
Effect of Isolation Methods on Physicochemical Properties of
Purple-fleshed Sweet Potato Starch
Elisa Julianti
1,2
, Herla Rusmarilin
1
, Ridwansyah
1,2
and Era Yusraini
1,2
1
Department of Food Science and Technology, Faculty of Agriculture, Universitas Sumatera Utara,
Jalan prof. A.Sofyan No. 3 Medan, Indonesia
2
Centre for Tuber and Root Crops Study Faculty of Agriculture, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Purple-fleshed Sweet Potato, Starch, Isolation Methods
Abstract: Pretreatment methods in starch manufacturing will influence starch properties. In the present study the
purple-fleshed sweet-potato (PFSP) starch was produced with different isolation agents: distilled water,
sodium metabisulfite, and citric acid, and their effect on yield and physicochemical properties of starch was
evaluated. Isolation of PFSP starch by sodium metabisulfite yielded the greatest recovery of starch
(12.12%). Isolation methods significantly affected the fat, total starch, amylose, amylopectin and crude
fiber content of PFSP starch. The isolation methods gave no significant effect on the color (lightness) and
whiteness of PFSP starch. The starch content of PFSP starches from distilled water, sodium metabisulfite
and citric acid isolation were 58.49; 50.20; and 62.93% respectively, while amylose content and whiteness
of PFSP starch varied from 20.69 – 28.34% and 64.09 – 66.93 respectively.
1 INTRODUCTION
Sweet potato (Ipomoea batatas L. Lam) is one of
tuber crops that has been grown in many areas in
Indonesia including in North Sumatera, since it has a
high adaptability to a wide variety of climatic
conditions. It does not require a lot of input and has
a shorter harvest period than other crops (Horton,
1989). It also has high starch content with low in
glycemic index {ILSI, 2008) and high fiber content
(Zhang, 2009). The common locally varities of
sweet potato in North Sumatera are purple-skin
white-fleshed, yellow-skin purple-fleshed, yellow-
skin yellow fleshed, yellow skin-orange fleshed, and
purple-skin purple fleshed (Hutasoit, 2018). Purple-
fleshed sweet potato (PFSP) had a high antocyanin
content, and therefore it showed stronger antioxidant
activity than other vegetable crops (Van Hall, 2000).
The most utilization of sweet potatoes in
Indonesia are consumed fresh such as boiled, fried
or processed into variety of snacks and cake. In the
other hand, sweet potato tubers have high post-
harvest losses, and these can be minimized if the
tubers can be converted into non- perishable forms
by drying or extracting the starch. The utilization of
sweet potato starch was determined by its
physicochemical and functional characteristics,
where they depend on the processing technologies
such as isolation methods of starch. The use of
enzyme or chemical agents such as sodium
metabisulfite, sodium hydroxide or citric acid) will
help to inactivate the indigenous enzyme (e.g.
polyphenol oxidase), so they can maintain the
quality of sweet potato starch (Jangchud, 2003). This
study was aimed to study the physicochemical
properties of starch that was isolated by different
isolation agents namely water, sodium metabisulfite
and citric acid.
2 MATERIAL AND METHODS
PFSP tubers were obtained from farmers in Phak
Phak Barat North Sumatera Province – Indonesia.
Tubers were washed and cleaned to remove the soil
and dirt by using tap water.
2.1 Isolation of PFSP Starch
Isolation of PFSP starch was done according to
(Tharise, 2014) with modification in isolation agent
of starch. This study uses the different isolation
Julianti, E., Rusmarilin, H., Ridwansyah, . and Yusraini, E.
Effect of Isolation Methods on Physicochemical Properties of Purple-fleshed Sweet Potato Starch.
DOI: 10.5220/0010079900370041
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
37-41
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
37
agents namely water, sodium metabisulfite, and
citric acid. The cleaned tubers were peeled
manually with stainless steel kitchen knife, shredded
by a grating machine, diluted 1 : 3 w/v with different
agents of starch isolation i.e. distillation water, 0,2%
sodium metabisulfite, and 0,2% citric acid, and then
filtered by using filter cloth. The filtrate was allowed
to settle for 12 hours at room temperature (27-30
o
C). The supernatant was poured while the starch
was collected and resuspended in water for 3 hours
and kept at room temperature for 3 hours to settle.
This process was repeated three times until the white
starch sediment was obtained. The collected starch
was dried in a convection oven at 50
o
C for 12 hours,
cooled to room temperature. Dry matter content of
the resulting starch for each treatment was calculated
to obtain the starch yield, and then finely ground,
sieve through a 80 mesh sieve, packed and sealed in
polyethylene plastic bags for further analyzed.
2.2 Determinonati Physicochemical
Properties of PFSP Starch Samples
Determination of starch yield (SY) was done by
using the following formula :
Extracted starch
SY (%) = --------------------------------------- x 100 (1)
Total amount of raw PFSP tubers
The starch color was determined by using a
Minolta Chromameter CR-400 type and the Hunter
color values (L*,a *, b*) were obtained. The
whiteness of starches were calculated as described
by Thao and Noomhorn (2011) by using following
formula :
Whiteness = 100 – [(100-L
2
) + a
2
+ b
2
]
1/2
The chemical properties of PFSP starches
including moisturem protein (N x 6,25), crude fat,
ash, and crude fiber were determined by using
AOAC methods (AOAC, 1995). The determination
of starch content was done by acid hydrolyzing the
PFSP starch samples with 25% HCl for a period 2.5
hours in water bath at 100
o
C. The quantification was
performed using 3.5 dinitrosalicylic acid (DNS)
spectrophotometric methods at 490 nm, using
glucose as standard (Dubois, 1956). Amylose
content (%) was determined by using IRRI method
(IRRI, 1996). Amylopectin was calculated by
difference method as follows :
Amylopectin (%) = (100 - Amylose)
2.3 Analysis of Data
A completely randomized design and analysis of
variance were employed to study the effect of
isolation agents on the physicochemcial properties
of PFSP starch. Least significant different (LSD)
tests at 95% confidence level (p<0.05)was used to
determine the differences between the ranges of
physicochemcial properties of PFSP starch.
3 RESULTS AND DISCUSSIONS
3.1 Starch Yield and Physical
Properties
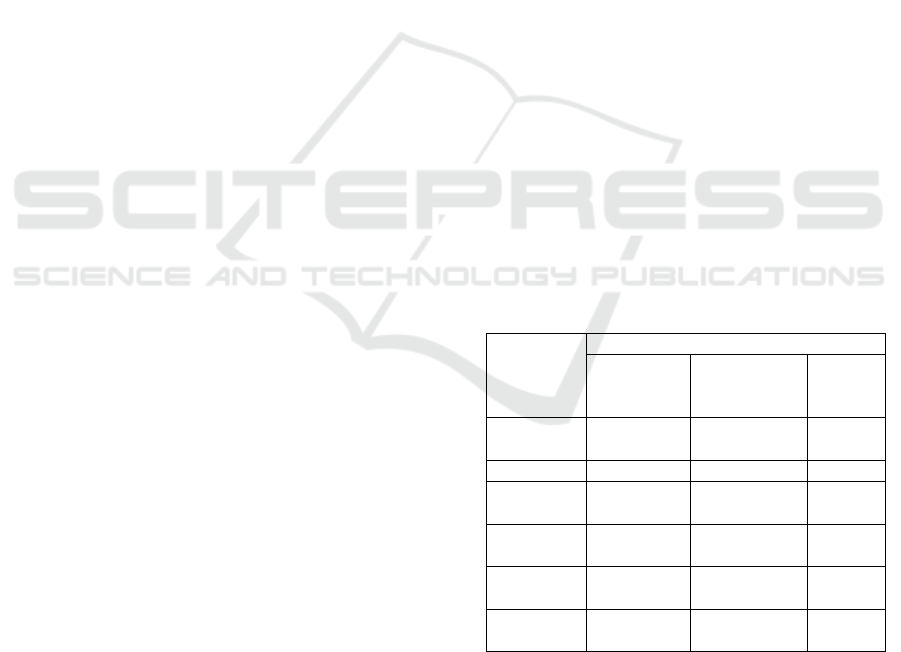
The isolation methods gave no significant effect on
starch yields, lightness and whiteness of PFSP starch
as shown in Table 1. The highest starch yield was
obtained by sodium metabisulfite isolation
(12.12%). The starch yiels obtained in this study
was similar to the study of (Soison, 2015) and
reporting the starch yield of 6-13% from four
varieties of sweet potato. But this result differ from
previous studies (Babu and Parimalavalli, 2014),
showing that starch isolation by using distillation
water produced higher starch yields than sodium
metabisulfite.
Table 1: The effect of isolation agents on starch yield,
lightness and whiteness of PFSP starch.
Parameter
s
a),b)
Isolation Agents of Starch
Distillation
Water
Sodium
Metabisulfit
e
Citric
Acid
Starch
yield (%)
11.53
±0.44
12.12
±1.18
10.38
±0.96
Colo
r
L* 64.33
±2.80
65.60
±3.31
67.43
±1.19
a* -1.17
±0.45
b
0.70
±0.36
a
-1.53
±0.46
b
b* 3.70
±1.84
3.33
±1.05
5.50
±0.36
Whiteness 64.09
±2.96
65.42
±3.35
66.93
±1.24
a)
Value reported as the mean ± Std. Dev. of three
replications
b)
Means followed by same letter superscripts within a row
are not significantly different (p<0.05)
Color was the most important characteristics for
determining the successful starch applications in
food products. The starch color was determined by
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
38
polyphenolic compounds and anthocyanin content of
PFSP starch (Glavez and Resureccion, 1993). There
was no significant difference observed for PFSP
starch color isolated by difference methods in terms
of lightness (L*), yellowness (b*) and whiteness but
there was slight difference in greenness (a*).
However PFSP starch which isolated by citric acid
found to be more lightness and whiteness followed
by sodium metabisulfite and distillation water. The
lightness and whiteness of PFSP starch observed in
this research were lower than those obtained by
Thao and Noomhorm (2011) and Babu and
Parimalavalli (2014). In their studies they found that
the lightness and whiteness of sweet potato starch
were ranged from 90.27-93.66. The lower of the
lightness and whiteness of PFSP starch obtained in
this research were due to anthocyanin pigment is
carried over the starch product during the starch
isolation (Glavez and Resureccion, 1993).
3.2 The Chemical Composition of
PFSP Starch
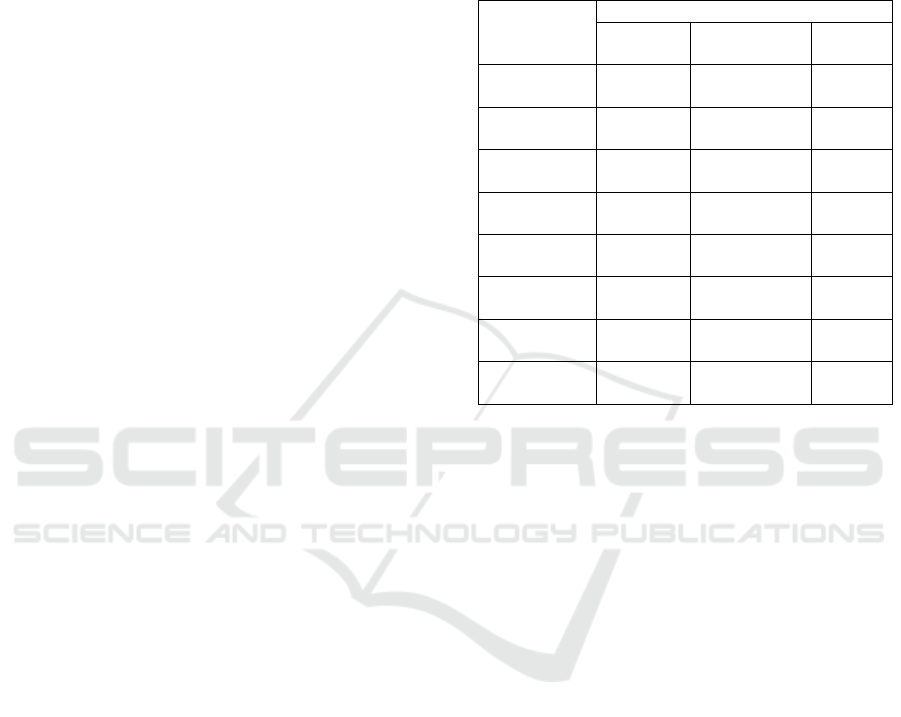
Table 2 showed that there was a significant
difference (p<0.05) in the moisture, crude fat,
starch, amylose, amylopectin and crude fiber content
of PFSP starch, but there is no significant difference
(p>0.05) in the protein and ash content among the
samples. Moisture content of PFSP starch ranged
from 8.52-14.86% similar to the results of Babu and
Parimalavalli (2014), and it was within the range of
recommended moisture content of commercial
starch i.e. 10-10% (Soni, 1993). Starch isolation by
using citric acid produced PFSP starch with the
lowest moisture content. However, basically the
starch moisture content depends mainly by the
drying methods and time, and also by the
surrounding humidity (Lawal, 2004).
The fat content was found to be 0,68% in sodium
metabisulfite isolation, 0,62% in citric acid isolation
and 0,50% in distillation water isolation, and these
values were similar with Thao and Noomhorm
(2011) studies i.e. 0.06-0.07%. The fat content in
PFSP starch that isolated by sodium metabisulfite
and citric acid were higher than distillated water
isolation. Ash content of PFSP starch ranged from
0.26 – 0.36, and the same result was found by (Babu
and Parimalavalli 2014) and (Abegunde, 2012). The
variation in the values of ash and fat content could
be attributed to extraction method and degree of
homogenization for isolation of starch (Kale, 2017).
Table 2 shows that the protein content of PFSP
starch ranged from 0.14-0.17%. The protein and fat
content of PFSP starch were lower than PFSP flours
that had 1.9-2.6% protein and 0.4-0.7% fat
(Jangchud, 2003). The high content of protein, lipid
and ash indicated the low purity of starch (Thao and
Noomhorm, 2011).
Table 2: Effect of isolation agents on chemical
composition of PFSP starch
Parameters
a),b)
Isolation A
g
ents of Starch
Distillatio
n Wate
r
Sodium
Metabisulfite
Citric
Aci
d
Moisture (%) 14.86
±3.83
a
16.93 ±0.82
a
8.52
±0.46
b
Crude fat (%) 0.50
±0.05
b
0.68 ±0.03
a
0.62
±0.04
a
Ash (%) 0.26
±0.01
0.28 ±0.04 0.36
±0.11
Protein (%) 0.17
±0.03
0.14 ±0.08 0.16
±0.04
Starch (%) 58.49
±1.84
a
50.20 ±2.80
b
62.93
±0.70
a
Amylose (%) 37.79
±0.94
a
30.87 ±2.72
b
34.59
±0.62
ab
Amylopectin
(%)
20.70
±2.98
b
20.85 ±1.17
b
28.34
±1.07
a
Crude Fiber
(
%
)
0.25
±0.11
b
0.56 ±0.16
b
2.59
±1.15
a
a)
Value reported as the mean ± Std. Dev. of three
replications
b)
Means followed by same letter superscripts within a row
are not significantly different (p<0.05)
The isolated PFSP starch had the lower starch
content (50.20-62.93%) than those in previous
studies i.e. 97-99% (Soison, 2015) and 92-96%
(Abegunde, 2013). The differences of starch content
may be due to a difference in variety and extraction
methods. PFSP starch isolated by citric acid
significantly had the higher purity of starch followed
by distillation water isolation and sodium
metabisulfite isolation had the lowest purity (Table
2).
Amylose content ranged from 30.87-37.79%,
while amylopection content ranged from 20.70-
28.34%. Isolation of PFSP starch by using
distillation water produced the high amylose content
of starch followed by citric acid isolation, and the
highest amylopectin content was found in citric acid
isolation. Various studies showed that the amylose
content of sweet potato starches varied greatly with
range of 8.5 to 38% (Abegunde, 2013; Collado.,
1999; Tian, 1991;Takeda, 1987). The difference in
amylose content of the PFSP starches will affect the
physicochemical properties of starches and
technological quality of starch-based foods (Ngoc,
2017). Amylose content in starch influences the
pasting properties and strength of starch gel, because
Effect of Isolation Methods on Physicochemical Properties of Purple-fleshed Sweet Potato Starch
39

of rapid retrogradation. The association and
interaction to lipids and amylopectin in forming
helical complex gave the strong structure of gel. The
high content of amylose in starches were desired for
manufacture of starch noodles (Tan, 2009; Jane,
1999).
Crude fiber content of PFSP starch from citric
acid isolation significantly was higher than those in
distillation water and sodium metabisulfite isolation.
The crude fiber content of PFSP starch ranged from
0.25-2.59%. Sweet potato was a significant source
of dietary fibre (Collins and Walter, 1982) and
therefore it plays a role in reducing the ocurence of
certain diseases such as diabetes, coronary heart
disease, colon cancer and various digestive disorders
(Augustin, 1978).
4 CONCLUSIONS
The physicochemical properties of PFSP starch
isolated from different kind of isolation agent
(distillation water, sodium metabisulfite, and citric
acid) were evaluation in this study. The result
showed that, each isolation method had its own
physicochemical characteristics, which affect the
end-use quality of starch based foods. The sodium
metabisulfite isolation produced the highest yield of
starch with higher lightness and whiteness of PFSP
starch color, but it had a lowest starch and amylose
content. While starch isolation by using citric acid
isolation produced the lowest yield of starch but had
the highest starch content. The isolation PFSP starch
by using distillation water produced the starch with
the lowest lightness and whiteness of color, but it
had the highest amylose content. It may be
concluded that purple fleshed sweet potato starch
can be applied for the development of food products.
ACKNOWLEDGEMENTS
We wish to thank to Directorate General of Research
Strengthening and Development, Ministry of
Research, Technology and Higher Education
Republic of Indonesia for funding this research
through “Penelitian Strategis Nasional 2018”
project.
REFERENCES
Abegunde, O.K., Mu, T., Arogundade, L.A., Deng, F., and
Chen, J. 2012. Physicochemical characterization of
starches from some Nigerian and Chinese roots and
tubers. African Journal of Food Science 6 317-329
Abegunde, O.K., Mu, T.H., Chen, J.W., and Deng, F.M.
2013 Physico-chemical characterization of sweet
potato starches popularly used in Chinese starch
industry. Food Hydrocolloids 33169-177
AOAC, 1995. Official Methods of Analysis of The
Association of Official Analytical Chemists
Washington.
Augustin, J., Johnson, G.K., Teitzel, C., True, R.H.,
Hogan, J.M., and Deutsch, R.M. 1978. Changes in
nutrient composition of potatoes during home
preparation. American Potato Journal 55 653-662
Babu, A.S. and Parimalavalli, R. 2014 Effect of starch
isolation method on properties of sweet potato starch.
The Annals of the University Dunarea de Jos of Galati
Fascicle VI – Food Technology 38 (1) 48-63
Collado, L.S., Mabesa, R.C., and Corke, H. 1999 Genetic
variation in the physical of sweet potato starch.
Journal of Agricultural and Food Chemistry 47 4195-
4201.
Collins, W.W. and Walter, W.M. 1982 Potential for
increasing nutritional value of sweet potatoes. In: R.L.
Villareal and T.D. Griggs, Sweet Potato. Tainan Int.
Symp., Taiwan, 355-363.
Dubois, M., Giles, K.A., Hamilton, J.K., Rebers, P.A., and
Smith, F. 1956 Colorimetric method for determination
of sugars and related substances. Analytical Chemistry
28 350-356
Glavez, F.C.F. and Resureccion, A.V.A. 1993 The effects
of decortications and method of extraction on the
physical and chemical properties of starch from mung
bean (Vignaradiate L. Wilcze). Journal of Food
Process Preservation 17 93-107
Horton, D., Prain, G., and Fregory, P. 1989 High-level
investment return for global sweet potato research and
development CIP Cir 17 1-11
Hutasoit, M.S., Julianti, E., and Lubiz, Z. 2018 Effect of
pretreatment on purple-fleshed sweet potato flour for
cake making. IOP Conference Series: Earth and
Environmental Science122 012086
http://iopscience.iop.org/article/10.1088/1755-
1315/122/1/012086. doi :10.1088/1755-
1315/122/1/012086
International Life Science Institute (ILSI). 2008
Nutritionally improved sweet potato. In: Assessment
of foods and feeds. Comprehensive Review in Food
Science and Food Safety 7 81-91
IRRI. 1996 Standard Evaluation System For Rice. INGER
Genetic Resources Center, International Rice Research
Institute, Manila, Philippine
Jane, J., Chen, Y.Y., Lee, L.F., McPherson, A.E., Wong,
K.S., Radosavljevic, M. and Kasemsuwan, T. 1999
Effects of amylopectin branch chain length and
amylose content on the gelatinization and pasting
properties of starch. Cereal Chemistry 76 629- 637.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
40

Jangchud, K., Phimolsiripol, Y., and Haruthaithanasan, V.
2003 Physicochemical Properties of Sweet Potato
Flour and Starch as Affected by Blanching and
Processing Starch/Stärke 55 258–264
Kale, R.V., Shere, D.M., Sontakke, M.D., and Gadhe, K.S.
2017 Effect of isolation methods on physicochemical
and functional properties of sweet potato (Ipomoea
batatasL.) starch. Journal of Pharmacognosy and
Phytochemistry 6(4) 223-227
Lawal, O.S. 2004 Composition, physicochemical
properties and retrogradation characteristics of native,
oxidized, acetylated and acid thinned new cocoyam
(Xanthosoma sagittifolium) starch. Food Chemistry 87
205-218.
Ngoc, L.B.B., Trung, P.T.B., Hoa, P.N., and Hung, P.V.
2017 Physicochemical properties and resistant starch
contents of sweet potato starches from different
varieties grown in Vietnam. International Journal of
Food Science and Nutrition 2 (1) 53-57
Soison, B., Jangchud, K., Jangchud, A., Harnsilawat, T.,
and Piyachomkwan, K. 2015 Characterization of
starch in relation to flesh colors of sweet potato
varieties. International Food Research Journal 22 (6)
2303-2308
Soni, P.L., Sharma, H., Dun, D., and Gharia, M.M. 1993.
Physicochemical properties of Quercus
leucotrichophora (Oak) starch. Starch/Starke 45 127-
130
Takeda, Y., Hizukuri, S., Takeda, C., and Suzuki, A. 1987
Structure of branched molecules of amylose of
various origins and molar fractions of branched and
unbranched molecules. Carbohydrate Research
165:139- 145.
Tan, H.Z., Li, Z.G., and Tan, B. 2009 Starch noodles:
History, classification, materials, processing, structure,
nutrition, quality evaluating and improving. Food
Research International 42 551-576.
Thao, H.M. and Noomhorm, A. 2011 Physiochemical
Properties of Sweet Potato and Mung Bean Starch and
Their Blends for Noodle Production. Journal of Food
Processing & Technology 2(1) 2-9
Tharise, N., Julianti, E., and Nurminah, M. 2014
Evaluation of physico-chemical and functional
properties of composite flour from cassava, rice,
potato, soybean and xanthan gum as alternative of
wheat flour. International Food Research Journal
21(4): 1641-1649
Tian, S.J., Rickard, J.E., and Blanshard, J.M.V. 1991
Physicochemical properties of sweet potato starch.
Journal of The Science of Food and Agriculture
57:459-491.
Van Hall, M. 2000 Quality of sweet potato flour during
processing and storage J Food Reviews Int 16 1-37
Zhang, Z.E., Fan, S.H., Zheng, Y.L., Lu, J., Wu, D.M.,
Shan, Q.,, and Hu, B. 2009 Purple sweet potato color
attenuates oxidative stress and inflammantory
response induced by D-galactose in mouse liver. Food
and Chemical Toxicology 47 (2) 496-501
Effect of Isolation Methods on Physicochemical Properties of Purple-fleshed Sweet Potato Starch
41
