
Isolation and Characterization of Microcrystalline Cellulose from
Coconut Fiber using Acid Hydrolysis Process
H. Nasution
1
, H. Harahap
1
, P. Suherman
1
and Kelvin
1
1
Department of Chemical Engineering, Faculty of Engineering, Universitas Sumatera Utara, Padang Bulan, Medan,
Indonesia
Keywords: Coconut fiber; microcrystalline cellulose; x-ray diffraction, Water soluble, Loss on drying.
Abstract: The object of this research was to explore the utilization of coconut fiber as a natural source for the production
of microcrystalline cellulose. Coconut fiber was treated with alkali at the first time then bleached before the
production of microcrystalline cellulose by acid hydrolysis (HCl). The produced materials were characterized
using X-ray Diffraction (XRD) and some physicochemical properties such as pH, water soluble substance
and loss on drying. XRD showed that acid hydrolysis process increase the crystallinity of microcrystalline
cellulose from α-cellulose. Meanwhile the physicochemical properties showed that the produced material was
close to British Pharmacopeia standard. The results proved that coconut fiber was a valuable source for the
production of microcrystalline cellulose.
1 INTRODUCTION
Natural fibers consist amorphous and semicrystalline
structure. Amorphous structure contain lignin and
hemicellulose while semicrystalline contain
cellulose. Cellulose fiber has better strength, stiffness
and thermal stability than natural fiber because
natural fiber still contains lignin and hemicellulose
(Melbi et al, 2018).
Cellulose is a linear chain of glucose molecules
with degree of polymerization between 10,000 to
15,000 and linked together through an oxygen
covalently bounded. During biosynthesis, van der
Waals and intermolecular hydrogen bonds between
hydroxyl groups and oxygen of adjacent molecules. It
makes cellulose relatively stable and gives high axial
stiffness. In cellulose there are regions where the
cellulose chains are arranged in a highly ordered
structure. This region is called crystalline region. On
the other side there are region that disordered. It is
known as amorphous region. Crystalline phase can be
extracted using acid catalyst hydrolysis. The result of
this extraction is microcrystalline cellulose (Robert et
al, 2011).
Microcrystalline cellulose is produced by
depolymerization of cellulose materials with
solutions of mineral acids at increased temperatures.
The acid catalyst destroys glycoside bonds mainly in
non crystalline domains. It makes cellulose lose its
degree of polymerization from 10,000-15,000
become 120 to 250. Microcrystalline cellulose is used
mainly as food products, cosmetic formulation,
inactive ingredients of tablets and special additive for
some technical applications (Michael and Alex,
2006). There are some natural resources that are used
to produce microcrystalline cellulose. That natural
resources are cassava bagasse (Panee et al,2015), corn
husk (Roshni and Yamini, 2015), rice straw (Chin et
al, 2016), empty fruit bunch palm oil (Nasution et al,
2017), cotton stalks (Hassan and El-Sakhawy, 2005).
In this study, coconut fiber was used as raw material.
The composition of coconut fiber can be seen at Table
1 (Khalil et al, 2006). Table 1 shows that the main
constituent of coconut fiber is cellulose.
Table 1: Composition of coconut fiber
Composition Percentage
Holocellulose 56.3
α – Cellulose 44.2
Lignin 20.5
Ash 2.2
There are some methods to produce
microcrystalline cellulose which is ultrasonication
222
Nasution, H., Harahap, H., Suherman, P. and Kelvin, .
Isolation and Characterization of Microcrystalline Cellulose from Coconut Fiber using Acid Hydrolysis Process.
DOI: 10.5220/0010077802220226
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
222-226
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

(Zailani et al, 2016), ultrafiltration membrane
(Nguyen and Rajesh, 2016), enzymatic hydrolysis
(Herman et al, 2017) and acid hydrolysis (Ravindra et
al, 2017). Acid hydrolysis was the conventional
method for produce microcrystalline cellulose.
Hydrochloric acid and sulfuric acid were common
used as mineral acid for acid hydrolysis (Ravindra et
al, 2017). With sulfuric acid hydrolysis, the sulphate
group are problematic because of the thermal stability
if compared to hydrochloric acid hydrolysis. So in
this study, we choose hydrochloric acid to hydrolyzed
the cellulose because microcrystalline produce with
hydrochloric acid has better thermal stability (Birgi
and John, 2009).
The main purpose of the present paper is to
characterize microcrystalline cellulose that extracted
from coconut fiber. The microcrystalline will be used
as reinforcement agent for composites.
2 METHODS
2.1 Materials
Coconut fiber was collected from local market in
Medan, Sumatera Utara, Indonesia. Other chemicals
such as nitric acid, sodium nitric, hydrochloric acid,
sodium hydroxide and sodium hypochloride were
supplied by Merck.
2.2 Extraction of α-Cellulose from
Coconut Fiber
Coconut fibers were cleaned from impurities and cut
into small pieces. 50 gram of coconut fiber were
added with 700 ml of 3,5% nictric acid that contained
8 mg of sodium nitric, then heated on temperature 90
°C using hotplate for 2 hours. Then, coconut fiber was
washed with distilled water until its filtrate neutral.
2% of sodium hydroxide solution were add to the
fiber and heated on temperature 50 °C for 1 hour.
Clean the filtrate using distilled water till its filtrate
neutral. Then the cellulose were added with 3,5%
sodium hypochloride and then heated until boiled for
10 minutes then cleaned the filtrate using distilled
water until its filtrate neutral.
Purified the cellulose using 340 ml sodium
hydroxide solution 17,5% for 30 munites on
temperature 80 °C. Cellulose was washed using
distilled water until its filtrate neutral then bleached
again for 30 minutes using sodium hypochlorite on
temperature 60 °C then washed with water until
filtrate neutral.
2.3 Isolation of Microcrystalline
Cellulose from α-Cellulose
The α-cellulose hydrolysed with hydrochloric acid
2,5 N at temperature 75 °C for 15 minutes. Cold water
were added and stirred strongly then placed at free air
area for one night until the solution formed a
suspension. Distilled water was used to washed the
suspension until neutral and then dried using oven for
1 hour at temperature 60 °C. Then Microcrystalline
Cellulose was saved in desiccator.
2.4 Yields of α-Cellulose and
Microcrystalline Cellulose
Yields of α-cellulose and microcrystalline cellulose
were determined by the following equations:
α-cellulose yield =
α-Cellulose weight
Coconut fiber weight
x 100 % (1)
Microcrystalline cellulose yield =
microcrystalline celluloseweight
α-celluloseweight
x 100 % (2)
3 CHARACTERIZATION OF
COCONUT HUSK FIBER,
Α-CELLULOSE AND
MICROCRYSTALLINE
CELLULOSE
3.1 Crystallinity
Crystalinity of coconut husk fiber, α-cellulose and
microcrystalline cellulose was determined by X-Ray
Diffraction (XRD) and recorded in 6100 Shimadzu.
The crystallinity index was determined with Segal’s
empirical method (Larissa et al, 2015):
C
IR
(%) = (I
200
– I
am
)/I
200
x 100 (3)
3.2 Determination of Phsycochemical
Properties of the Microcrystalline
Cellulose
Some of the properties were pH, water-soluble
substance and moisture content identification.
3.2.1 pH
Shake 1 g of microcrystalline cellulose for 5 minutes
in 50 ml of distilled water the pH was determined with
a pH meter (Achor et al, 2014).
Isolation and Characterization of Microcrystalline Cellulose from Coconut Fiber using Acid Hydrolysis Process
223
3.2.2 Water Soluble Substances
5 g of microcrystalline cellulose was shook in 80 ml
of distilled for 10 minutes, then filtered using filter
paper Whatman no.1. Then dried at 100-105 °C for 1
hour to evaporate the water and weight the
microcrystalline cellulose (Paul, 2008).
3.2.3 Loss on Drying
5 g of microcrystalline cellulose was dried at 100-105
°C for 1 hour then cooled in a desiccator. After then
the microcrystalline cellulose was weighed. The %
loss on drying was then determined as the ratio of
weight of mass loss to weight of sample expressed as
a percentage (Achor et al, 2014).
4 RESULT AND DISCUSSION
4.1 Yield of Process
From the process, the yield of α-cellulose was 45.44%
and the yield of microcrystalline cellulose was
42.74%. From 50 g of coconut fiber, 22.72 g of
cellulose was obtained. Then from 22.72 g of
cellulose, 9.71g of microcrystalline cellulose was
obtained. Here the results show concluded that
coconut fiber contains a lot of amorphous phase in
cellulose.
4.2 Crystallinity
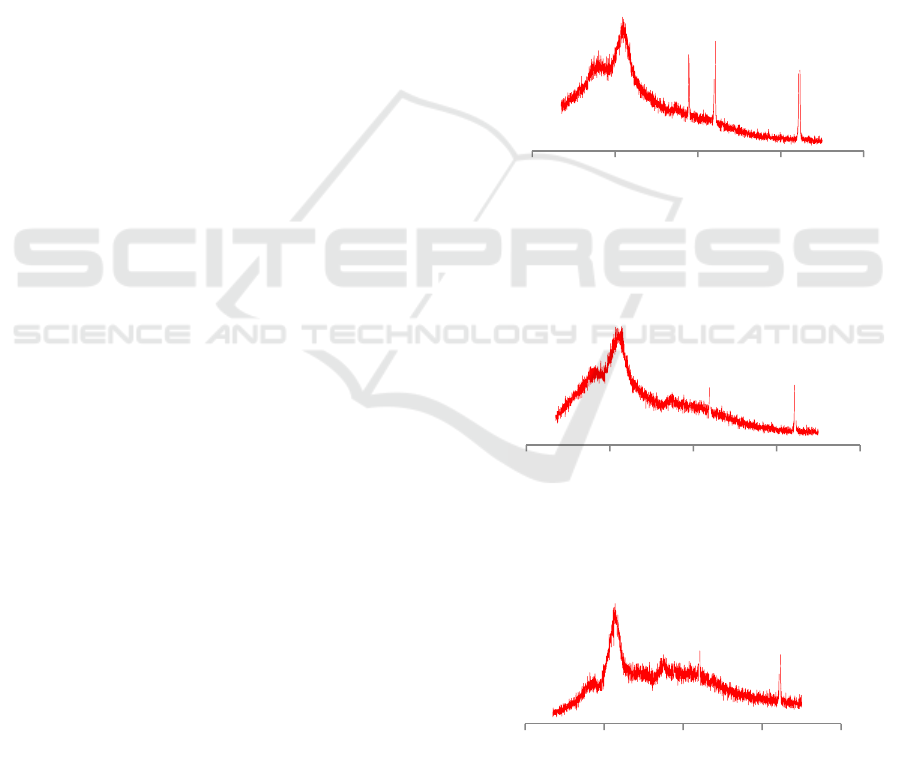
Figure 1, Figure 2 and Figure 3 show the XRD
patterns of microcrystalline cellulose, α-cellulose and
coconut fiber. The sharp peak indicative the
crystallinity (Larissa et al, 2015). From fig 1(a), the
amorphous peak still high and the difference between
amorphous peak and crystalline peak still small and it
means the crystallinity still low. From fig 1(b), the
amorphous peak of cellulose was lower than coconut
fiber, it means the most of the lignin and
hemicellulose was removed from coconut fiber and
the crystallinity increased. From fig 1(c) the
amorphous peak already low and the difference peak
between amorphous and crystal peak was large, it
means most of the amorphous region already
removed from the crystal region.
From the for coconut fiber XRD characteristics, a
sharp peak is resolved which is indicative of
crystalline at 2θ = 21.80°. The α-cellulose XRD
characteristics, a sharp peak is resolved which is
indicative of crystalline at 2θ = 22.80°. Then
microcrystalline cellulose XRD characteristics, a
sharp peak is resolved which is indicative of
crystalline at 2θ = 22.80°. The index crystallinity of
coconut fiber, α-cellulose and microcrystalline
cellulose was determined by Segal empirical. The
results show that the crystalinity of coconut fiber, α-
cellulose and microcrystalline cellulose were
43.45%, 46.54% and 75%. The results show that the
crystallinity increases in the series of transformation
from coconut fiber to microcrystalline cellulose.
Some studies about microcrystalline cellullose
percent crystallinity by hydrochloric acid hydrolysis
have been report such as empty fruit bunch palm oil
is 73% (Nasution et al, 2017), cotton stalks is 77%,
bagasse is 76% and rice straw is 77% (Hassan and El-
Sakhawy, 2005).
Figure 1: XRD patterns of coconut fiber.
Figure 2: XRD patterns of α-cellulose.
Figure 3: XRD patterns of microcrystalline cellulose.
0 20406080
0 20406080
0 20406080
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
224

4.3 Physicochemical Properties of
Microcrystalline Cellulose
By using universal pH indicator, the pH of the
microcrystalline cellulose was 7. The water soluble
substance was 0.2% and the loss on drying was 5%.
From British Pharmacopeia, the standard pH for
microcrystalline cellulose was 5-7, the water soluble
substance was <0.25% and the loss on drying was
<7% (British Pharmacopoeia, 2009). From the results
can be concluded that the microcrystalline cellulose
in accordance with British Pharmacopeia’s standard.
5 CONCLUSIONS
Microcrystalline cellulose has been isolated from
coconut fiber by acid hydrolysis, after coconut fiber
treated by alkali and bleaching process. it showed that
the crystallinity of microcrystalline cellulose has
increased because the exposure of crystalline phase
after the removal of lignin via alkaline treatment and
removal amorphous region of cellulose via acid
hydrolysis. The physicochemical properties of
microcrystalline cellulose showed that the produced
material is close to British Pharmacopeia standard.
Meanwhile XRD showed that increasing crystallinity
of microcrystalline cellulose from α-cellulose after
acid hydrolysis. The results obtained here suggest that
coconut fiber is capable of being a source for
production microcrystalline cellulose which can be
used as reinforcing fillers in various industries.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge that the present
research is supported by Directorate of Research and
Community Service Director General Strengthening
Research and Development Ministry of Research and
Technology and The Higher Education Republic of
Indonesia on year of grant 2018.
REFERENCES
Achor M, Oyeniyi Y J and Yahaya A 2014 Extraction and
Characterization of Microcrystalline Cellulose
Obtained from the back of the Fruit of Lageriana
siceraria (water gourd) Journal of Applied Science
Pharmaceutical Science 4 pp 57-60
Birgi B and John R D 2009 Single Step Method for the
isolation and Surface Functionalization of Cellulosic
Nanowhiskers Biomacromolecules 10 pp 334-341
British Pharmacopoeia 2009 The Commission office
London 111 pp 6578-6585
Chin K, Sam S T, Ong H L and Wei T O 2016 Extraction
of Microcrystalline Cellulose from Rice Straw and Its
Effect on Polyvinyl Alcohol Biocomposites Film
Proceeding of the 3
rd
International Conference of
Global Network for Innovative Technology pp 1-6
Hassan M L and El-Sakhawy M 2005 Physical and
Mechanical Properties of Microcrystalline Cellulose
Prepared from Local Agriculture Residues 8
th
Arab
International Conference on Polymer Science &
Technology pp 1-17
Herman S, Sutriyo, Hasty R S and Dianah R 2017
Preparation of Microcrystalline Cellulose from Water
Hyacinth Powder by Enzymatic Hydrolysis Using
Cellulase of Local Isolate Journal of Young
Pharmacists 9 pp 19-23
Khalil H P S A, Alwani M S and Omar A K M 2006
Chemical Composition, Anatomy, Lignin Distribution,
and Cell Wall Structure of Malaysian Plant Waste
Fibers BioResorces 1 pp 220-232
Larissa A D C, Ananda F F, Fabiano V P and Janice D 2015
Extraction and Characterization of Cellulose
Nanocrystals from Corn Stover Cellulose Chemical
Technology 49 pp 127-133
Melbi M, Hairul A, Anwar K, Syukri A and Mochamad A
2018 Production of Nanocellulose from Pineapple Leaf
Fibers via High_Shear Homogenization and
Ultrasonication Fibers 6 pp 1-12
Michael I and Alex L 2006 Formation Nano-Structure of
Microcrystalline Cellulose Cellulose Chemistry and
Technology 40 pp 231-317
Nasution H, Yurnaliza, Veronicha, Irmadani and Sitompul
S 2017 Preparation and Characterization of Cellulose
Microcrystalline (MCC) from Fiber of Empty Fruit
Bunch Palm Oil 1
st
Annual Applied Science and
Engineering Conference 180 pp 1-8
Nguyen H T T and Rajesh N 2016 Fractionation of
Hydrolyzed Microcrystalline Cellulose by
Ultrafiltration Membrane Journal of Engineering
Science and Technology 11 pp 136-148
Panee P, Naiyasit Y and Prakit S 2015 Isolation and
Characterization of Microcrystalline cellulose from
Cassava bagasse Proceedings of the Burapha
University International Congerence pp 601-608
Paul M E 2008 Investigation of the Physicochemical
Properties Of Microcrystalline Cellulose From
Agricultural Wastes I: Orange Mesocarp Cellulose 15
pp 141-147
Ravindra D K, Prabhat S B and Vikrant G G 2017
Extraction of Microcrystalline Cellulose from Cotton
Sliver and Its Comparison with Commercial
Microcrystalline Cellulose Journal of Polymers and
Environment DOI: 10.1007/s10924-017-0936-2
Isolation and Characterization of Microcrystalline Cellulose from Coconut Fiber using Acid Hydrolysis Process
225

Robert J M, Ashlic M, John N, John S and Jeff Y 2011
Cellulose Nanomaterial Review : Structure, Properties
and Nanocomposites Chem Soc Rev 40 pp 3941-3994
Roshni S V and Yamini D S 2015 Production of Micro
Crystalline Cellulose from Corn Husk and Its
Evaluation as pharmaceutical Excipient International
Journal of Research and Scientific Innovation 2 pp 69-
74
Zailani I S A, Aviceena, Jimat D N and Jami M S 2016
Extraction of Microcrystalline Cellulose (MCC) from
Cocoa Pod Husk via Alkaline Pretreatment Combined
with Ultrasonication International Journal of Applied
Engineering Research 11 pp 9876-9879.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
226
