
In Silico Analysis of Boron Derivate Compounds as Potential ER-α
Inhibitor
Urip Harahap
1
*, Ginda Haro
1
, Hari Purnomo
2
and Denny Satria
3
1
Department of Pharmacology, Universitas Sumatera Utara, Medan, Indonesia.
2
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Gadjah Mada University, Yogyakarta, Indonesia
3
Department of Pharmacology, Universitas Sumatera Utara, Medan, Indonesia.
Keywords: BornUSU I, BornUSU II, ER-α, Inhibitor, In silico.
Abstract: Background :BornUSU I or Boronhafagama I (1,5-bis(4-hydroxyphenyl)-3-oxa-1,5-diaza-2,4-diboropentane-
2,4-diol) and BornUSU II or Boronhafagama II (1-(4-hydroxynaphthalen-1-yl)-5-(4-hydroxyphenyl)-3-oxa-
1,5-diaza-2,4diboropentane-2,4 diol) are boron derivate compounds which are boron neutron captured therapy
(BNCT) candidates. Estrogen receptor alpha (ER-α) appear a crucial assignment in the growth and
development of bone, breast and uterine pathology especially in human cancers, including breast
cancer.Tamoxifen has been used as a cure for women who have been identified breast cancer for around four
decades. Tamoxifen has high risks, such as the risk endometrial malignancy and hyperplasia varies from 1.5
to 6.9 fold after cumulative and long duration usage. Methods: In silico docking using PLANTS programme
and visualized by Pymol programme. The model of three dimension enzyme structures used in this research
was ER-α, binding pocket with the Protein Data Bank (PDB) code 3ERT. Results: Two and three dimension
of compounds and 4-hydroxytamoxifen as the standard were generated using Marvin Sketch program. Both
compoundsand standards inhibitedER-α with docking score -92.1697; -
1 INTRODUCTION
Breast cancer is the most incidence cancer and the
second famous cause of cancer death in females
(Jemal, et al., 2010). Then, breast cancer ranks as the
fifth cause of death from cancer on the whole and the
most frequent cause of cancer death in women in less
developed countries, and the second cause of cancer
death in developed countries after lung cancer. A
recent study published which breast cancer is leading
in the estimate new cancer cases, and the second most
general death cause among women suffering from
cancer in the America (Siegel, et al., 2010).
Estrogen plays a critical role in the growth and
development of bone, breast and uterine pathology.
There are two subtypes of estrogen receptor, ER-α
(Estrogen Receptor alpha) and ER-β (Estrogen
Receptor beta). ER-α plays a role in cell proliferation
and has been found in the endometrial, breast cancer
and ovarian stromalcell, as well as in the
hypothalamus (Levin, 2005). Tamoxifen is also
prescribe for breast cancer patients as hormonal
inhibitor. The tamoxifen-bound ER complex inhibits
the genes from being switched on by Estrogen,
leading to the prevention of the Estrogenic leverage
which accountable for cancer cell proliferation
(Chang, 2012). Tamoxifen has high risks in women
who have been it in their therapy (Subarnas, et al.,
2015) after cumulative and long duration wear
(Cohen, et al., 2003). With all of these risks many
patients regardless this therapy. Thus, alternative
treatments are needed.
Boron Neutron Capture Therapy (BNCT) is an
progress form of radiotherapy technique which is
potentially supreme to all conventional techniques for
cancer treatment, as it is targeted at killing individual
cancerous cells with minimal harm to surrounding
healthy cells (Payudan. Et al., 2016). Boronic
compounds has been before used in imaging and
medicinal chemistry offering unique advantages
associated with its low toxicity and stability (Trippier,
and McGuigan, 2010). BornUSU 1 (1,5-bis(4-
hydroxyphenyl)-3-oxa-1,5-diaza-2,4-diboropentane-
2,4-diol) or Boronhafagama I and BornUSU 2 or
Boronhafagama II (1-(4-hydroxynaphthalen-1-yl)-
5-(4-hydroxyphenyl)-3-oxa-1,5-diaza-
2,4diboropentane-2,4-diol). The chemical structures
814
Harahap, U., Haro, G., Purnomo, H. and Satria, D.
In Silico Analysis of Boron Derivate Compounds as Potential ER- Inhibitor.
DOI: 10.5220/0010091808140817
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
814-817
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
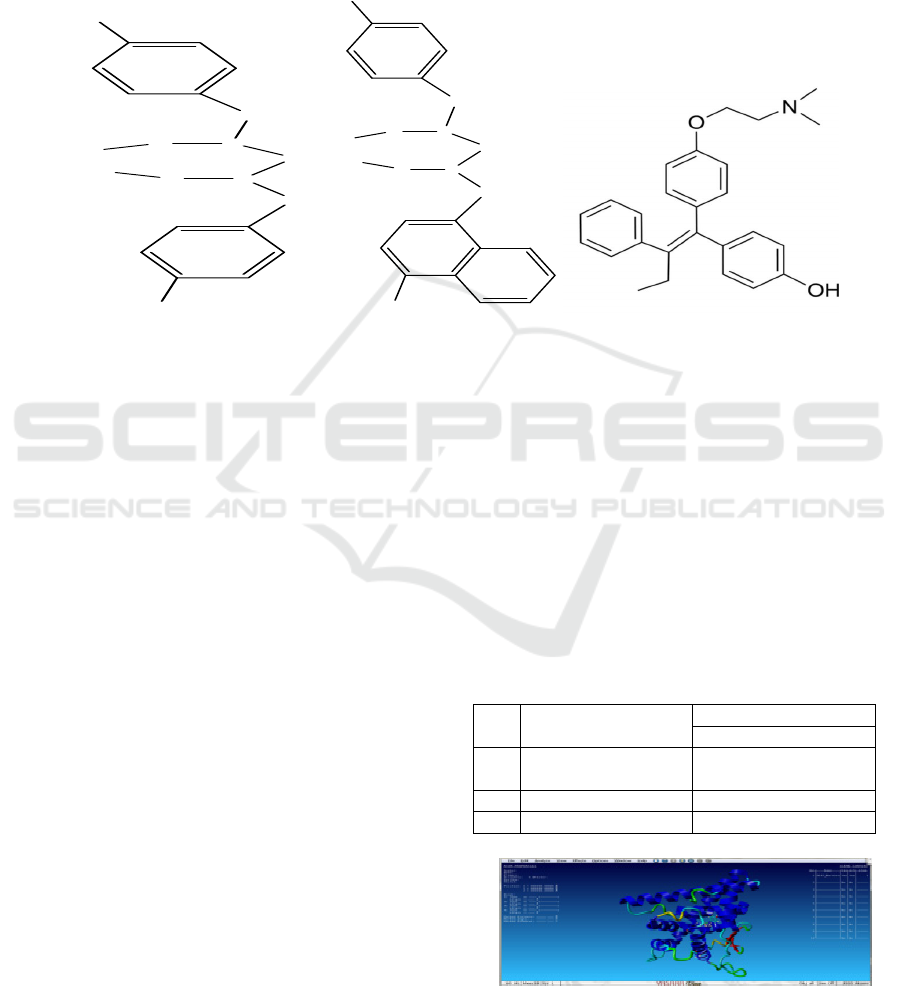
of BornUSU I, BornUSU II, tamoxifen and 4-
hydroxy tamoxifen are showed in Figure 1.
Computational analysis are being expanded to
evaluation in predict the compounds activity. In silico
approaches contribute significantly to beginning
pharmaceutical research and notable in object
discovery. (Bharath, et al., 2011). The purpose of our
study was to identify how ER-α inhibitors are
working for cancer using in silico molecular docking
method. The purposes of this research was to assess
the activity of BornUSU I, BornUSU II, tamoxifen
and 4-hydroxy tamoxifen in inhibiton ER-α with in
silico method.
HO
NH
B
O
O
BO
NH
HO
H
H
HO
NH
B
O
O
BO
NH
HO
H
H
a b c
Figure 1. Structure of (a) BornUSU I, (b) BornUSU II, and (c) 4-hydroxy tamoxifen.
2 METHODS
Aspire E1-470 series operated by Windows 7 Home
Premium, Intel
®
Core
TM
i3 -3217U (1,8 GHz, 3MB
L3 cache), 32-bit, hard disc drive 500 GB and RAM
memory 2 GB DDR3 L were used to run the
molecular docking process.
In silico docking using PLANTS program and
visualized by pymol programmee. Co Pen Drive
Linux KDE program was used to connecting
Windows operation system to Linux operation
system. The model of three dimension of enzyme
structure used in this research was ER-α binding
pocket with the Protein Data Bank (PDB) code 3ERT.
It was obtained through from
http://www.rscb.org/pdb. Two and three dimension
conformation models of BornUSU 1, BornUSU 2 and
4-hydroxy tamoxifen as the standard inhibitor were
generated by Marvin Sketch program.
3 RESULT
The Root Mean Square Deviation (RMSD) values
resulted from these ligand docking was 1.6276Å for
3ERT. The RMSD was obtained less than 2.0000 Å
indicating that the docking methods were valid
(Terstappen., and Reggiani, 2001). In silico docking
between BornUSU 1, BornUSU 2, tamoxifen and 4-
hydroxy tamoxifen into 3ERT binding pocket result
inthe docking scorein Table 1 isshowed the results of
docking score into 3ERTbinding pocket. Figure 2 and
3 are showed the results of visualization of BornUSU
1, BornUSU 2 and 4-hydroxy tamoxifen to ER-α
using pymol.
Table 1. Docking score between ligand and protein target
No Ligand Name
Dockin
g
Score
ER-α
1 4-hydroxy
tamoxifen
-99.0879
2 BornUSU I -92.1697
3 BornUSU II -100.1940
A
In Silico Analysis of Boron Derivate Compounds as Potential ER- Inhibitor
815
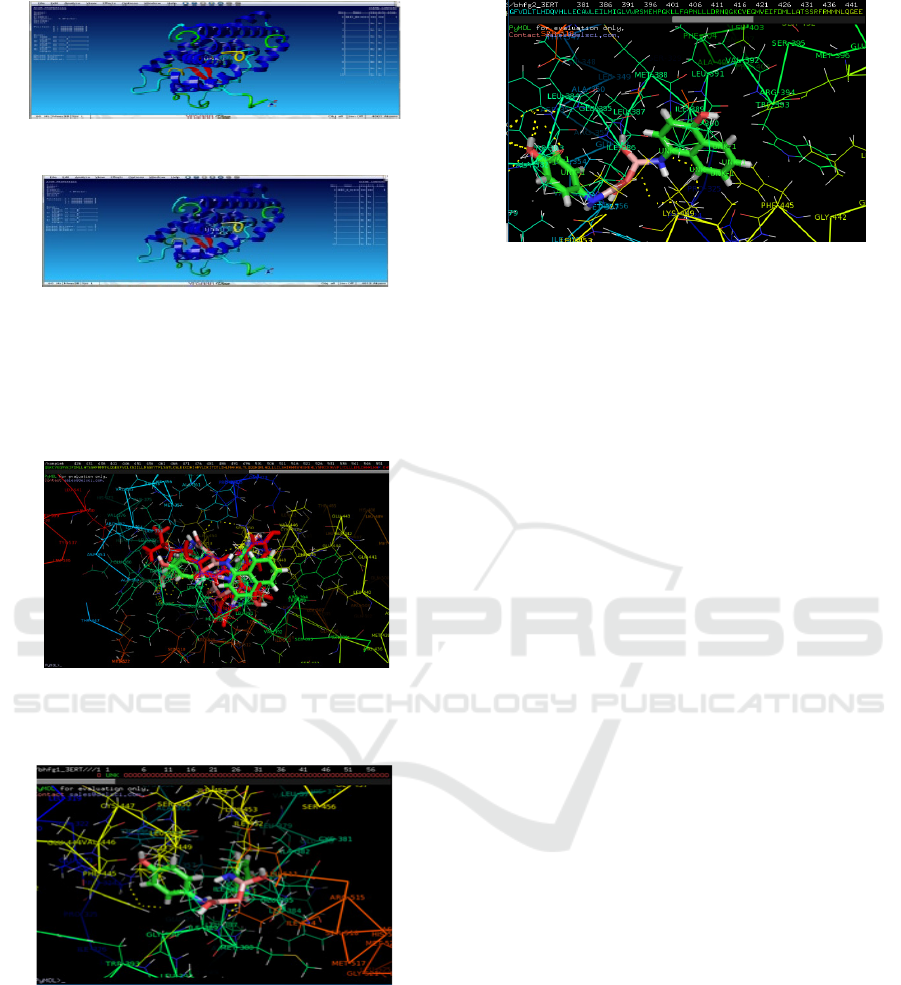
b
c
Figure 2. Visualization of interaction between.
(a) BornUSU 1 with ER-α
(b) BornUSU 2 with ER-α
(c) 4-hydroxy tamoxifen with ER-α
Figure 3. Overlay interaction of 4-hydroxy tamoxifen,
BornUSU 1 and 2 with Receptor of Estrogen Alpha with
pymol.
a
b
Figure 4. Interaction of BornUSU I and BornUSU 2with
Receptor of Estrogen Alpha with pymol.
Visualization interaction of 4-hydroxy tamoxifen
with Estrogen alpha receptor (ER-α) shown at Picture
4. Some of amino acids from ER-α which is role in
the mechanism of action of 4-hydroxy tamoxifen are:
Met-242, Leu-346,Thr-347, Leu-349, Ala-350, Asp-
351, Glu-353, Trp-383, Leu-284, Leu-387, Met-388,
Leu-391, Arg-394, Phe-404, Glu-420, Met-421, and
Leu-428. Oxygen from hydroxyl group to form
hydrogen bond with water, Arginin-394(Arg-394)
and Glutamat-353(Glu-353), while Leusin- 387(Leu-
387) participate in stabilize hydrogen bond between
oxygen with both of amino acids which mentioned
above.
Several amino acids from ER-α which is role in
BornUSU I or in Boronhafagama I are Ile-386, Met-
357, Ala-382, Trp-383, Leu-384, Glu-385, Leu-387,
Met-388, Ile-389, Gly-390, Phe-445, Val-446, Lys-
449, Ile-452 and Ile-514.Several amino acids from
ER-α which is role in BornUSU II or Boronhafagama
II are Met-357, Trp-360, Ala-361, Leu-378, Leu-379,
Glu-380, Cys-381, Ala-382, Trp-383, Leu-384, Glu-
385, Ile-386, Leu-387, Gly-390, Phe-435, Leu-440,
Phe-445, Val-446, Leu-448, Lys-449, Ile-452, Leu-
453, Ser-456 and Ile-514.Although docking score of
BornUSU 2 is higher than 4-hydroxy tamoxifen but
this score is not significance. BornUSU 2 interaction
with 24 amino acids from ER-α while 4-hydroxy
tamoxifen interaction with 17 amino acids from ER-
α.
4 DISCUSSION
The docking score represents the binding affininty of
the ligand to the target protein. The docking of ER-α
target with compounds using docking procedure was
mentioned that all the computationally predicted
lowest energy complexes of ER-α is stabilized by
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
816

intermolecular hydrogen bonds and stacking
interactions (Levin, 2005).
Docking score of BornUSU II were lower than
tamoxifen and 4-hydroxy tamoxifen but BornUSU I
is lower than 4-hydroxy tamoxifen and higher than
tamoxifen. In silico drug design can play a significant
role in all of stages of drug development from
preclicial assesment to the end of clinical
development (Levin, 2005). The results were
obtained at in silico screening have shown that it
represents the best step (way) to get an accurate result
in a short time and saving manner (Terstappen and
Reggiabi, 2001).
5 CONCLUSIONS
BornUSU I and II are boron derivate compounds.
They were showed to have the activity in inhibition
of cancer growth through ER-α pathways and they are
potential to develop as anticancer.
ACKNOWLEDGEMENT
We gratefully thank to Research CenterUniversity of
Sumatera Utara through Hibah Talenta “Hibah
Penelitian Guru Besar” for financial support in the
study.
REFERENCES
Bharath, E.N., Manjula, S.N., Vijaychand, A. 2011. In
Silico Drug Design Tool for Overcoming the Innovation
Deficit in the Drug Discovery Process, Int. J Pharm
Pharm Sci, 3(2): 8-12.
Chang, M. 2012. Tamoxifen resistance in breast cancer.
Biomol. Ther., 20, 256–267.
Cohen, L.H.; Remley, M.J.; Raunig, D.; Vaz, A.D. 2003. In
vitro drug interactions of cytochrome p450: An
evaluation of fluorogenic to conventional substrates.
Drug Metab. Dispos. 31, 1005–1015.
Jemal A, Rebecca S, Xu J, Elizabeth W. 2010. Cancer
statistics. CA Cancer J Clin; 60: 277-300.
Levin, E.R. 2005. Integration of the extranuclear and
nuclear actions of estrogen. Mol. Endocrinol. 19,
1951–1959
Payudan, A; Abdullah, N.A; Yohannes, S. 2016. Basic
Principle Application and Technology of Boron
Neutron Capture Cancer Therapy (BNCT) Utilizing
Monte Carlo N Particle 5’S Software (MCNP 5) with
Compact Neutron Generator. Ind J Phys Nuc App. 1,
20-33.
Siegel, R.L., Miller, K.D., Jemal, A. 2015.Cancer statistics
.CA Cancer J Clin, 2015, 65(1): 5-29.
Subarnas, A.; Diantini, A.; Abdulah, R.; Zuhrotun, A.;
Hadisaputri, Y.E.; Puspitasari, I.M.; Yamazaki, C.;
Kuwano, H.; Koyama, H. 2015. Apoptosis induced in
mcf-7 human breast cancer cells by 20,40-dihydroxy-6-
methoxy-3,5-dimethylchalcone isolated from eugenia
aquea burm f. Leaves. Oncol. Lett. 9, 2303–2306.
Terstappen, G.C., Reggiani, A. 2001. In Silico Research in
Drug Discovery. Trends. Pharmacol. Sci, 22: 23-6.
Trippier, P. C.; McGuigan, C. 2010. Boronic acids in
medicinal chemistry: Anticancer, antibacterial and
antiviral applications. Med. Chem. Commun. 1,
183−198.
In Silico Analysis of Boron Derivate Compounds as Potential ER- Inhibitor
817
