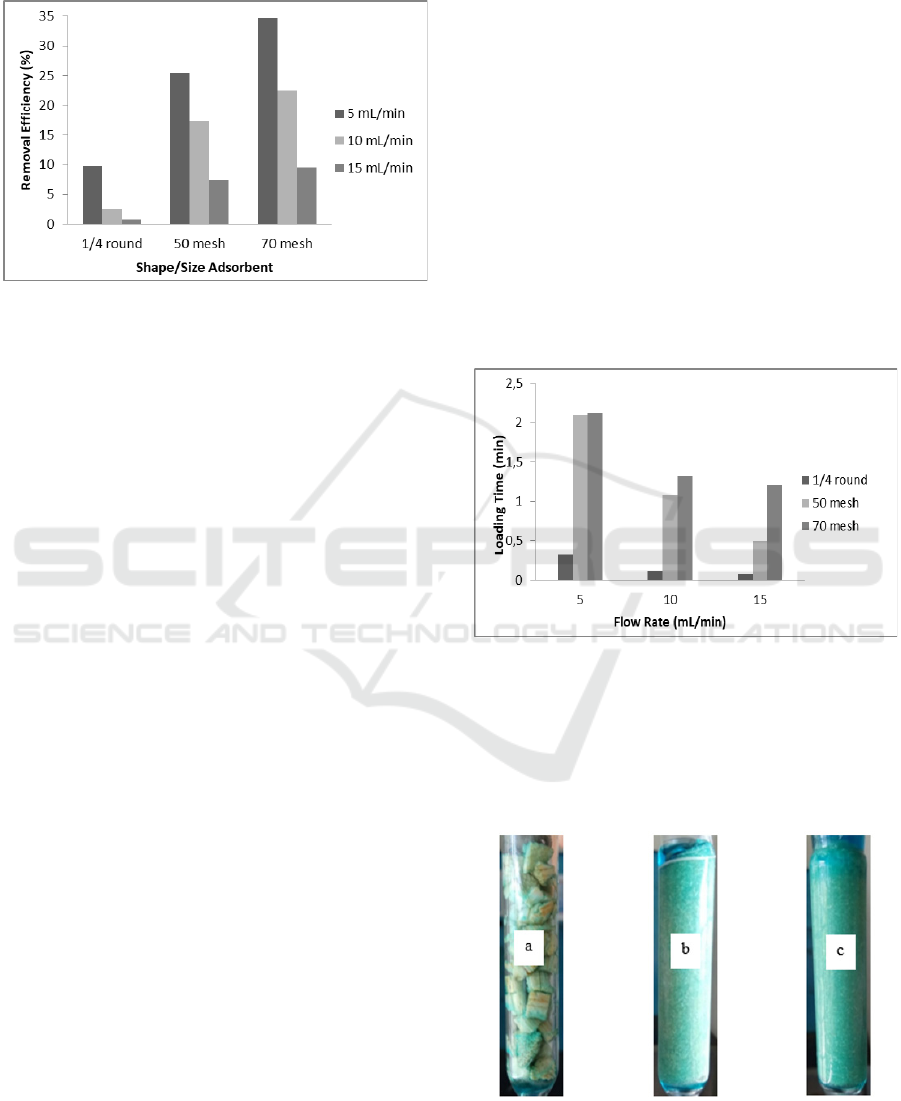
efficiency when compared with the size of 50 mesh
and 1/4 round.
Figure 3: Influence adsorbent shape/size on removal
efficiency.
The removal efficiency decreases with increasing
flow rate. In the Flow rate is an important parameter
as a metaphor of contact time of solution with
adsorbent in adsorption column. With increasing
flow rate then removal efficiency will decrease. At
high flow rates the contact time of the inlet solution
with the adsorbent is ineffective to exclude the metal
ion as the solution leaves the column before the
equilibrium is reached, resulting in a high effluent
solution concentration (Kumar, 2015). High flow
rates cause limited intraction between the pores and
the inlet solution resulting in low removal
efficiency. In this study the best flow rate is 5
mL/min.
As the particle diameter increases, the stagnant
film thickness around the particles increases
resulting in the kinetics of the process decreasing as
the time for the adsorbent absorbs the short ionic
molecule (Ensar and Muhammed, 2014). Increased
absorption rate is affected by small particle size,
since small particles have large surface of
adsorbents. The breaking of large particles becomes
smaller aims to open the gaps on the surface of the
adsorbent so that the diffusion process is more easily
achieved (Kartthikeyan, 2004). In this study the best
adsorbent size is 70 mesh.
3.3 Effect of Loading Time and
Channeling Effect
Loading time is the time required for the solution to
penetrate the pores of the adsorbent until it exits
from the adsorption column. Determination of
loading time can also be affected by channeling
effect (Haryanto, 2018) The effect of loading time
and channeling effect is shown in Figure 4.
In Figure 4 with variation of adsorbent size at
increasing flow rate obtained loading time
decreasing. In the shape of 1/4 round with flow rate
5; 10; 15 (mL / minute) obtained loading time 0,32;
0.11; 0.08 (minutes). At the size of 50 mesh with a
flow rate of 5; 10; 15 (mL / min) obtained loading
time 2.09; 1.08; 0.50 (minutes). At size 70 mesh
with flow rate 5; 10; 15 (mL / min) obtained loading
time 2.12; 1.32; 1.20 (minutes).
Increasing the flow rate and down flow flow
direction influenced by the force of gravity causes
the time required by the solution to exit from the
shorter column. Because the time required by the
solution to come out short then loading time will
decrease. Loading time is the time required for the
solution to penetrate the pores of the adsorbent until
it exits from the adsorption column (Haryanto,
2018).
Figure 4: Effect of loading time on shapes variation.
The shape and size of the adsorbent also affects
the time required by the solution to exit the column.
The larger the shape and size of the adsorbent will
result in the formation of a gap that causes the
solution to rapidly exit the column.
Figure 5: Image of channelling effect on shapes variation.
Corn Stalk (Zea Mays L.) Ability on Copper Removal in Continuous Column (Down Flow)
325
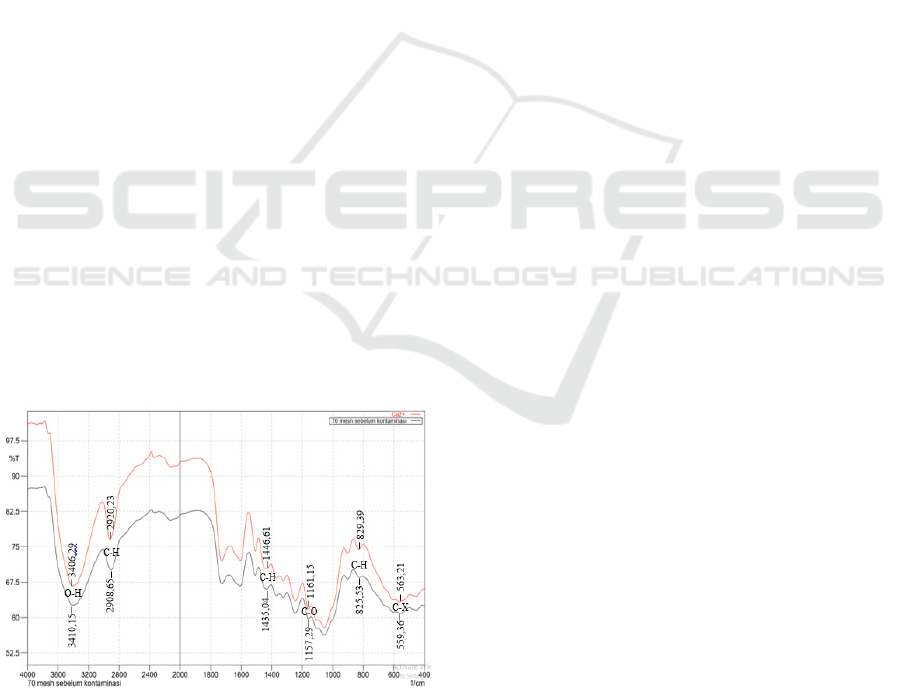
In Fig. 5 with different shapes and sizes of
adsorbents showing the presence of an channelling
effect. The phenomenon of channeling effect is
evident in the shape of 1/4 round. In the figure with
the shape of a 1/4 round forming a large gap, so that
when the solution comes in there is a portion of the
surface of the non-wetted adsorbent as a whole. This
can be seen from the color difference in the
adsorbent. At the size of 50 mesh and 70 mesh
channeling effect also occur, but the phenomenon of
channeling effect is not obvious because the gap
formed on the size of 50 mesh and 70 mesh is very
small.
Different shapes and sizes of adsorbents can affect
the porosity of the adsorbent associated with the
fluid velocity that flows in the column. The varying
porosity of the adsorbent may result in a difference
in drag force in the fluid stream which causes the
fluid flow tendency to move freely, resulting in
channeling effect (
Vafai, 1986).
Loading time and channeling effect will affect the
ability of adsorption of corn stalk. With the increase
in flow rate then loading time will decrease. High
flow rates cause limited intraction between the pores
and the inlet solution resulting in low removal
efficiency. Given the phenomenon of channeling
effect resulting in absorption capacity at large
adsorbent size is not maximal because the formation
of a large gap between the adsorbents on the column
resulted in the metal solution out quickly before
interacting on the surface of the adsorbent. In this
study the best flow rate is 5 mL / min and the best
adsorbent size is 70 mesh.
3.4 Results FT-IR and SEM Analyzes
Figure 6: Figure 6 FT-IR Analysis results.
Fourier Transform Infra Red (FT-IR) analyzes of 70
mesh corn stalk adsorbent before and after
contamination of Cu
2+
metal ions were performed to
identify the functional groups present in each
sample. From the functional group analysis using
FT-IR obtained spectrum results are presented in
Figure 6.
Figure 6 shows the increase and decrease of wave
numbers before and after contamination. Increasing
the wave number before and after the contamination
occurred at the wave number 2908.65 cm-1 to
2920,23 cm-1 is the C-H bond wave number; the
wave number 1435.04 cm-1 to 1446,61 cm-1 is the
number of the C-H bond wave; the wave number
1157.29 cm-1 to 1161.15 cm-1 is the number of the
bond wave C-O; the wave number 825.53 cm-1 to
829.39 cm-1 is the number of the C-H bond wave;
the wave number 559.36 cm-1 to 563.21 cm-1 is the
number of the C-X bond wave. The decrease of
wave numbers before and after contamination
occurred at the wave number 3410.15 cm-1 to
3406.29 cm-1 is the O-H bond wave number
(
Skoog, 1998). The shift in wavelength indicates
there is an interaction of adsorption absorption
between functional groups and Cu
2+
ions. Cu
2+
ions
may bind to carboxyl groups and hydroxyl groups
(Rifaqat and Umra, 2017).
The result of Scanning Electron Microscope
(SEM) analysis on 70 mesh corn stalk adsorbent
before and after contaminated Cu
2+
metal ion was
done to identify morphological structure of corn
stalk. From the analysis of the morphological
structure using SEM the results obtained are
presented in Figure 6.
4 CONCLUSIONS
The conclusion that can be obtained is kinetics%
removal efficiency of 98.30; 62,78; 34.74 (%) with
84 mL sampling volume has reached saturation
point. The highest efficiency removal was obtained
34,74% at 5 mL/minute flow rate with 70 mesh corn
stalk adsorbent. The shortest loading time is
obtained at a flow rate of 15 mL/min with a corn
stalk 1/4 round adsorbent. The phenomenon of
channeling effect is evident in the shape of a 1/4
round corn stalk adsorbent.
ACKNOWLEDGMENTS
The authors wish to express sincere gratitude to
Lembaga Penelitian Universitas Sumatera Utara on
the DRPM Project 2018, No:24/UN5.2.3.1/PPM
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
326

/KP-DRPM/2018, for the financial support for this
research project.
REFERENCES
Dalia, M S, Ewa, C, & Hlanganani, T., 2015. ‘Column
adsorption studies for the removal of U by
phosphonated cross-linked polythylenimine:
modelling and optimazation’, Appl Water Sci, vol.5,
pp. 57-63.
Ensar, O, & Muhammed, E., 2014. ‘Biosorption of Cobalt
(II) with sunflower biomass from aquueous solutions
in a fixed bed column and neural networks
modelling’, Ecotoxicology and Environmental Safety,
vol. 99, pp. 54-60.
Guyo, U, Makawa, T, Moyo, M, Nharingo, T, Nyamunda,
BC, & Mugadza, T., 2015. ‘Application of reponse
surface methodoogy for Cd(II) adsorption on maize
tassel-magnetite nanohybrid adsorbent’, Journal of
Environmental Chemical Engineering, vol.3, pp.
2472-2483.
Haryanto, B, Singh, WB, Barus, ES, Ridhom A, & Rawa
MR., 2017. ‘Pseudo order kinetics model to predict the
adsorption interaction of corn-stalk adsorbent with
metal ion adsorbate Cu(ii) and Fe(ii)’, Journal of
Physic: Conference Series, vol. 801, pp. 012098.
Haryanto, B, Tambun, R, Haloho, H, Panjaitan, F, &
Sitorus, S., 2017. ‘Investigation on the ability of a
natural adsorbent corn stalk in removing heavy metal
ions from aqueous solution’, ARPN Journal of
Engineering and Applied Science vol.12, no.18, pp.
5263 – 5270.
Haryanto, B, Singh, WB, Chang, CH, Chairunnisa, A, &
Butarbutar, FR., 2018. ‘Loading time and channeling
effect in removing copper ions from sand surface by
surfactin solution in flushing column’, IOP
Conference Series: Material Science and
Engineering, vol. 308, pp. 012021.
Hui, H, Jubin, Z, Kangli, L, & Yayun, T., 2015.
‘Characterization of acidosasa edulis shoot shell and
its biosorption of copper ions from aqueous solution’,
Journal of Environmental Chemical Engineering, vol.
3, pp. 357-364.
Kartthikeyan, G, Anbalagan, K, & Andal NM., 2004.
’Adsorption dynamics and equilibrium studies og
Zn(II) onto chitosan’, Indian J. Chem. Sci., vol.116
no.2, pp. 119-127.
Kumar, P S, Deepthi, ASLS, Bharani, R, & Rakkesh, G.,
2015. ‘Study of adsorption of Cu(II) ions from
aqueous solution by surface-modified eucalyptus
globulus seeds in a fixed-bed column: experimental
optimization and mathematical modeling’, Res Chem
Intermed, vol. 41, no.11, pp. 8681–8698.
Maksudur, M R K, Wasikur, M R, Salatul, M I M, Kaniz,
F, Huei, R O, Chan, K M, & Prasad, RDM., 2015.
‘Peformance of a submerged adsorption column
compared with conventional fixed-bed adsorption’,
Desalination and Water Treatment, vol. 57 no. 21, pp.
9705-9717.
Malihe, F, Masoud, B, & Hassan S., 2015. ‘Single and
binary adsorption of nickel and copper from aqueous
solutions by γ-alumina nanoparticles: equilibrium and
kinetic modeling’, Journal of Molecular Liquid, vol.
211, pp.1060-1073.
Martin-Lara, MA, Blazquez, G, Calero, M, Almendros,
AI, & Ronda, A., 2016. ‘Binary biosorption of copper
and lead onto pine cone shell in batch reactors and in
fixed bed columns’, International Journal of Mineral
Processing, vol.148, pp. 72-82.
Rehab, M A, Hesham, A H, Mohamed, M H, & Gihan, F
M., 2016. ‘Potential of using green adsorbent of heavy
metal removal from aqueous solutions: adsorption
kinetics, isotherm, thermodynamic, mechanism and
economic analysis’, Ecological Engineering, vol. 91,
pp. 317-332.
Rifaqat, A K R, & Umra, K., 2017. ‘Adsorption studies of
Cu(II) on boston fern (nephrolepis exaltata schott cv.
bostoniensis) leaves’, Applied Water Science, vol. 7,
no. 4, pp. 2051–2061.
Shahram, A, Madhumita, B R, & Argyrios, M., 2016.
‘Copper ion removal by acer saccharum leaves in a
regenerable continuous-flow column’, Chemical
Engineering Journal, vol. 287, pp. 755-764.
Skoog, Holler, & Neiman., 1998. Principles of
Instrumental Analysis, Orlando: Harcourt Brace 7
Co. 5th edition.
Sunil, J K, & Jayant, P K., 2015. ‘Analysis of packed bed
adsorption column with low cost adsorbent for
cadmium removal’, Int. J. Of Thermal &
Environmental Engineering vol. 9, no.1, pp.17-24.
Wang, JY, Han, C, Cui, CW, & Xing, DF., 2016.
‘Bosorption of copper (II) from aqueous
solution by
aspergillus niger-treated rice straw’.
Ecological Engineering, vol. 95, pp. 793-
799.
Vafai, K., 1986. ‘Analysis of the channeling effect in
variable porosity media’, Journal of Energy Resources
Technology, vol. 108 no. 2, pp. 131-139.
Corn Stalk (Zea Mays L.) Ability on Copper Removal in Continuous Column (Down Flow)
327
