
Preparation and Evaluation of Sunscreens Nanoemulsions Containing
Avobenzone and Octyl Methoxycinnamate
Anayanti Arianto
1
, Hakim Bangun
1
, Josephine Yauvira
1
1
Department of Pharmaceutical Technology, Faculty of Pharmacy, Nanomedicine Centre,
Universitas Sumatera Utara, Medan Indonesia
anayantia@yahoo,com
Keywords: Avobenzone, Octyl methoxycinnamate, Nanoemulsion, Sunscreen
Abstract: The use of sunscreen for the purpose of effectively absorbing sunlight in order to prevent the occurrence of
premature aging and skin cancer. Nanoemulsion is very effective to be applied as cosmetic preparation due
to their characteristic properties of small droplet size with high surface area enables effective delivery of the
active ingredients. It is transparent appearance and thermodynamically stable. The formulation of the
nanoemulsions was prepared in various ratios of Tween 80 as a surfactant and ethanol as co-surfactant using
avobenzone and octyl methoxycinnamate as sunscreen agent.The nanoemulsion was evaluated for particle
size, centrifugation, stability during experiment for 12 weeks of storage at room temperature, pH, viscosity,
and SPF value. Nanoemulsion in the ratio of tween 80 and ethanol (34:26) had the smallest average particle
size of 163.31 nm with yellowish color, clear and transparent appearance, pH value (7.46 ± 0.00), viscosity
value (75cP ± 25), did not show any separation or creaming in the centrifugation, stable during experiment
for 12 weeks of storage at room temperature. The sunscreen nanoemulsion preparations containing
avobenzone and octyl methoxycinamate with the ratio of Tween 80 and ethanol 34:26 contributed to give SPF
value of 16.80 ± 0.08. This formulation could be considered efficient for sunscreen cosmetic use
1 INTRODUCTION
The skin is on the outer surface of the body so often
exposed to sunlight. Every year, about a million
people are diagnosed with skin cancer and about
10,000 die from malignant melanoma. In 2018, an
estimated 9,320 deaths from melanoma will occur.
The harmful effects of solar radiation caused by
solar radiation consist of UVA rays from 320 to 400
nm, UVB from 290 to 320 nm and UVC of 200-290
nm. UVC radiation is filtered by the atmosphere
before it reaches the earth. UVB radiation is not
perfectly filtered by the ozone layer and is
responsible for sunburn damage. UVA radiation
reaches the deeper layers of the epidermis and
dermis and causes premature aging of the skin. UV
radiation is one of the leading causes of skin cancer
(Dutra, et al. 2004; Mitsui, 1997; Parkin, et al.
2011).The formulation of sunscreen that is efficient,
stable and can be marketed is a challenge. The
stability of product appearance obtained during
storage is a problem of efficacy and consumer safety
(Lionetti and Rigano, 2017). Sunscreen products on
the market mostly available in the form of lotions,
gels, emulsions and creams. Nanoemulsion is very
attractive to be applied in cosmetics (sunscreen
products) because nanoemulsi has droplet size
smaller than emulsion ie in nano size (20-500 nm)
so it is more stable, can prevent creaming,
sedimentation or coalescence, besides also increase
solubility of an insoluble active ingredient in water.
Nanoemulsion is very useful to be applied in
cosmetic because of it is more stable, with low
viscosity, and transparent visual aspect, and a high
surface area allows effective delivery of the active
ingredient for the skin, thereby increasing the
efficacy (SPF value) of the sunscreen
product.(Rhein, 2007, Devarajan and Ravichandran,
2011). The Nanoemulsion is formed spontaneously,
generally without high-energy input. This research
uses chemical sunscreen, which is avobenzone as
absorbent of UVA and octyl methoxycinnamate as
UVB absorbent. The maximum concentration of
avobenzone used is generally 3% and the
concentration of octyl methoxycinnamate is 7.5%
(Rieger, 2000).The aim of this study was to obtain
nanoemulsion of avobenzone and octyl
methoxycinnamate (OMC), and to evaluate their
Arianto, A., Bangun, H. and Yauvira, J.
Preparation and Evaluation of Sunscreens Nanoemulsions Containing Avobenzone and Octyl Methoxycinnamate.
DOI: 10.5220/0010087207470751
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
747-751
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
747
physical stability and in vitro sunscreen activity
through Sun Protection Factor (SPF) determination
by spectrophotometric methods. in vitro testing
methods by spectrophotometric methods have been
developed because they are more rapid, less
expensive and above all because they prevent the
involvement of human volunteers with the related
ethical problems. The nanoemulsion in this study
was made by low energy spontaneous emulsification
method using 3% avobenzone and 7.5% octyl
methoxycinnamate as sunscreen agent and Tween
80 as surfactants and ethanol as co-surfactant.
Tween 80 is widely used as surfactant in the
preparation of nanoemulsion. In addition to having a
large HLB of 15, Tween 80 is stable against
electrolytes, weak acids, and bases (Rowe et al.,
2009). However, the use of Tween 80 singly is not
enough to reduce surface tension to form
nanoemulsion.Therefore, in the preparation of
nanoemulsion, surfactants are often combined with
cosurfactants.
2 MATERIALS AND METHODS
2.1 Materials
Avobenzone, octyl methoxcinnamate (India),
Tween 80, ethanol 96%, paraffin liquid, propylene
glycol were purchased from PT.Brataco (Medan,
Indonesia). Methyl paraben, propyl paraben, butyl
hydroxyl toluene and buffer pH solution purchased
from CV Rudang (Medan Indonesia).
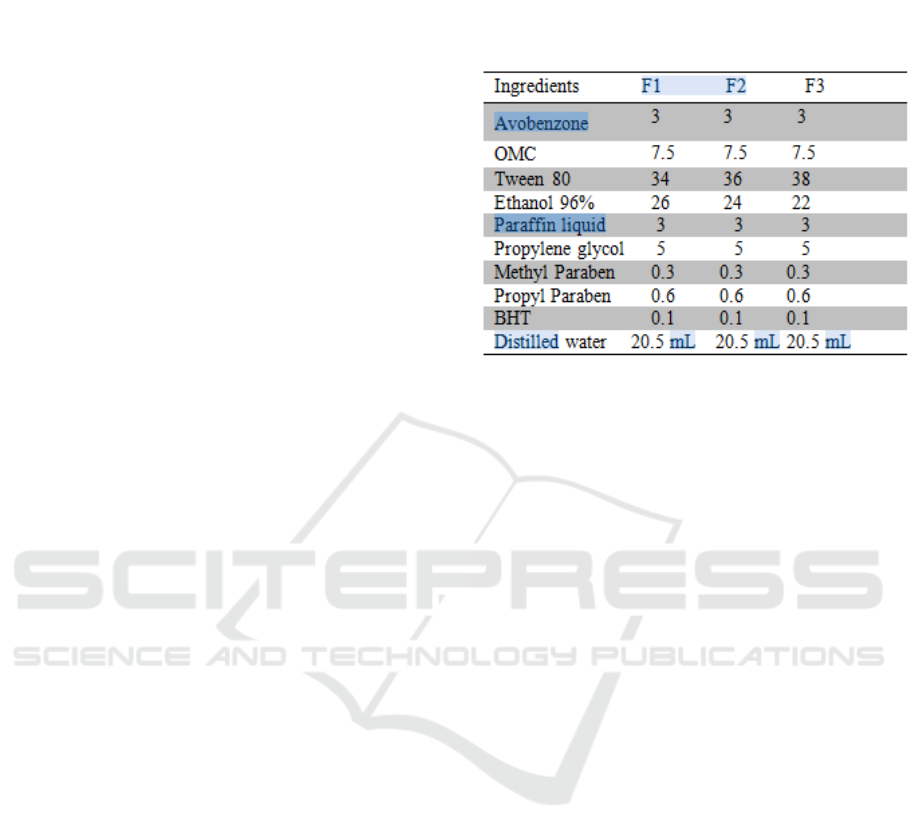
2.2 Preparation of Nanoemulsion
In the preparation of nanoemulsion, Tween 80 as
surfactant combined with ethanol as cosurfactant.
The ratio of surfactant (Tween 80) and co surfactant
(ethanol) mixtures in nanoemulsion formulation as
shown in Table 1. The nanoemulsions were prepared
according the spontaneous emulsification method
(Cinar, 2017), where the oil phase (avobenzone
dissolved in ethanol, octyl methoxycinnamate, butyl
hidroxyl toluene, paraffin liquid) were mixed with
water phase (Tween 80, methyl paraben and propyl
paraben dissolved in hot distilled water, propylene
glycol) and stirred,then add with deonized water to
provide 3% w/w paraffin liquid in final
nanoemulsion and then stirred gently at 3000 rpm
(magnetic stirrer HI 190 M) for 4 hours until a clear
nanoemulsion was produced. Then the
nanoemulsion preparation was sonicated for 1 hour
(Ultrasonic Cleaner 1510 E-MT)) until a transparent
nanoemulsion was produced.
Table 1: Composisiton of nanoemulsions
2.3 Physical Stability Assessment
Prepared formulations were subjected to
centrifugation at 3750 rpm for 5 hours and were
observed for phase separation. (Lachman, et al.,
1994). The stability studies were performed by
keeping the selected formulation of nanoemulsion at
room temperature (25±2ᵒC) for a period of 3 months.
The viscosity, and pH were determined at 0, 1, 2, and
3 months (Alam, S M et al. 2015).
2.4 Determination of SPF Value
The SPF value is calculated using the Mansur
equation. The sample absorption spectrum was
obtained by using a UV-Vis spectrophotometer at
290-400 nm wavelength with 96% alcohol as blank,
the absorption value recorded per 5 nm interval
wavelength 290-320 nm and 10 nm interval
wavelength 320-400 nm. The value of absorption
obtained was multiplied by erythemal effects
spectrum (EE) x I for each interval. The value of EE
x I per interval could be seen in Table 2. The amount
of EE x I obtained multiplied with the final correction
factor, then the SPF value of the tested sample would
be obtained. The value of EE x I and correction factor
is a constant where the value of EE x I from the
wavelength 290-320 nm and every 5 nm difference
and the correction factor 10 has been determined by
(Sayre, 1979),
SPF = CF Σ
EE (ʎ) x I (ʎ) x Abs (ʎ)
CF = Correction factor
EE = Erythemal Effect Spectrum
I = Solar Intensity Spectrum
Abs = Sample absorption
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
748

Table 2. Correlation between the erythemogenic effect (EE)
and the radiation intensity at each wavelength (I) ( Mansyur
et al. 1986).
The value of EE x I and correction factor is a
constant where the value of EE x I from the
wavelength 290-320 nm and every 5 nm difference
and the correction factor 10 has been determined by
(Sayre, 1979).
2.5 Determination of Nanoemulsion
Droplet Size
The mean droplet size of the nanoemulsion was
determined by Vasco
γ
CORDOUAN Technologies
Particle Size Analyzer Measurements were
performed at room temperature.
2.6 pH Determination
The pH of the formulation was measured using a
digital pH meter (Hanna Instrument). One g of
nanoemulsion was dissolved in 100 ml distilled
water. The measurement of pH of each formulation
2.7 Viscosity Measurement
The Viscosity of nanoemulsion was measured by
using Brookfield DV-E viscometer. The sample was
filled in a beaker and the viscosity was measured by
using Spindle number S62.
3 RESULT AND DISCUSSION
The result of all formulations showed a light yellow,
clear, and transparent nanoemulsion as shown in
Figure 1. One characteristic of nanoemulsions is a
bluish brightness and translucent aspect, whereas
macro emulsions display milky appearance
(Mason et al., 2006) The preparations of
nanoemulsion by spontaneous emulsification
method. This method can produce nanoemulsions at
room temperatures and no special devices are
required. When an oil phase with a water soluble
substance is mixed with water, oil droplets
spontaneously forms. The mechanism depends on
the movement of water dispersible substance from
the oil phase to the water phase. This leads to
interfacial turbulence and thus formation of
spontaneous oil droplets. The nanoemulsions stable
during experiment for 12 weeks of storage at room
temperature (Figure 2).
Figure 1: Appearance of the prepared sunscreen
nanoemulsion (F1, F2, F3) containing avobenzone and
octyl methoxycinnamate.
Figure 2: Appearance of the prepared sunscreen
nanoemulsion (F1, F2, F3) containing avobenzone and
octyl methoxycinnamate after storage for 12 weeks at room
temperature
The centrifugation test was performed to determine
the stability of nanoemulsion. The centrifugation test
describes the stability of the preparation because of
the effect of gravitational force equivalent to one year
of storage. All of the nanoemulsions (Figure 3) were
stable, did not show any a phase separation or
creaming.
Figure 3: Appearance of the prepared sunscreen
nanoemulsion (F1, F2, and F3) containing avobenzone and
octyl methoxycinnamate before and after centrifugation
Preparation and Evaluation of Sunscreens Nanoemulsions Containing Avobenzone and Octyl Methoxycinnamate
749

Table 2: Globule size and globule size distribution
distribution of different nanoemulsion samples during
storage for 12 weeks at room temperature.
The results of the globule size determination show
the nanoemulsion (F1) that used the lowest surfactant
concentration with ratio of surfactant Tween 80 and
co-surfactant ethanol 34:26 was the smallest globule
size and was increased during storage for 12 weeks
at room temperature. (Table 2). The results of
viscosity and pH measurements of nanoemulsion
containing avobenzone and octyl methoxycinnamate
during experiment for 12 weeks of storage at
different temperature as shown in Table 3. The
viscosity of nanoemulsion was increased during 12
weeks of storage and with the decrease in
temperature the viscosity will increase and the
preparations become more viscous. The pH of
nanoemulsion was decreased during 12 weeks of
storage and with increasing storage temperature
(Table 3.).
Table 3: Viscosity and pH of nanoemulsion (F1) during
storage for 12 weeks at different temperature
From the experimental results, the nanoemulsion F1
that used the lowest surfactant concentration and the
small globule droplet size, while still maintaining
stability, centrifugal stability that selected for further
in vitro SPF value determination. Based on the result
that the SPF values resulted from the sunscreen
nanoemulsion F1 containing avobenzone and octyl
methoxycinnamate was 16.80 ± 0.08. This sunscreen
nanoemulsion preparation already has sun protection
activity in medium protection level (Lionetti and
Rigano, 2017).
4 CONCLUSION
The sunscreen nanoemulsion preparations containing
avobenzone and octyl methoxycinamate are
physically stable during experiment for 12 weeks of
storage at room temperature and characterized by the
absence of discoloration, creaming, or phase and
contributed to give SPF value of 16,80. This
formulation could be considered efficient for
sunscreen cosmetic use
.
ACKNOWLEDGEMENTS
This work was supported by University of Sumatera
Utara through the TALENTA Research, scheme of
Penelitian Guru Besar 2018.
REFERENCES
Alam, S M., Ali, S M., Alam I M., Anwer, T., and Safhi A
M M. (2015). Stability Testing of Beclomethasone
Dipropionate Nanoemulsion. Tropical Journal of
Pharmaceutical Research 14(1): 15-20.
Cinar, K. A. (2017). Review on Nanoemulsions:
Preparation Methods and Stability. Trakya University
Journal of Engineering Sciences, 18(1): 73-83.
Devarajan, V., and Ravichandran, V., (2011)
Nanoemulsions: As Modified Drug Delivery Tool.
Pharmacy Globale (IJCP) 2(4): 1-6.
Dutra, A., Alamanca, D., and Hackmann, E. (2004).
Determination of sun protector factor (SPF) of
sunscreen by ultraviolet spectrophotometry. Brazilian
Journal of Pharmaceutical Sciences. Brazil
:Universidade de Sao Paulo. 40(3): 381-382.
Lionetti, N. and Rigano, L.(2017). The New Sunscreen
among Formulation Strategy, Stability Issues,
Changing Norms, Safety and Efficacy Evaluations.
Cosmetics 4(15):111.
Mansur, J S., Breder, M N R., Mansur, M. C. A., Azulay,
R. D. (1986). Determinação do fator de proteção solar
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
750

por espectrofotometria. An. Bras. Dermatol., Rio de
Janeiro, 61, p. 121-124.
Mason, T G,Wilking, J N., Meleson, K., Chang, C B.,
Graves, S M. (2006). Nanoemulsions: formation,
structure, and physical properties. J. Phys.-Condens.
Matter., 18:635-666.
Mitsui, T. (1997). New Cosmetic Science. 1th Edition.
Amsterdam : Elsevier Sciences B.V. Page: 38-45.
Parkin, DM, Mesher, D., and Saseini, P. (2011). Cancers
attributable to solar (ultraviolet) radiation exposure in
the UK in 2010. British Journal of Cancer 105: 566-569.
Rieger, M.M. (2000). Harry’s Cosmetology. 8th Edition.
New York : Chemical Publishing Co., Inc. Page:. 420-
421
Rowe, R.C., Sheskey, P.J., and Quinn, M.E. (2009).
Handbook of Pharmaceutical Excipients. 6th edition.
Washington D.C : Pharmaceutical Press and American
Pharmacists Association. Page. 540-553, 600-605.
Sayre, R. M., Agin, P. P., Levee, G. J., and Marlowe, E.
(1979). Comparison of in vivo and in vitro testing of
sunscreening formulas. Journal of The Society of
Cosmetic Chemists. Oxford: Photochem Photobiol.
29(3): 559-56
Preparation and Evaluation of Sunscreens Nanoemulsions Containing Avobenzone and Octyl Methoxycinnamate
751
