
The Effect of Arenga Pinnata Merr. Polysaccharide Extract on Blood
Glucose Level
Juliati Br. Tarigan,
1*
Aminah Dalimunthe
2
and Sabarmin Peran
g
in-an
g
in
1
1
Department of Chemistry, University of Sumatera Utara, Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Pharmacology, University of Sumatera Utara, Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
Keywords: Arenga pinnata, blood glucose concentration level, anti-diabetic.
Abstract:
An observational experiment was designed to study the effect of Arenga pinnata polysaccharide
extract on blood glucose level of the rat. The rats weigh from 151 – 207 g were fed by the extract
from the different hardness level of Arenga pinnata endosperm. Three different weight of 50, 100
and 200 mg containing 1% of Arenga pinnata endosperm extract were studied and compared
simultaneously with a common diabetic drug (glibenclamide) and glucose 50%. The blood
glucose level was determined in the range time of 0 – 120 min using a glucometer. The result
shows that the endosperm extract of Arenga pinnata could reduce blood glucose due to high fiber
contained. Arenga pinanata endosperm extract 1 and 3 with a level of 200 and 50 mg,
respectively, significantly reduced blood glucose after 90 min treatment compared to diabetic
rats. Even though glibenclamide showed a better result, this finding opens the possibility to use
natural Arenga pinnata endosperm as diabetic controlled.
1 INTRODUCTION
Dietary fiber has been recognized playing an crucial
role in reducing the risk of chronic diseases such as
diabetic type 2, lever and obesity (2010, Mann and
Cummings, 2009). Diabetic is a metabolic chronic
which identified from hyperglycaemic and insulin
deficiency (Watanabe et al., 2010). It was predicted
that in 2030 more than 552 million people in the
world will suffer from diabetic (Whiting, 2011).
Even though the synthetic diabetic drug has been
widely used for diabetic type 2, some side effects
such as hypoglycemia, drug-resistant, edema and
increasing body weight have limited the utilization
(Tahrani, 2010). Therefore another alternative
therapeutics drug must be developed not only by
using synthetic material but also from a natural
product. The polysaccharide of Pleorutus ostreatus
has been identified and used as a traditional
antidiabetic drug (Zhang, 2016). Another
polysaccharide like galactomannan fenugreek
potentially can be used as a diabetic drug (Madar,
1988) as well as their combination with pectin citrus
(Shtriker, 2018).
One of the abundant sources of galactomannan in
Indonesia is Arenga pinnata endosperm (APE)
which usually sell in the traditional market as
“kolang-kaling” (Mogea, 1991). The utilization of
APE is limited for a cocktail and food (Orwa, 2009).
APE contains a high amount of fiber (Tarigan, 2012)
and has water dissolved and not dissolved fraction.
Dissolved water fraction compose of carbohydrate at
62.49% and crude fiber (1.11%) (Tarigan and
Kaban, 2010). The main component of
polysaccharide in APE is galactomannan that can
dissolve in water (Rao, 1961) which contains
galactose and mannose ratio of 1:1.33 with the
antioxidant activity of IC
50
= 22.109 mg/mL
(Tarigan, 2012), (Tarigan, 2014). To the date, no
studies have been done regarding to galactomannan
as an antidiabetic drug.
Based on that, this study aims to explore the
possibility of APE powder as antidiabetic. The APE
was categorized into three different groups based on
their texture (hard, medium and soft). This
systematic study provides greater understanding and
information about the prospect of APE endosperm
extract as an antidiabetic food supplement or drug.
964
Tarigan, J., Dalimunthe, A. and Perangin-angin, S.
The Effect of Arenga Pinnata Merr. Polysaccharide Extract on Blood Glucose Level.
DOI: 10.5220/0010075909640968
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
964-968
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All r ights reserved

2 EXPERIMENTAL
2.1. Materials
The APE was brought from a local traditional
market in Medan, North Sumatera – Indonesia. All
the chemicals used were brought from local
chemicals dealers and was used without any
purification.
2.2. Procedures
2.2.1. Extraction of Galactomannan from
APE
Preparation of dry APE and extraction of
galactomannan from APE based on their texture
were conducted based on our previous study with
slight modification (Tarigan, 2012). The APE was
categorized based on their texture of (1) hard, (2)
medium, and (3) soft. Qualitative analysis of
monosaccharide component in dissolved and
undissolved water fraction were done following
procedure from (Mulimani and Prashanth, 2002). A
100 mg galactomannan was mixed with 50 mL 1M
HCl and heated in a water bath for 14 h followed by
thin layer chromatography analysis. After 14 h
hydrolysis, the solution was discarded and
neutralized with barium carbonate. The filtrate was
evaporated to form syrup and was dropped in
chromatography paper (20 x 20 cm) using glass
pipette. Separation of the thin layer chromatography
was conducted using two solvent systems in one
phase. Firstly, a mix of n-butanol : ethanol : water
(BEW) solvent at a ratio of 4:1:1 was added and the
plate was drying in the dryer. Next a mix of n-
butanol : acetic acid : water (BAW) solvent at a ratio
of 4:1:1 was added. The appearance of sugar was
detected by spraying with a p-anisidin solvent in
methanol and dry in oven drying for 20 min. The
brown spot signed the appearance of sugar.
2.2.2. Preparation of APE Extract
The APE extract was prepared from mixed of 1%
dry APE with water stirred at 50 - 55ºC until all the
APE dissolved followed by a shaker for 30 min at
the same temperature.
2.2.3 Determination of Blood Glucose Level
using a Blood Glucose Test Meter
This procedure was conducted based on previous
researcher (Thomson, 1985). The blood glucose
level of fasting rats was determined for 18 h. Blood
from each rat was taken from vena vessel which
firstly the end tail of rats was disinfected by ethanol
70%. The first drop of rat blood was discarded, and
the next blood drop was absorbed to the strip layer
of blood glucose test meter and the blood glucose
level determined in mg/dL.
2.2.4. Determination of the Effect of APE
Extract in Blood Glucose Level using
Tolerant Method
Rats weight ranging from 150 – 200 g had food
fasting for 24 h and was measured the as fasting
blood glucose level categorized in 5 different groups
which each group contain three rats.
Group 1, rats fed by 1% hydrogel from APE 1 with a
dosage of 50, 100, 200 mg.
Group 2, rats fed by 1% hydrogel from APE 2 with a
dosage of 50, 100, 200 mg.
Group 3, rats fed by 1% hydrogel from APE 3 with a
dosage of 50, 100, 200 mg.
Group 4, rats fed by glibenclamide with a dosage of
0.65 mg.
Group 5, rats fed by glucose dosage 50%
3 RESULTS AND DISCUSSION
The APE endosperm was categorized into three
different groups based on their texture. The
percentage yield of dry APE had reported in the
previous studied (Tarigan, 2018). Table 1 depicts the
yield of dry APE which contains dissolved water
fraction (galactomannan) and undissolved water
fraction (mannan). Separation of galactomannan and
mannan could be done through a simple method
using water in the neutral condition. Extraction
process in this condition does not require further
purification since it uses alcohol as a solvent and
could produce material with good quality and
environmentally friendly (Cerqueira, 2009).
Commonly crude galactomannan is used in
pharmaceutical and cosmetical industry (Üner and
Altınkurt, 2004). Galactomannan and mannan could
easy to separate with centrifugation which
galactomannan is in supernatant and mannan which
is in undissolved water fraction. In addition of
alcohol, both fractions will form precipitation which
further drying to form a dry extract.
Dissolved water fraction obtained highly in APE
2 while undissolved water fraction occurred mostly
in APE 3. Based on our previous study, the hard
texture is obtained in APE 3 which is ripened.
Usually immature APE contains more
The Effect of Arenga Pinnata Merr. Polysaccharide Extract on Blood Glucose Level
965
galactomannan than mannan (Kooiman, 1971). A
polysaccharide containing in mannan is mannose
range from 85 – 95% (Aspinall, 1959). Releasing
galactosyl residue by α-galactosidase in endosperm
increasing ratio mannose in galactomannan (Iglesias,
2014).
Table 1. The yield of APE
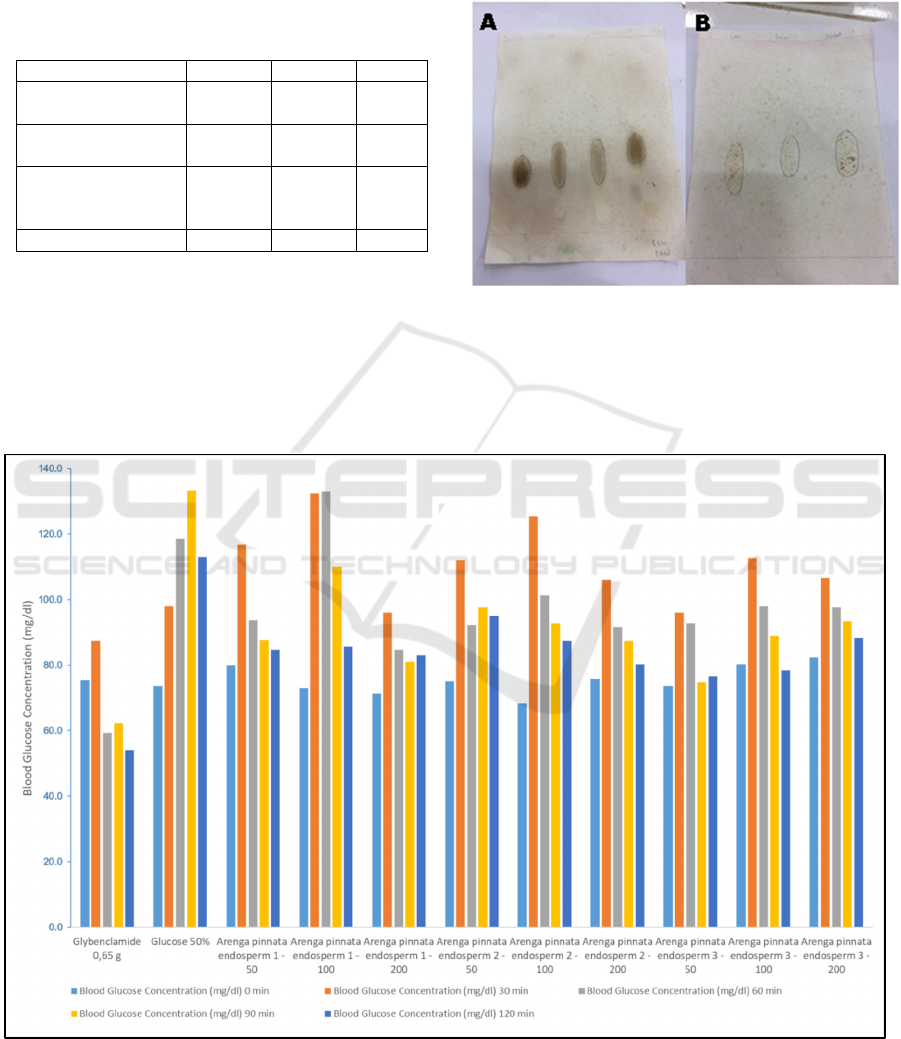
Figure 1 depicts the thin layer chromatography
result for standard galactose, mannose, dissolved
water fraction, undissolved water solvent and
mannose + glucose. As shown, both dissolved water
and undissolved fraction contains galactose which
their spots appeared in between spot of mannose and
galactose (Fig. 1b & 1c). This was supported by the
appearance spots as showed in figure 1B. Therefore
it can be concluded that undissolved water fraction
contains a high amount of mannan. This result
similar with the previous researcher reported
(Kaban, 2018).
Figure 1. Thin layer chromatography spectrum of (A1)
galactose standard; (A2) dissolved water fraction; (A3)
undissolved water fraction and (A4) mannose; and (B1)
dissolved water fraction; (B2) undissolved water fraction;
and (B3) mannose + galactose.
Figure 2. Graph the effect of APE extract on blood glucose level.
Paramete
r
APE 1 APE 2 APE 3
Dry weight APE
(
%
)
2,785 5,650 7,144
Weight of dissolved
water fraction
(
%
)
1,688 4,267 3,158
Weight of
undissolved water
fraction
(
%
)
0,504 1,140 2,966
Wei
g
ht loss
(
%
)
2,965 1,215 5,100
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
966

The effect of APE extract on blood glucose level
of rats was presented in figure 2. Some researchers
have demonstrated that galactomannan could
decrease blood glucose level (Madar, 1988),
(Shtriker, 2018), (Kendall, 2010), (Mann and
Cummings, 2009). However no study is found for
APE in decreasing blood glucose level. Three
different weights of 50, 100 and 200 mg containing
1% of APE extract were studied and compared
simultaneously with a common diabetic drug
(glibenclamide) and glucose 50%. The blood
glucose level was determined in the range time of 0
– 120 min using a glucometer. The result shows that
the APE extract could reduce blood glucose due to
high fiber contained. Most of the rats fed by APE
extract showed slightly higher blood glucose level
than rats fed by the antidiabetic drug
(glibenclamide). This is because the concentration of
APE used is lower than glibenclamide. APE extract
1 and 3 with a level of 200 and 50 mg, respectively,
significantly reduced the blood glucose level after 90
min treatment compared to diabetic rats. Therefore it
can be concluded that APE could be used to reduce
blood glucose level.
4 CONCLUSIONS
Galactomannan obtained from APE 1 – 3 were
1.688, 4.267, and 3.158%, while mannan was
occurred at 0.504, 1.140, and 2.966%, respectively.
APE extracts 1 and 3 with a dosage of 200 and 50
mg, respectively significantly reduce blood glucose
level after 90 min treatment compared to diabetic
rats. APE potentially could be used to reduce blood
glucose level and do not have any side effect.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support from
Directorate General of Higher Education – Ministry
of Research, Technology and Higher Education,
Indonesia and the Rector of University of Sumatera
Utara by TALENTA USU on
2590/UN5.1.R/PPM/2018, date of 16 March 2018.
REFERENCES
Aspinall, G. O. 1959. Structural Chemistry of the
Hemicelluloses. In: WOLFROM, M. L. (ed.)
Advances in Carbohydrate Chemistry. Academic
Press.
Cerqueira, M. A., Pinheiro, A. C., Souza, B. W. S., Lima,
Á. M. P., Ribeiro, C., Miranda, C., Teixeira, J. A.,
Moreira, R. A., Coimbra, M. A., Gonçalves, M. P. &
Vicente, A. A., 2009. Extraction, purification and
characterization of galactomannans from non-
traditional sources. Carbohydrate Polymers. 75, 408-
414.
Iglesias, J., Melero, J. A., Bautista, L. F., Morales, G. &
Sánchez-Vázquez, R., 2014. Continuous production of
biodiesel from low grade feedstock in presence of Zr-
SBA-15: Catalyst performance and resistance against
deactivation. Catalysis Today. 234, 174-181.
Kaban, J., Reveny, J., Tarigan, J. & Zebua, N. F., 2018.
Modificated Extraction And Purity Test Of Arenga
Pinnata Gum. Asian Journal of Pharmaceutical and
Clinical Research. 3.
Kendall, C. W. C., Esfahani, A. & Jenkins, D. J. A., 2010.
The link between dietary fibre and human health.
Food Hydrocolloids. 24, 42-48.
Kooiman, P., 1971. Structures of the galactomannans from
seeds of Annona muricata, Arenga saccharifera, Cocos
nucifera, Convolvulus tricolor, and Sophora japonica.
Carbohydrate Research. 20, 329-337.
Madar, Z., Abel, R., Samish, S. & Arad, J., 1988. Glucose-
lowering effect of fenugreek in non-insulin dependent
diabetics. Eur J Clin Nutr. 42, 51-4.
Mann, J. I. & Cummings, J. H., 2009. Possible
implications for health of the different definitions of
dietary fibre. Nutrition, Metabolism and
Cardiovascular Diseases. 19, 226-229.
Mogea, J., Seibert, B. & Smits, W., 1991. Multipurpose
palms: the sugar palm (Arenga pinnata (Wurmb)
Merr.). Agroforestry Systems. 13, 111-129.
Mulimani, V. H. & Prashanth, S. J., 2002. Investigating
plant galactomannans. Biochemistry and Molecular
Biology Education. 30, 101-103.
Orwa, C., Mutua, A., Kindt, R., Jamnadass, R. & Simons,
A., 2009. Agroforestree database: a tree species
reference and selection guide version 4.0. World
Agroforestry Centre ICRAF, Nairobi, KE.
Rao, K. V., 1961. Development and life history of a
nudibranchiate gastropod Cuthona adyarensis Rao.
Journal of the Marine Biological Association of India.
3, 186-197.
Shtriker, M. G., Hahn, M., Taieb, E., Nyska, A., Moallem,
U., Tirosh, O. & Madar, Z., 2018. Fenugreek
galactomannan and citrus pectin improve several
parameters associated with glucose metabolism and
modulate gut microbiota in mice. Nutrition. 46, 134-
142.e3.
Tahrani, A. A., Piya, M. K., Kennedy, A. & Barnett, A.
H., 2010. Glycaemic control in type 2 diabetes:
Targets and new therapies. Pharmacology &
Therapeutics. 125, 328-361.
Tarigan, J. B. 2014. Karakterisasi Edible Film Yang
Bersifat Antioksidan Dan Antimikroba Dari
Galaktomanan Biji Aren (Arenga pinnata) Yang
Diinkorporasi Dengan Minyak Atsiri Daun Kemangi
The Effect of Arenga Pinnata Merr. Polysaccharide Extract on Blood Glucose Level
967

(Ocimum basilicum L.). Doktor Disertasi, Universitas
Sumatera Utara.
Tarigan, J. B., Barus, T., Kaban, J. & Marpongahtun.
Characteristic and Study of Antioxidant Activity
Galactomanan from "Kolang-Kaling" (Arenga
pinnata). Asian International Conference on
Materials, Mineral and Polymer, 23 - 24 March 2012
2012 Penang. Penang.
Tarigan, J. B. & Kaban, J. Karakterisasi Ekstrak Kolang-
kaling. SEMIRATA MIPA BKS PTN Wilayah Barat,
10-11 Mei 2010 2010 Pekan Baru.
Tarigan, J. B., Kaban, J. & Zulmi, R., 2018.
Microencapsulation of vitamin e from palm fatty acid
distillate with galactomannan and gum acacia using
spray drying method. IOP Conference Series:
Materials Science and Engineering. 309, 012095.
Thomson, R. G., 1985. Semi-quantitative measurement of
blood glucose levels: An undergraduate laboratory
study. The American Biology Teacher. 47, 159-162.
Üner, M. & Altınkurt, T., 2004. Evaluation of honey
locust (Gleditsia triacanthos Linn.) gum as sustaining
material in tablet dosage forms. Il Farmaco. 59, 567-
573.
Watanabe, K., Kamata, K., Sato, J. & Takahashi, T., 2010.
Fundamental studies on the inhibitory action of
Acanthopanax senticosus Harms on glucose
absorption. Journal of Ethnopharmacology. 132, 193-
199.
Whiting, D. R., Guariguata, L., Weil, C. & Shaw, J., 2011.
IDF Diabetes Atlas: Global estimates of the
prevalence of diabetes for 2011 and 2030. Diabetes
Research and Clinical Practice. 94, 311-321.
Zhang, Y., Hu, T., Zhou, H., Zhang, Y., Jin, G. & Yang,
Y., 2016. Antidiabetic effect of polysaccharides from
Pleurotus ostreatus in streptozotocin-induced diabetic
rats. International Journal of Biological
Macromolecules. 83, 126-132.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
968
