
Supplementation of Turmeric Extract Does Not Improve
Neurological Function Following Repetitive Mild Traumatic Brain
Injury in the Rat
Andre Marolop Pangihutan Siahaan
1
and Iskandar Japardi
1
1
Department of Neurosurgery, Faculty of Medicine, Universitas Sumatera Utara, Jl. Dr. Mansyur No. 5, Medan, Indonesia
Keywords: Repetitive Traumatic Brain Injuries, Curcumin, Psychomotor Assessment.
Abstract: Turmeric has been in use since ancient times as a condiment and due to its medicinal properties. Curcumin,
the yellow coloring principle in turmeric, is a polyphenolic and a major active constituent. Besides the anti-
inflammatory, thrombolytic, and anti-carcinogenic, curcumin was also reported to have therapeutic potential
in Alzheimer’s disease by inhibiting the amyloid-β-protein aggregation. Inflammation and early degeneration
are two of main processes believed happened after repetitive mild traumatic brain injury. The effect of
curcumin was evaluated in weight drop model of repetitive mild traumatic brain injury. Male Sprague dawley
rats (n=10) were given multiple brain injury (40 gr mass drop from 1 m heights, 3 times daily on day 0,1,3,
and 7). Curcumin (500 mg/kg) were given orally. On the last day of injury, psychomotor assessment (beam
walk assessment and exit circle test) were performed. Control injured rats had a significant neurological deficit
(p<0.01). No significant different found control and treatment group. The study does not demonstrate the
efficacy of curcumin in rat with repetitive mild traumatic brain injury model.
1 INTRODUCTION
Traumatic brain injury (TBI) results from impact to
the head. The severity of this injury is varied, from
mild (brief and little change in consciousness or
mental status) to severe (prolonged loss of
consciousness and coma to fatal). Either mild of
severe TBI can result in short and long-term
disability. On a global scale, TBI is a serious health
concern and is the leading cause of mortality and
disability among individuals in young-age
population. TBI is one of the most common
neurological diagnoses in the US and the CDC has
estimated that 1.7 million people sustain TBI
annually (Rutland-Brown, 2006).
Repetitive mild TBI (rmTBI) is the form of TBI
that has gained public awareness, as well as within
military, scientific, and medical communities. TBI
accounts for around 28% of all combat casualties in
Iraq and Afghanistan (Okie, 2005). Persistent
accounts of rmTBI suffered by athletes have also
directed much attention. It is estimated that 1.8-3.8
million sports-related TBIs occur every year (Halstead
& Walter, 2010). About 60% of retired professional
football players sustained at least 1 concussion during
their careers and approximately 25% experienced
repeated injury (Guskiewicz, 2005).
Repetitive mild TBIs generally produce a
constellation of symptoms (e.g. headache, dizziness,
confusion) collectively known as post-concussive
syndrome (Halstead and Walter, 2010). Reports of
more serious consequences of rmTBI such as chronic
traumatic encephalopathy and increased co-morbidity
of neurodegenerative disorders (Omalu, 2010),
(Guskiewicz, 2007). This situation becomes even
more complex by the fact that rmTBI is extremely
difficult to detect. For the most part, routine imaging
approaches (CT and MRI) contribute little to the
evaluation and management of mild concussion
(Boven, 2009). The more advanced and specialized
approaches such as diffusion tensor imaging are
showing promise (Donald, 2011).
Histologically, there is protein aggregation
happened after rmTBI. The primary proteinopathy is
tau protein, in form of neurofribrillary tangle (NFT).
The most common secondary proteinopathies are
TDP-43 and amyloid-β-protein aggregation (McKee,
2013).
Turmeric (Curcuma longa) is a traditional
medicinal plant that also is commonly used as spice
in South as well as Southeast Asia. Curcumin, the
active ingredient of this plat, had been isolated since
long time ago and is considered as a potent
antiinflammation. In animal model, curcumin will be
bounded to beta structure in amyloid, so that reduces
486
Siahaan, A. and Japardi, I.
Supplementation of Turmeric Extract Does Not Improve Neurological Function Following Repetitive Mild Traumatic Brain Injury in the Rat.
DOI: 10.5220/0010075504860490
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
486-490
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

the plaque formation. Curcumin also will decrease the
aggregation process and stimulate the clearance.
Curcumin was also reported decreased the
accumulation of soluble NFT and inhibit kinases that
are involved in tau formation. Moreover, also in
animal model, curcumin supplementation was proved
to be effective in Alzheimer’s disease (Darvesh,
2012).
Until now, there is no proved effective therapy for
rmTBI. Curcumin, that was proved to be effective in
neurodegenerative disease, might potential to be
therapeutic agent in rmTBI. The aim of this study was
to prove the role of curcumin in neurological function
after rmTBI.
2 MATERIAL AND METHODS
2.1 Animal Model and Experimental
Groups
Male Sprague dawley rats (n=30) weighing 350 to
400 gr were housed in polycarbonate cages
maintained at 50 ± 10% humidity with a 12-hour light
and dark cycle. Rats were fed with standard
laboratory chow and water ad libitum. The
experimental protocol was approved by an
Institutional Review Committee of Universitas
Sumatera Utara, Medan, Indonesia. The animals were
also acclimatized to the laboratory condition prior to
experimentation for two weeks.
The rats were randomly allocated into three
groups (n=10) as following; a control (sham-
operated) group, a trauma group, and turmeric extract
(TE) groups. The rats in controlled group were placed
only in stereotactic apparatus, with neither trauma nor
drug treatment. The control group underwent trauma
protocol and the TE group underwent trauma protocol
and turmeric extract treatment
2.2 Drug Treatment
Turmeric extract (Sido Muncul, Semarang,
Indonesia) was given in 500 mg/kgBW dose,
suspended in 2 cc of double-distilled water. All rats
were weighed before the extract was given. The
extract was given orally via oral gavage every day for
consecutively eight days, at least two hours before
trauma protocol.
2.3 Weight Drop Brain Injury Model
Rats were placed under a stereotaxic frame. This
protocol was done without any anaesthesia. A 40-
gram weight was dropped from a height of 1 m unto
5 mm diameter pipe resting on the vertex. To prevent
skull fracture, a round metal with 3 cm diameter was
placed on the rats’ vertex. The trauma was given three
times daily, every 4 hours on day 0,1,3, and 7. Every
rat in control and TE groups underwent 12 traumas in
this research.
2.4 Outcome Assessment
2.4.1 Mortality and Body Weight
Death of the rats following trauma protocol were
noted. Every rat was weighed on day-0 before the
protocol started and on day -7, i.e on the last trauma
protocol.
2.4.2 Beam Walk Assessment
Rat was placed on a 1.5 m length plywood with 10 cm
width. For baseline data, we recorded the time needed
to cross the plywood and the maximal walking distant
before did the protocol. We also recorded maximal
time before rats fell down and marked whether there
was sign of disequilibrium. We repeated the
assessment
2.5 Statistical Analysis
The total time needed were reported in mean and
standard deviation. When comparisons were made
between groups, significance in between-group
variability was analysed using the one-way Anova
test with Tukey as post hoc test. Differences were
considered significant at the P <0.05.
3 RESULT
3.1 Mortality and Weight
There was no mortality in all groups. The rats still
survived, moved actively, and had good appetite after
three days protocol. We did not find significant
weight changes between day 0 and day 7 either in
negative sham control, trauma, or TE group (p>0.05;
table 1.
Supplementation of Turmeric Extract Does Not Improve Neurological Function Following Repetitive Mild Traumatic Brain Injury in the Rat
487
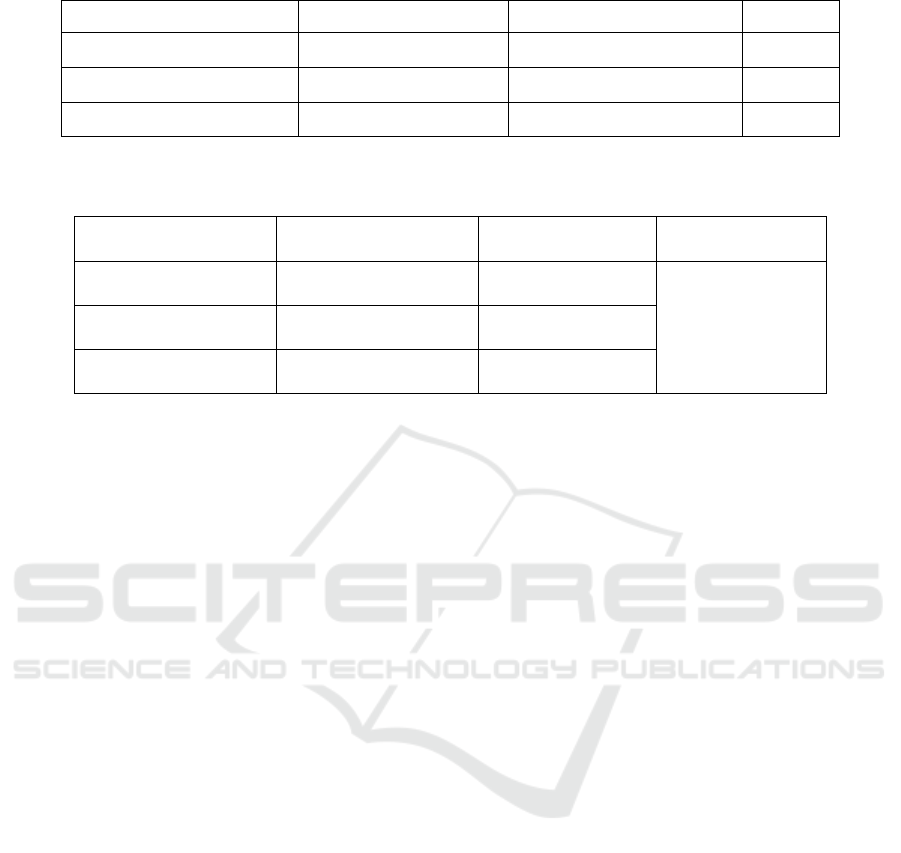
Table 1. Weight Change (gr)
Group Before traumas After traumas p
Negative sham
377.22 ± 29.72
378.44 ± 29.66
0,910
Trauma
351.78 ± 29.89 349.33 ± 38.90
0,482
TE
367.89 ± 36.70 357.89 ± 39.89
0,950
One way Annova, significant if p<0.05
Table 2. Time needed to walk along the stick
Group n
x ± SD (s)
p
Negative sham 10
9,7 ± 1,95
0,342
Trauma 5
12,20 ± 3,89
TE 5
8,80 ± 1,30
One way Annova, significant if p<0.05
3.2 Disorder of Equilibrium
Repetitive TBI generated disorder of equilibrium. On
the negative sham control group, none of the rat
showed any sign of disequilibrium. On the trauma
group, 90% of the showed sign of disequilibrium one
day after trauma. On the TE group, 60% of the rats
showed the same sig, whether 30% among them
showed psychomotor depression, and only 10%
showed no sign of disequilibrium.
3.3 Walking Length and Fell Down
On negative sham control group, all of the animals
could walk for 1.5-metre long. Conversely, only 50%
of the animals in the trauma group that could walk for
1.5-metre long. 40% of them could not walk for 1.5
metre and 10% of them did not move at all. On the
TE group, 50% of animals could walk for 1.5-metre
length. 20% of the could not walk till the edge and
30% did not move at all.
The same finding could be seen regarding fell
down. None of the negative sham control group fell
down while walking on the plywood. Conversely,
40% animals on the control group fell down while
walking on the stick and 50% animals on the
treatment group fell down while walking.
Regarding the time needed to walk along the stick,
there was no significant different in all three groups
(table 2).
4 DISCUSSION
Repetitive mild traumatic brain injury is one of major
concerns in neurology right now. Many people are
prone to suffer this condition, such as athlete and
military personnel. In animal study, it is said that the
cellular as well as molecular balance will be back to
normal state in around 7-10 days (Giza and Hovda,
2014). This fact makes hypothesis arising, that brain
will be in prone-to-injury condition if the second
impact happened in this time period.
We used the modified weight drop model in this
study with modification from Marmarou procedure.
We showed zero mortality rate, compared to report by
Marmarou (64%) or by Kane (10%) (Marmarou,
1994), (Kane, 2012). Our finding was consistent with
the next study. They also reported the same mortality
rate (Xu, 2016). The other unique feature in our
model is no need of anesthesia. All the trauma
procedures were performed without anesthesia, even
this method could produce a non-uniform injury site.
Even so, this situation fitted contact injury happened
during sport.
We also did not find changes in body weight in all
groups following trauma. In many reports of animal
model of traumatic brain injury, there are significant
body weight decrease that probably happened due to
injury affecting feeding behavior and injury to the
anterior hypothalamus (Wei, 2012), (Samini, 2013),
(Moon, 2009).
We found significant different regarding motoric
and equilibrium. Rats in traumatic brain injury and
turmeric extract group could not maintain the stability
in the plywood. After trauma, only half of groups in
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
488

either trauma or TE group that could maintain the
stability. A study reported that motoric disfunction
was one of common manifestation in Chronic
traumatic encephalopathy, a sequel of repetitive mild
traumatic brain injury (Montenigro, 2015).
Turmeric extract is a well-known potent
antioxidant and antiinflammation. On animal study,
many reports showed the effectivity in degenerative
disease, but it was failed in human clinical study
(Darvesh, 2012), (Tang, 2017). In our study, we did
not find significant different regarding outcome in
trauma and turmeric extract group. One of the main
problems of crude turmeric extract is the low
bioavailability. If the challenge of the low
bioavailability is overcome, curcumin as medication
in repetitive mild traumatic brain injury may still be
in horizon.
The main limitation of this study is the outcome
that only limited to clinical. For the next research, it
is advised to do biochemistry analysis to determine
the cellular status and cell expression. Besides, a long
follow up period is advisable to make sure the long-
term outcome in this condition.
In conclusion, we found no significant
improvement after curcumin supplementation in
repetitive mild traumatic brain injuries.
ACKNOWLEDGEMENTS
This research was fully funded by Research
Committee of Universitas Sumatera Utara in
TALENTA Grant 2017.
REFERENCES
Darvesh, A.S., Carroll, R.T., Bishayee, A., Novotny, N.A.,
Geldenhuys, W.J., Van der Schyf, C.J. (2012).
Curcumin and neurodegenerative diseases: a
perspective. Expert Opin Invest Drugs, 21(8), 1123–
1140.
Giza, C.C., Hovda, D.A. (2014). The New Neurometabolic
Cascade of Concussion. Neurosurgery, 75, S24–S33.
Guskiewicz, K.M., Marshall, S.W., Bailes, J., McCrea, M.,
Harding Jr, H.P., Matthews, A., Mihalik, J.R., Cantu,
R.C. (2007). Recurrent concussion and risk of
depression in retired professional football players. Med
Sci Sports Exerc, 39, 903–9.
Guskiewicz, K.M., Marshall, S.W., Bailes, J., McCrea,
M., Cantu, R.C., Randolph, C., Jordan, B.D. (2005).
Association between recurrent concussion and late-life
cognitive impairment in retired professional football
players. Neurosurgery, 57, 719–26.
Halstead, M.E., Walter, K.D. (2010). Sport-related
concussion in children and adolescents. Pediatrics,
126, 597–615.
Kane, M.J., Angoa-Pérez, M., Briggs, D.I., Viano, D.C.,
Kreipke, C.W., Kuhn, D.M. (2012). A mouse model of
human repetitive mild traumatic brain injury. J
Neurosci Methods 203(1), 41–49.
Mac Donald, C.L., Johnson, A.M., Cooper, D., Nelson,
E.C., Werner, N.J., Shimony, J.S., Snyder, A.Z.,
Raichle, M.E., Witherow, J.R., Fang, R., Flaherty, S.F.,
Brody, D.L. (2011). Detection of blast-related
traumatic brain injury in US military personnel. N Engl
J Med, 364, 2091–100.
Marmarou, A., Foda, M.A., van den Brink, W., Campbell,
J., Kita, H., Demetriadou, K. (1994). A new model of
diffuse brain injury in rats. Part I. Pathophysiology and
biomechanics. J Neurosurg, 80, 291–300
McKee, A.C., Stein, T.D., Nowinski, C.J., Stern, R.A.,
Daneshvar, D.H., Alvarez, V.E., et al. (2013). The
spectrum of disease in chronic traumatic
encephalopathy. Brain, 136(1), 43–64.
Montenigro, P.H., Bernick, C., Cantu, R.C. (2015). Clinical
Features of Repetitive Traumatic Brain Injury and
Chronic Traumatic Encephalopathy. Brain Pathol,
25(3), 304–317.
Moon, R.J., Wilson, P., Kirkham, F.J., Davies, J.H. (2009).
Growth monitoring following traumatic brain injury.
Arch Dis Child, 94, 699–701.
Okie, S. Traumatic brain injury in the war zone. (2005). N
Engl J Med, 352, 2043–7.
Omalu, B.I., Hamilton, R.L., Kamboh, M.I., DeKosky,
S.T., Bailes, J. (2010). Chronic traumatic
encephalopathy (CTE) in a National Football League
Player: case report and emerging medicolegal practice
questions. J Forensic Nurs, 6, 40–6.
Rutland-Brown, W., Langlois, J.A., Thomas, K.E., Xi,
Y.L. (2006). Incidence of traumatic brain injury in the
United States. J Head Trauma Rehabil, 21, 544–8.
Samini, F., Samarghandian, S., Borji, A., Mohammadi, G.,
Bakaian, M. (2013). Curcumin pretreatment attenuates
brain lesion size and improves neurological function
following traumatic brain injury in the rat.
Pharmacology, Biochemistry and Behavior, 110(C),
238–244.
Tang, M., Taghibiglou, C. (2017). The Mechanisms of
Action of Curcumin in Alzheimer's Disease. J
Alzheimers Dis, 58(4), 1003-1016.
Van Boven, R.W., Harrington, G.S., Hackney, D.B., Ebel,
A., Gauger, G., Bremner, J.D., D’Esposito, M., Detre,
J.A., Haacke, E.M., Jack Jr, C.R., Jagust, W.J., Le
Bihan, D., Mathis, C.A., Mueller, S., Mukherjee, P.,
Schuff, N., Chen, A., Weiner, M.W. (2009). Advances
in neuroimaging of traumatic brain injury and
posttraumatic stress disorder. J Rehabil Res Dev, 46,
717–57.
Wei, J., Pan, X., Pei, Z., Wang, W., Qiu, W., Shi, Z., et al.
(2012). The beta-lactam antibiotic, ceftriaxone,
Supplementation of Turmeric Extract Does Not Improve Neurological Function Following Repetitive Mild Traumatic Brain Injury in the Rat
489

provides neuroprotective potential via anti-
excitotoxicity and anti-inflammation response in a rat
model of traumatic brain injury. J Trauma Acute Care
Surg, 73, 654–60.
Xu, L., Nguyen, J.V., Lehar, M., Menon, A., Rha, E.,
Arena, J., et al. (2016). Repetitive mild traumatic brain
injury with impact acceleration in the mouse:
Multifocal axonopathy, neuroinflammation, and
neurodegeneration in the visual system. Experimental
Neurology, 275(Part 3), 436–449.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
490
