
Inulin Extraction and Characterisation of Fresh and Chip Gembili
(Dioscorea Esculenta) Extract by Ultrasound-assisted Extraction
A Hilman
1
*, E. Harmayani
2
, M. N. Cahyanto
2
1
Postgraduate Program of Food Science and Technology, Faculty of Agricultural Technology, Universitas Gadjah Mada,
Sleman 55281, Yogyakarta, Indonesia
2
Department of Food and Agricultural Products Technology, Faculty of Agricultural Technology, Universitas Gadjah
Mada, Sleman 55281, Yogyakarta, Indonesia
Keywords: Inulin, prebiotic, fresh gembili, chip gembili, ultrasound-assisted extraction.
Abstract: Inulin has the prebiotic component to improve health and reduce the risk of digestive tract disorders.
Gembili (Dioscorea esculenta) is one of inulin source found in Indonesia. Fresh Gembili has a relatively
short shelf-life so it needs to be drying into chips. Inulin extraction was the factor that can affect the
characteristics of gembili extract. The aim of this research was to determine the effect of ultrasound-assisted
extraction on the inulin extraction stage to the yield and the characteristics of fresh and chip gembili extract.
The study was conducted in two stages: (1) inulin extraction from fresh and chip gembili with ultrasound-
assisted extraction and compared by non-ultrasound extraction; (2) characterisation of physical and
chemical properties. The results showed that inulin extraction by ultrasound-assisted extraction was not
different significantly to the yield (30.78%-32.47%); degree of white (92.18-93.69); pH (6.55) and
solubility at 25
o
C (11.26%-12.75%), 60
o
C (22.50%-25.97%), 90
o
C (36.34%-37.71%) compared by non-
ultrasound extraction. Gembili extract from fresh and chip gembili by ultrasound-assisted extraction have
inulin content was about 10.00%-21.13%; inulin purity was about 61.57-119.22 mg/kg; and viscosity
becomes smaller as the temperature increases. It could be concluded that inulin can be extracted from fresh
and chip gembili.
1 INTRODUCTION
Inulin is a prebiotic component consisting of a D-
Fructose unit monomer connected by a β-(2→1)
bond and has a D-Glucose terminal group connected
to an α-(1→2) bond. The arrangement of fructose
monomers makes inulin cannot be hydrolyzed in the
digestive system. However, inulin can be fermented
by microbiota in the digestive tract in the colon by
Bifidobacterium and Lactobacillus (Li et al., 2015).
The results of inulin fermentation in the digestive
system are short-chain fatty acids that can be used
by cells to stimulate the growth of intestinal mucosal
cells and become the main source of cell energy
(Pompei et al., 2008). In addition, the results of
inulin fermentation have immunomodulatory effects
and increase the mineral absorption (Dominguez et
al., 2014; Panchev et al., 2011). The food industry
has been using inulin in various applications in
various products today.
This sparked a great deal of research and
publication about the production of inulin from
different types of plants (Roberfroid, 2005). Inulin
has been commercially produced from chicory root
(Chicorium intybus) and Jerusalem artichoke tubers
(Helianthus tuberosus) in some western countries
such as America, England and some European
countries.
According to the research that has investigated
by Winarti et al. (2011) and also Zubaidah and
Akhadiana (2013) reported that one of the major
sources of inulin in Indonesia can be found in plants
such as gembili (Dioscorea esculenta) tuber. Winarti
et al. (2011) reported that the content of inulin in
fresh gembili was 14.77% (db). While Ciptaningrum
(2015) reported that the inulin extraction method of
gembili chip with water addition ratio resulted in a
yield of 36.40% (db) with an inulin content of
21.64%.
According to previous study, gembili has the
potential to serve as a raw material on inulin
extraction. However, gembili has a relatively long
Hilman, A., Har mayani, E. and Cahyanto, M.
Inulin Extraction and Characterisation of Fresh and Chip Gembili (Dioscorea esculenta) Extract by Ultrasound-assisted Extraction.
DOI: 10.5220/0010084000470053
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
47-53
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
47

harvest time of about seven to nine months. In
addition, the gembili that is kept in fresh condition
only has a relatively short shelf-life of about 10 to
14 days in room temperature (Kasno, Saleh and
Ginting, 2006). These problems can be solved by
giving preliminary treatment at fresh gembili
processed into the chip. Only a few studies have
studied the extraction of inulin from the materials of
the chip, among others, from the Jerusalem artichoke
chip (Saengthongpinit, 2005; Bekers et al., 2008)
and Cichorium intybus L dried (Park, de Oliveira,
Brod, 2006). Current research has studied the
extraction of inulin from raw materials in the form
of gembili chip (Ciptaningrum, 2015).
Arumdinari (2015) reported that the inulin
extraction method performed was the development
of the method Gupta, Kaur and Kaur (2003) which
used the principle of extraction by heating in boiling
water (90
o
C) for 20 min, filtrate freezing process for
24 h, inulin precipitation with ethanol 20% and
drying used by cabinet drying in overnight at 50
o
C.
The study obtained in inulin yield of 43.39% (db)
and inulin content of 28.89%.
Therefore, research is needed to improve the
inulin yield of the gembili on an industrial scale. The
ultrasound-assisted extraction is used as a new
method capable of extracting inulin from various
plant tissues. The aim of this study was to determine
the effect of ultrasound-assisted extraction on the
stage of inulin extraction to the yield and the
characteristics of fresh and chip gembili extract.
2
MATERIALS AND METHOD
The research was conducted in two stages: 1) inulin
extraction from fresh and chip gembili by
ultrasound-assisted extraction and compared by non-
ultrasound extraction and 2) characterization of
physical and chemical properties.
2.1
Materials
The research material was harvested from fresh
gembili tubers obtained from Watubonang Village,
Tawangsari District, Sukoharjo Regency, Central
Java Province. Gembili chip was a fresh gembili that
has been sliced thin chip-shaped and dried at 50
o
C in
cabinet drying for 12 hours. Another materials used
are inulin (C6H10O5)n standard produced by Sigma
Aldrich and fructooligosaccharides for inulin purity
analysis using HPLC (High Liquid Performance
Chromatography).
2.2
Instruments
The research instruments used were waterbath
shaker, HPLC Shimadzu, chromameter CR-400
(Konica Minolta), viscosity Brookfield LVDV-II+P,
column ion-exclusion Aminex HPX-87H 150X7.8
mm ID (BioRad, Watford, Herts), freeze drying,
centrifuge, sonicator bath.
2.3
Statistical Analysis
The research method was conducted by Complete
Randomize Experimental Design. The treatment
factor was the type of material consist of 1) fresh
gembili with non-ultrasound extraction (G1) and by
ultrasound-assisted extraction (G2); 2) chip gembili
with non-ultrasound extraction (G3) and by
ultrasound-assisted extraction (G4). Each treatment
was repeated 3 times to minimize experimental
error. The analysis was performed by analysis of
variance (ANOVA) and followed by Duncan's test
with a significance level of 5%. Statistical data is
calculated by SPSS 17.0 software.
2.4
Inulin Extraction
Inulin extraction from fresh and chips gembili in this
study used the method by Arumdinari (2015) with
modification. Fresh gembili cleaned, peeled and
mixed in 800 ml of hot water (80
o
C). Gembili
crushed with blender for 3 min to get the puree. The
puree was extracted used ultrasound-assisted in a
sonicator bath for 5 min. As a comparison, the other
puree was extracted used the treatment non-
ultrasound extraction. Both of the puree were
extracted used hot water extraction on waterbath
shaker at 80
o
C for 60 min at 80 rpm. The hot puree
was filtered with four layers of cotton to get the
filtrate. The filtrate was added to 1000 ml of hot
water (80
o
C) and reheated by the same method. In
the third stage of heating, the filtrate was added 500
ml of ethanol 96% and reheated on a waterbath
shaker at 80°C for 60 min at 80 rpm. The filtrate was
then frozen at -20°C for 24 h. The frozen filtrate was
thawed for 12 h and centrifuged at 1500 rpm for 15
min to obtain the natan. The supernatant section is
separated from natan as it contains the impurities
component. White natan is taken and washed with
ethanol 20% and centrifuged again (Kaur and Gupta,
2015). Furthermore, natan was washed by used
aquades as a neutralizer. Natan was dried in freeze
drying at -87
o
C for 48 h. (The same experiment was
also done for the material of gembili chip).
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
48

2.5
Calculation of the Yield
The yield is the ratio between the weight of the dried
gembili extract (a) and the weight of the gembili
material (b). The yield can be used to determine the
depreciation or addition of weight after processing.
2.6
Analysis of Moisture Content
The sample of 1-2 g was inserted into an aluminum
foil plate that has been dried in an oven at 105°C. It
was known to be constant to weight (a). The sample
dried at 105°C for 3-5 h and cooled in a desiccator,
then weighed. Heated again in the oven for 30 min,
chilled and weighed. This treatment was repeated
until it reaches a constant (b).
𝑎𝑏
𝑎
100%
(1)
2.7
Analysis of Inulin Content (Li et al.,
2015)
Inulin content can be calculated by differences
between the difference in total sugar content used by
phenol-sulfuric acid and inulin as standard (Dubois
et al., 1956) with reducing sugar content used by the
method of 3,5-dinitrosalicylic acid (DNS) and D-
fructose as standard (Miller, 1959).
2.8
Analysis of Solubility (Jiang et al.,
2013)
Solubility assay was performed by heating 50 ml
inulin solution 1% w/v (S). The solution put into the
water at 25°C, 60°C and 90°C and stirred for 15 min.
The solubility time was calculated used the
stopwatch until all dissolves. The solution filtered by
a filter paper that has been known the weight (K1).
The solution left in the filter paper dried at 105°C for
3 h and weighed (K2).
𝑆𝑇𝑆𝑆
𝐾2𝐾1
𝑆𝑇𝑆𝑆
100%
(2)
2.9
Analysis of Viscosity
Prepared fresh and chip of gembili extract solution
of 10% with aquades and heated while stirring to a
temperature at 100°C. The viscosity was measured
used by Brookfield LVDV-II+P viscosimeter.
Spindles were used adjusted to the viscosity of
solution. The analysis began with temperatures of
90°C, 80°C, 70°C, 60°C, 50°C, 40°C and 30°C. The
results were compared with inulin standard solution.
2.10
Analysis of White Degree (Takeuchi
and Nagashima, 2011)
Coordinate of L* a* b* is measured by chromameter
CR-400 (Konica Minolta) with visual angle 20. The
color parameters were expressed as follows:
brightness (L*), redness (a*) and yellowish (b*).
The lowest value of L* was 0 which indicate by
blackness and the highest was 100 indicate by white.
a* negative value indicate by green and positive
showed by red. While the negative b* value showed
by blue and positive colors indicate by yellow.
100
100𝐿
𝑎
𝑏
(3)
2.11
Analysis of Inulin Purity
(Retnaningtyas, 2012)
The inulin purity test was measured used by HPLC
with Aminex Ion-Exclusion HPX-87C (250mm x
4mm) column, 410 model water refractive detector
and LCHE Waters model M-45 pump. Aquades
water were used as a mobile phase with a speed of
0.5 ml/min, injection volume 20 μl. The column
temperature was set at 60°C and detector at 40°C.
The inulin standard was used by inulin (C6H10O5)n
obtained from Sigma Aldrich.
2.12
Measurement of pH
The pH measurement was performed by dissolving
the sample with a concentration of 10% (w/v). The
pH measurement analysis aims to determine the
condition of substrate acidity in the dissolved
sample. The pH Measurement was made in
triplicate. The pH measurement was performed used
by pH-meters.
3
RESULTS AND DISCUSSION
3.1
Yield
The average yield of fresh and chip gembili extract
by ultrasound-assisted extraction were 32.47% and
30.78% respectively. The ultrasound-assisted
extraction was not different significantly on the yield
of fresh and chip gembili extract. However, the yield
of fresh and chip gembili extract obtained increased
Inulin Extraction and Characterisation of Fresh and Chip Gembili (Dioscorea esculenta) Extract by Ultrasound-assisted Extraction
49
through the extraction process used by ultrasound-
assisted extraction (Table 1). Lingyun et al. (2007)
reported that the efficiency of inulin extraction will
increase efficiency by ultrasound-assisted extraction.
ultrasound extraction with the cleaning bath is non-
destructive to the sample which will eliminate the
possible contamination and loss of the extract.
Moreover, the cleaning bath is usually much quieter
than the probe horn during the operation. Therefore,
an ultrasonic cleaning bath might be more
convenient and efficient for the inulin extraction.
Table 1: Yield, inulin content, solubility at 25
o
C, inulin
purity and degree of white from fresh and chip of gembili
extract by non-ultrasound and ultrasound-assisted
extraction.
Characteristic of
physico-chemistry
Fresh gembili extract
non-
ultrasoun
d
ultrasound
Yield (%)
26.91 ± 5.56
ab
32.47 ±
0.61
b
Inulin content (%) 6.35 ± 0.13
a
0.00 ± 0.08
c
Solubility 25
o
C (%) 11.26 ± 0.03
a
11.48 ±
0.09
a
White degree 92.37 ± 0.32
a
2.44 ± 0.30
a
Inulin purity (mg/kg) 56.55 ± 19.80 61.57 ± 0.56
Characteristic of
physico-chemistry
Chip gembili extract
non-
ultrasoun
d
ultrasound
Yield (%)
25.23 ± 1.18
a
30.78
±4.21
ab
Inulin content (%) 7.67 ± 0.14
b
1.13 ± 0.18
d
Solubility 25
o
C (%) 12.41 ± 0.02
b
12.75 ±
0.32
b
White degree 92.18 ± 0.49
a
2.69 ± 0.20
a
Inulin purity (mg/kg) 96.70 ± 5.70
119.22
±0.74
Description: different superscripts on the same line
showed significant differences (p <0.05)
3.2
Moisture Content
The moisture content of fresh and chip gembili
extract by ultrasound-assisted estraction were
11.48% and 11.28% respectively. Winarti et al.
(2011) reported that inulin extracted from fresh
gembili tubers dried by cabinet drying method with
temperature at 60
o
C had a moisture content of up to
13.5%. While Franck (2007) reported that the
percentage of inulin standard from chicory had dry
material of 95%, which means that inulin standard
from chicory only had moisture content of 5%.
Inulin moisture content of information should be
known to limit the amount of water in the material
that will affect the resistance to damage caused by
microorganisms.
3.3
Inulin Content
Fresh and chip gembili extract by ultrasound-
assisted extraction have inulin levels of 10.00% and
21.13% respectively. Ciptaningrum (2015) reported
that the inulin content of gembili chip ranged
between 21.13% to 21.64%. The ultrasound-assisted
extraction has provided an increase in inulin content
of fresh and chip gembili extract (Table 1).
Ultrasound-assisted extraction released of inulin
compounds within the plant cell vacuoles and
diffuses out of the cell with kinetic waves derived
from the resulting vibration (Vinatoru, 2001).
Analysis of inulin content by the spectrophotometric
method still reads all the sugars present in the
material. Thus the readable inulin content may be a
component other than inulin, such as starch, soluble
fiber and other carbohydrates. Therefore, it is
necessary to continue a more accurate analysis of
inulin levels by using methods other than
spectrophotometry, such as HPLC.
3.4
Solubility
The quality of inulin can be measured by the level of
solubility in water. This parameter is one of the
physical properties possessed by inulin. Fresh and
chip gembili extract by ultrasound-assisted
extraction have a solubility at 25
o
C by 11.48% and
12.75% respectively. Franck (2007) reported that the
rate of inulin solubility at 25°C was 12% (w/v).
However, the solubility of inulin will increase as the
temperature increases as well. The statistical results
show that there is increased solubility of the gembili
extract between the fresh and chip, where the
solubility of the chip gembili extract is higher than
that of fresh gembili extract. Lee and Cheng (2006)
reported that drying techniques using hot
temperatures can lead to smaller particle sizes that
exhibit better redispersing properties.
3.5
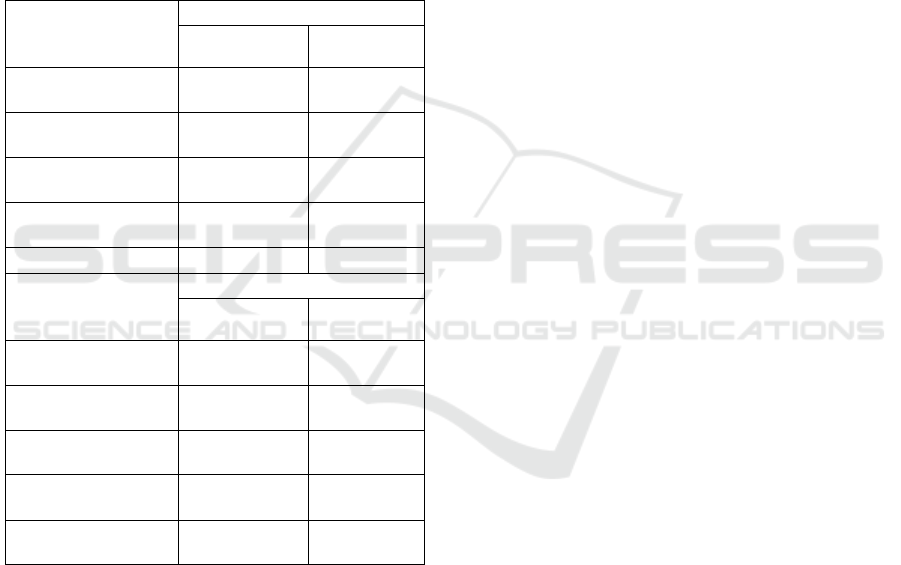
Viscosity
The viscosity is measured at a concentration of 10%
(w/v) and starts at 90°C until the temperature drops
at 30°C. The viscosity of fresh and chip gembili
extract by ultrasound-assisted extraction can be seen
in Figure 1. The results showed that the higher
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
50
temperature so the viscosity value will become
smaller. The temperature factor becomes the
determinant of the small value of the inulin viscosity
as the distance between the molecules further and
the frictional force decreases. Wada et al. (2005)
reported that the viscosity value may increase due to
the increase in molecular weight and temperature
drop. Bouchard, Hofland and Witkamp (2007)
reported that the value of inulin viscosity at 37°C
with a 10% concentration of 1.12 mpa-s. The low
level of inulin viscosity is a characteristic of
standard inulin physical properties commonly used
in food products (Franck, 2007).
Figure 1: The viscosity of gembili extract from fresh
gembili by non-ultrasound (G1) and ultrasound
(G2), chip gembili by non-ultrasound (G3) and
ultrasound (G4), and inulin
.
3.6
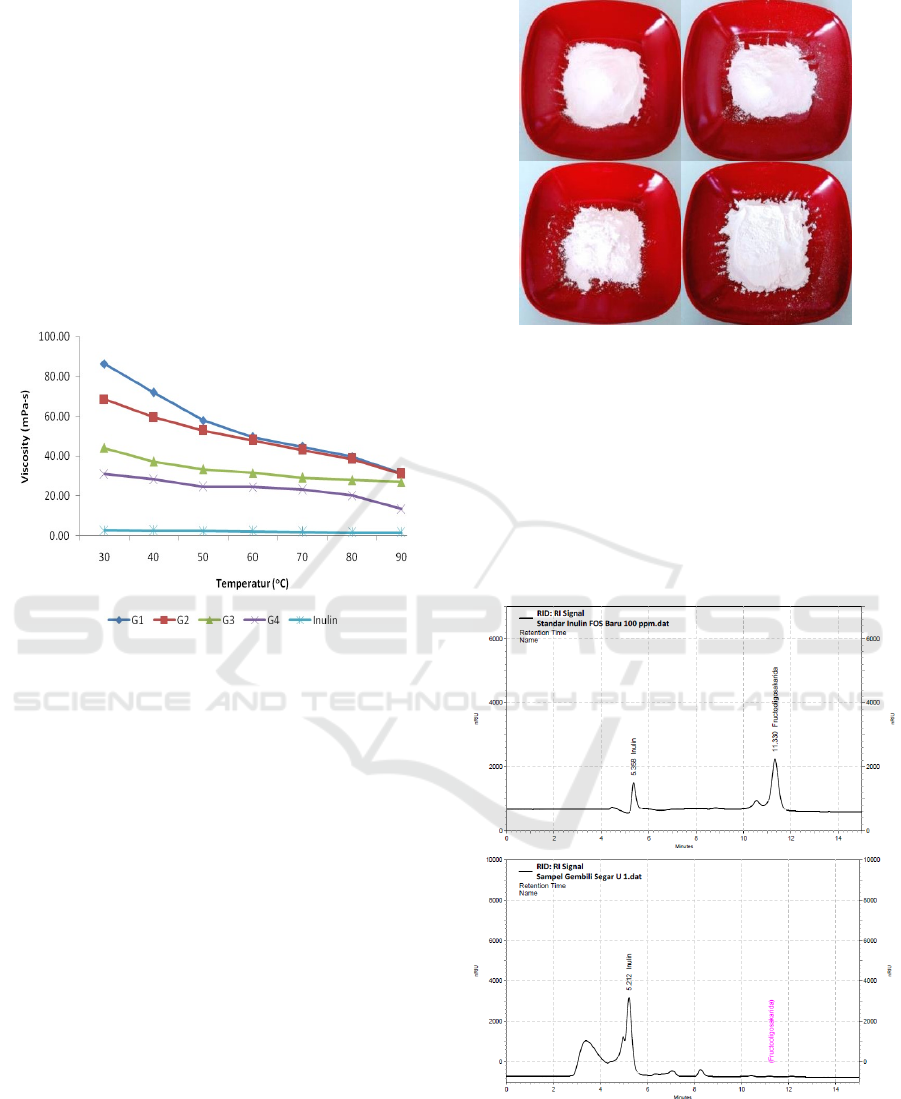
White Degree
Fresh and chip gembili extract by ultrasound-
assisted extraction have a white degree of 92.44 and
92.69 respectively. The results showed that the
ultrasound-assisted extraction did not affect a white
degree of fresh and chip gembili extract. The
gembili extract can be seen in Figure 2. Color is one
of the standard physical properties of inulin. Visual
appearance is a determinant of inulin quality. Franck
(2007) reported that the appearance of chicory and
arthicoke colors is white. However, the white degree
obtained was better than previous studies using
cabinet drying with a white degree of 81.39
(Ciptaningrum, 2015).
Figure 2: Gembili extract from fresh gembili by non-
ultrasound (a) and ultrasound (b), chip gembili by non-
ultrasound (c) and ultrasound (d).
3.7
Inulin Purity
Figure 3. point (a) showed that there were two main
peaks used as peaks of inulin and FOS standard with
a retention time of 5.3 and 11.3 min respectively.
Figure 3. points of (b) and (c) were the result of
chromatograms of gembili extract that have the same
retention times as inulin and FOS standard.
(b)
(a)
(a) (b)
(c) (d)
Inulin Extraction and Characterisation of Fresh and Chip Gembili (Dioscorea esculenta) Extract by Ultrasound-assisted Extraction
51
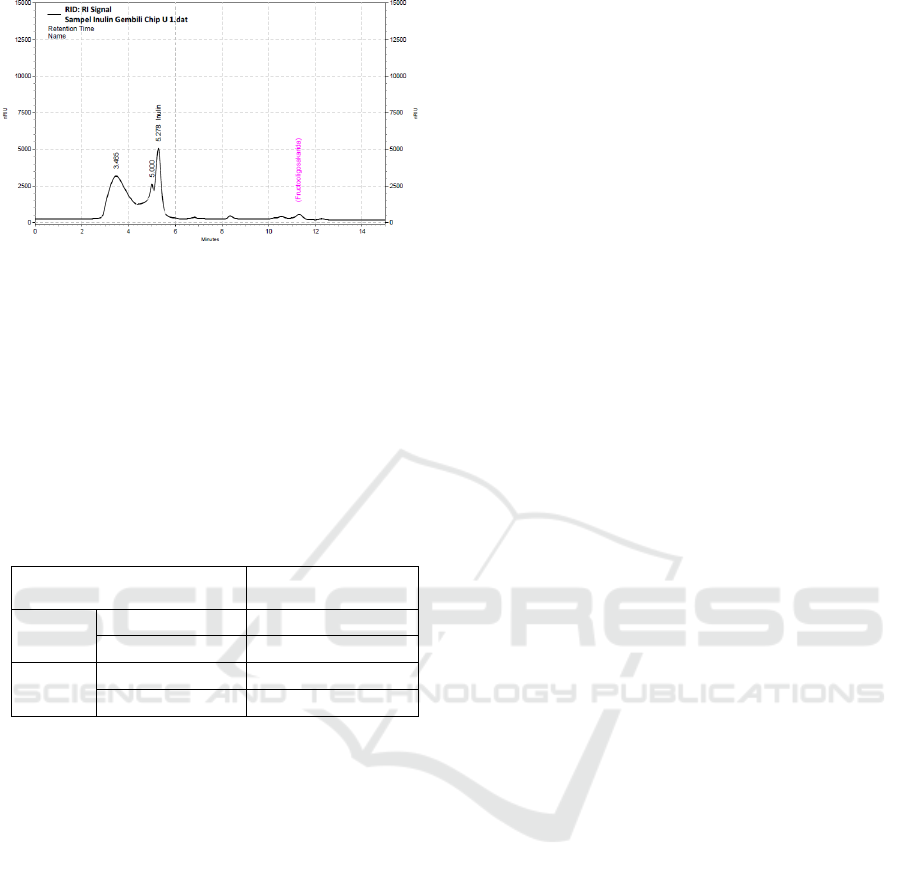
Figure 3: Chromatograms of inulin and FOS standard (a),
fresh gembili extract by ultrasound (b) and chip gembili
extract by ultrasound (c).
However, since the concentrations of FOS
standard still exist that have not been detected in
some chromatograms then the data is not shown.
The inulin purity is calculated by the multiplication
of concentration obtained by the diluting factor of
the gembili extract and divided by the initial weight
of the gembili extract. The inulin purity from
gembili extract is presented in Table 2.
Table 2: Inulin purity of gembili extract
Gembili extract
u
lin purity (mg/kg)
Fresh
Non-ultrasound
56.55 ± 19.80
Ultrasound
61.57 ± 0.56
Chip
Non-ultrasound
96.70 ± 5.70
Ultrasound
119.22 ± 0.74
Data as mean values ± standard deviation (n = 2)
Table 2. showed that there is an increase in inulin
purity of fresh and chip gembili extract by
ultrasound-assisted extraction. Fresh and chip
gembili extract by ultrasound-assisted extraction
have an inulin purity of 61.57 mg/kg and 119.22
mg/kg respectively. Zubaidah and Akhadiana (2013)
reported that inulin concentration in the fresh
gembili extract of 67.66 mg/kg.
This suggests that ultrasound-assisted extraction
of inulin extraction from fresh and chip gembili can
improve inulin purity. Lingyun et al. (2007) reported
that extraction by ultrasound-assisted extraction
effective can cause disruption of plant cell walls to
break and release compounds extracted from within
the cell wall.
3.8
pH
The pH parameter becomes important because inulin
is an additive in various food products to increase
functional value. So the base-acid information from
gembili extract can be used as a basic knowledge in
its use in various food products. The average pH
value of gembili extract from fresh gembili non-
ultrasound, fresh gembili ultrasound, chip gembili
non-ultrasound, chip gembili ultrasound extract were
6,55. The pH value of 6.55 is still within the normal
pH range. Franck (2007) reported that the standard
inulin pH range of 10% (w/v) concentration was 5-7.
So this can make the gembili extract as fortification
or food additives into food products.
4
CONCLUSIONS
The inulin extraction of fresh and chip gembili by
ultrasound-assisted extraction was not different
significantly to the yield (30.78%-32.47%); degree
of white (92.18-93.69); pH (6.55) and solubility at
25
o
C (11.26%-12.75%), 60
o
C (22.50%-25.97%),
90
o
C (36.34%-37.71%) compared by non-ultrasound
extraction. Gembili extract from fresh and chip
gembili by ultrasound-assisted extraction have inulin
content was about 10.00%-21.13%; inulin purity
was about 61.57-119,22 mg/kg; and the viscosity
becomes smaller as the temperature increases. It
could be concluded that inulin can be extracted from
fresh and chip gembili. Study of inulin extraction
from fresh and chip gembili by ultrasound-assisted
extraction needed to be developed by adding a
longer time variation in the future.
ACKNOWLEDGEMENTS
This work was financially supported by funded and
part of Prof. Dr. Ir. Eni Harmayani, MSc research
with the theme of Development of Functional Food
from Local Tuber.
REFERENCES
Arumdinari, R. (2015). Karakterisasi dan evaluasi sifat
prebiotik inulin hasil ekstraksi dari tepung gembili.
MSc. Universitas Gadjah Mada.
Bekers, M., Grube, M., Upite, D., Kaminska, E.,
Danilevich, A., Viesturs, U. (2008). Inulin syrup from
dried Jerusalem artichoke. Proceedings of the Latvia
University of Agriculture, 21(315), pp. 116-121.
Bouchard, A., Hofland, G. W., Witkamp, G. J. (2007).
Properties of sugar, polyol, and polysaccharide
water−ethanol solutions. Journal of Chemical &
Engineering Data, 52, pp. 1838–1842.
(c)
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
52

Ciptaningrum, A. B. (2015). Ekstraksi inulin dari chip
umbi gembili (Dioscorea esculenta) dengan variasi
rasio chip dan air serta evaluasi potensinya sebagai
prebiotik. MSc. Universitas Gadjah Mada.
Dominguez, A. L., Rodrigues, L. R., Lima, N. M.,
Teixeira, J. A. (2014). An overview of the recent
developments on fructooligosaccharide production and
applications. Food Bioprocess Technology, 7, pp. 324–
337.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A.,
Smith, F. (1956). Colorimetric method for
determination of sugar and related substances.
Analytical Chemistry, 28, pp. 350–356.
Franck, A. (2007). Technological functionality of inulin
and oligofructose. British Journal of Nutrition, 87, pp.
S287–S291.
Gupta, A. K., Kaur, N., Kaur, N. (2003). Preparation of
inulin from chicory roots. Journal of Sciencetific &
Industrial Research, 62, pp. 916 - 920.
Jiang, Q., Gao, W., Shi, Y., Li, X., W., Haiyang, H., Luqi,
Xiao, P. (2013). Physicochemical properties and in
vitro digestion of starches from different Dioscorea
plants. Food Hydrocolloids, 23, pp. 432-439.
Kasno, A., Saleh, N., Ginting, E. (2006). Pengembangan
pangan berbasis kacang-kacangan dan umbi-umbian
guna pemantapan ketahanan pangan nasional. Buletin
Palawija, (12), pp. 43-51.
Kaur, N., Gupta, K. (2002). Applications of inulin and
oligofructose in health and nutrition. Journal of
Biosciences, 27(7), pp. 703–714.
Lee, J., Cheng, Y. (2006). Critical freezing rate in freeze
drying nanocrystal dispersions. Journal of Controlled
Release, 111, pp. 185–192.
Li, W., Zhang, J., Yu, C., Li, Q., Dong, F., Wang, G., Gu,
G., Guo, Z. (2015). Extraction, degree of
polymerization determination and prebiotic effect
evaluation of inulin from Jerusalem artichoke.
Carbohydrate Polymers, 121, pp. 315–319.
Lingyun, W., Jianhua, W., Xiaodong, Z., Da, T., Yalin, Y.,
Chenggang, C., Tianhua, F., Fan, Z. (2007). Studies on
the extracting technical conditions of inulin from
Jerusalem artichoke tubers. Journal of Food
Engineering, 79, pp. 1087–1093.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent
for determination of reducing sugar. Analytical
Chemistry, 31, pp. 420–428.
Panchev, I., Delchev, N., Kovacheva, D., Slavov, A.
(2011). Physicochemical characteristics of inulins
obtained from Jerusalem artichoke (Helianthus
tuberosus L.). European Food Research and
Technology, 233, pp. 889–896.
Park, K. J., de Oliveira, R. A., Brod, F. P. R. (2006).
Drying operational parameters influence on chicory
roots drying and inulin extraction. Institution of
Chemical Engineering, 85, pp. 184-192.
Pompei, A., Cordisco, L., Raimondi, S., Amaretti, A.,
Pagnoni, U. M. (2008).
in vitro comparation of the
prebiotic effect of two inulin type fructans. Aerobe,
14(5), pp. 280-286.
Retnaningtyas, Y. (2012). Determination of inulin from
multivitamin syrup product by high performance
liquid chromatography with RI detector. Indonesia
Journal of Chemical, 12(2), pp. 201 – 205.
Roberfroid, M. B. (2005). Inulin-Type Fructans:
Functional Food Ingridients. Boca Raton: CRC Press.
Saengthongpinit, W. (2005). Influence of harvest time and
storage temperature on characteristics of inulin from
jerusalem artichoke and physicochemical properties of
inulin-starch mixed gel. PhD. Kasetsart University.
Takeuchi, J., Nagashima, T. (2011). Preparation of dried
chips from Jerusalem artichoke (Helianthus tuberosus)
tubers and analysis of their functional properties. Food
Chemistry, 126, pp. 922 – 926.
Vinatoru, M. (2001). An overview of the ultrasonically
assisted extraction of bioactive principles from herbs.
Ultrasonics Sonochemistry, 8, pp. 303– 313.
Wada, T., Sugatani, J., Terada, E., Ohguchi, M., Miwa, M.
(2005). Physicochemical characterization and
biological effects of inulin enzymatically synthesized
from sucrose. Journal of Agricultural and Food
Chemistry, 53, pp. 1246–1253.
Winarti, S., Harmayani, E., Nurismanto, R. (2011).
Extraction of inulin from various yam tubers
(Dioscorea, spp.). In: The 12
th
ASEAN Food
Conference. Bangkok: BITEC.
Zubaidah, E., Akhadiana, W. (2013). Comparative study
of inulin extracts from Dahlia, Yam, and Gembili
tubers as prebiotic. Food and Nutrition Sciences, 4,
pp. 8-12.
Inulin Extraction and Characterisation of Fresh and Chip Gembili (Dioscorea esculenta) Extract by Ultrasound-assisted Extraction
53
