
The
R
ole of Rhaphidophora pinnata (L.f) Schott Water Extract on
Dissolution of Calcium Containing Renal Stones
Masfria
1,2
, Marianne
1,2
, and Y. M. Permata
1
1
Faculty of Pharmacy, Universitas Sumatera Utara, Medan 20155, Indonesia
2
Nanomedicine Centre of Inovation, Universitas Sumatera Utara,
Medan 20155, Indonesia
Keywords: Kidney srones, invitro, antinephrolithiasis, Rhaphidophora pinnata,
Abstract: Kidney stone disease is a common chronic disorder in humans and the most common type of renal stone is
made of calcium oxalate. Calcium oxalate stones are made of calcium oxalate monohydrate and dihydrate,
often combined with calcium phosphate which is the original cause of stone formation. Growth and
aggregation of calcium stone inhibits by coating the surface of growing calcium crystals or by complexing
with calcium and oxalate, such as potassium ion and magnesium ion. Rhaphidophora pinnata (L.f) Schott
leaves contain high potassium mineral that is 847.9 mg / 100g and calcium 474.7 mg / 100g. This study was
to investigate the effect of water extracts of Rhaphidophora pinnata (L.f) Schott leaves toward solubility of
kidney stone. The water extract of Rhaphidophora pinnata (L.f) Schott leaves, simplicia, and nano-simplicia
was made in three different concentration, 5%, 10%, and 20%. Dissolution of calcium-containing renal
stone was performed by incubating each water extract with 0.1% calcium containing renal stone for 4 hours.
Nano-simplicia water extract was shown the highest dissolution ability, 80.55% calcium of kidney stones
decomposed within 4 hours of incubation.
1 INTRODUCTION
The search for the source of medicinal plants
continues to produce chemical compounds used in
various diseases, including from plants that have the
potential in the treatment of kidney stone disease
(Vina, 2010). Kidney stone disease is one of
common chronic disorder in humans. Renal stone
(calculi) is made of calcium oxalate, and also
contain other crystals and organic component that
formed in the renal pelvis or calyces. This process
called urolitiasis (litiasis renalis, nefrolitiasis)
(Pratomo, 2008), (Basavaraj, 2007), (Worcester and
Coe, 2008), (Cunningham, 2016).
Treatment of kidney stones is carried out
depending on the size of the stone. If kidney stones
are still relatively small or medium, and still can
pass through the urinary tract without surgery,
doctors usually recommend drinking lots of water
(Nouvenne, 2013). Handling of kidney stones with
special procedures (eg laser energy, ultrasound, or
surgery) is usually applied only if the stone is larger
enough to clog the patient's urinary tract.
Calcium oxalate stones are made of calcium
oxalate monohydrate and dihydrate, often combined
with calcium phosphate which is the original cause
of stone formation. Calcium oxalate monohydrate is
thermodynamically most stable form and observed
more frequently in clinical stones than calcium
oxalate dihydrate, at a ratio of >2:1. The stone will
be more quickly formed when the urine is very
concentrated and cause the supersaturating of
calcium oxalate. These condition will gradually form
a solid mass and hard resemble a stone (Pramono,
1988), (Brown, 1989), (Basavaraj, 2007),
(Worcester and Coe, 2008).
Growth and aggregation of calcium stone inhibits
by coating the surface of growing calcium crystals
or by complexing with calcium and oxalate, such as
potassium ion, magnesium ion. citrate,
pyrophosphates, inter-alpha-trypsin inhibitor family
of proteins, Tamm-Horsfall protein (THP),
glycosaminnoglycans, and renal lithostathine.
Potassium and magnesium ions can inhibit the
formation of stones by binding with oxalate, it will
form a water-soluble oxalate salt. Similarly, citrate
binding with calcium ions to form calcium citrate
472
Masfria, ., Marianne, . and Permata, Y.
The Role of Rhaphidophora pinnata (L.f) Schott Water Extract on Dissolution of Calcium Containing Renal Stones.
DOI: 10.5220/0010074004720475
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
472-475
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
which is water-soluble, and these condition lead to
decreasing of calcium oxalate supersaturation
(Basavaraj, 2007), (Putra and Fauzi, 2016),
(Maharani, 2012). Besides phytochemicals as
antioxidant, dietary phyto-phenols were found to be
effective for the prevention the growth of renal stone
by inhibits the stone formation process in the urinary
tract (Nirumand, 2018).
Supersaturation of urinary salts and crystal
retention in the urinary tract initiated the formation
of renal stones. Deficiency or abundance of
inhibitors are almost certain to predispose to stone
disease. Some herbal plants have been detected as a
deterrent of kidney stones allegedly contain high
potassium mineral such as tempuyung leaves, nasty
shard, kapok, corn hair and lettuce (Alvin, 2015),
(Girsang, 2016), (Muhgni, 2013), (Nessa, 2013),
(Permata, 2017). R. pinnata leaves contain high
potassium mineral that is 847.9 mg / 100g and
calcium 474.7 mg / 100g. According to these results,
allegedly the potassium ion can act as an agent to
bind oxalate (water-soluble), so as not to form stones
in the kidney. The kidneys will pass trough
potassium oxalate and remove it through the urine.
Based on the description above, since the human
nephron is a dynamic system, in vitro crystal
behaviour is too simplistic a concept to entirely
explain renal calculus formation. This study was to
see how the water extracts of simplicia leaves of R.
pinnata predispose to kidney stone
2 MATERIALS AND METHODS
2.1 Materials
The materials used in this research were R. pinnata,
calcium-containing renal stone, nitric acid, distillate
water, calcium standard solution. Those chemicals
and solvents used were analytical grade and are
commercially available from Merck. R. pinnata leaf
plants were cleansed and dried, then crushed to
obtain simplicia powder and nano simplisia powder.
The nano simplicia powder was analyzed by LIPI
(Lembaga Ilmu Pengetahuan Indonesia) using
particle size analyzer nano.
2.2 Methods
2.2.1 Water Extract Preparation
The water extract of R. pinnata leaves, simplicia R.
pinnata, and simplicia nano R. pinnata was made in
three different concentration-5%, 10%, and 20%- in
water, using infused technique according to
Farmakope Indonesia 4
th
Edition.
2.2.2 Dissolution of Calcium-containing
Renal Stone
Dissolution of calcium-containing renal stone was
performed by incubating each water extract with
0.1% calcium-containing renal stone for 4 hours.
The levels of calcium free ions present in the water
extract before and after incubation were measured
using atomic absorption spectrophotometry methods.
The dissolution percentage of calcium was
calculated by the formula below:
DCa (%) =
(1)
Where DCa is the dissolution percentage of calcium;
CAD is the calcium content after dissolution; CBD
is the calcium content in water extract; and CaRS is
the calcium contain in renal stone (1 gram of renal
stone contain 10.29 mg calcium).
3 RESULT AND DISCUSSION
The following Table 1 was the result of calcium
analysis in water extract of R. pinnata leaves,
simplicia of R. pinnata and nano-simplicia of R.
pinnata..
Table 1: The effect of treatments on calcium content in
water extract before and after dissolution.
No Treatments
Water
extract
(%)
Calcium level
(μg/ml)
in water
extract
Calcium
(μg/ml)
after
dissolution
1 R. pinnata
water
extract
5 2.22 2.77
10 3.79 4.18
20 5.11 5.70
2 Simpicia R.
pinnata
water
extract
5 6.21 7.84
10 17.57 19.78
20 26.56 40.09
3 Nano-
simplicia
R. pinnata
water
extract
5 61.91 105.62
10 112.20 169.28
20 206.53 436.05
The Role of Rhaphidophora pinnata (L.f) Schott Water Extract on Dissolution of Calcium Containing Renal Stones
473
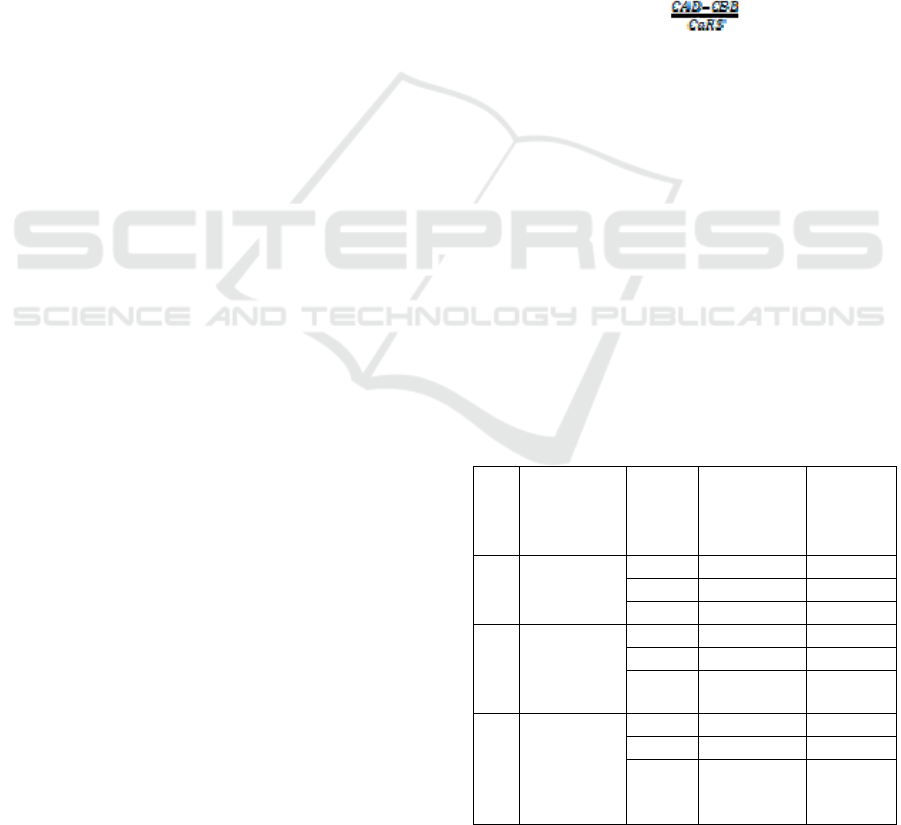
The effect of water extract on dissolution
percentage of calcium-containing renal stone result
is presented in the Figure 1.
Figure 1: The effect of water extract on dissolution
percentage of calcium-containing renal stone in the R.
pinnata. RPWE = R. pinnata water extract; SRPWE =
Simplicia of R. pinnata water extract; NSRPWE = Nano-
simplicia R. pinnata water extract.
From the data Table 1 and Figure 1 can, be seen
there are differences in calcium levels in R. pinnata
water extract incubated with kidney stones. After
incubation the levels of calcium in the water extract
as a whole increased. The highest dissolution was
shown by a 20% nano-simplicia water extract,
80.55% calcium of kidney stones decomposed
within 4 hours of incubation. The lowest dissolution
was shown by fresh water extract of R. pinnata
where for the three concentrations of water extract
did not show a significant difference that is below
1%.
Based on research conducted by (Alvin, 2015)
and (Girsang, 2016), reports that plants with high
potassium content, such as tempuyung and keji
beling has good solubility to calcium oxalate. In
terms of reactivity of alkaline ions, the position of
potassium in the voltaic series is more left than
calcium so that potassium is more reactive (the more
easily the electrons are released) then the potassium
gets rid of calcium to join the carbonate compound,
oxalate or urate from the calcium compound to
dissolve. In a supersaturated calcium oxalate
solution 2 mmol/L magnesium reduced particle
number by 50%. It was found that magnesium exert
a fine kinetic control on the precipitation and growth
of calcium oxalate monohydrate (Girsang, 2016),
(Permata, 2017), (Desmars and Tawashi, 1973),
(Basavaraj, 2007). Magnesium can form complexes
with oxalate and decreases supersaturation. Oral
intake of magnesium will decline the oxalate
absorption and urinary excretion, in a manner
similar to calcium by binding to oxalate in the
intestines (Liebman and Costa, 2000).
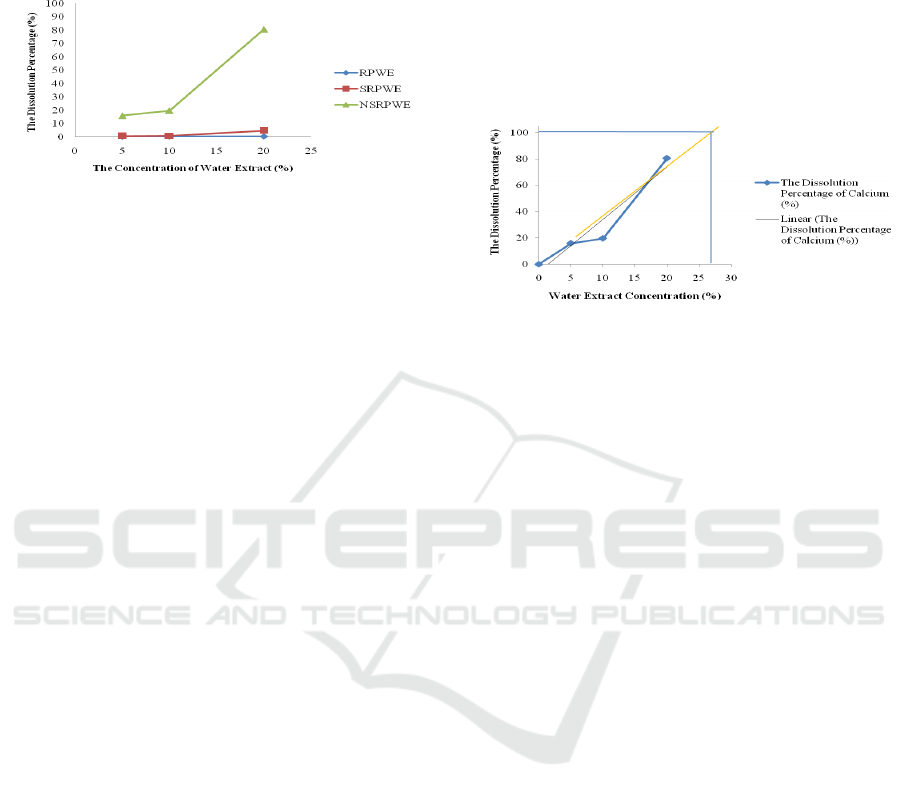
The figure 2 illustrated the extrapolation of the
dissolution effect of nano-simplicia of R. pinnata
water extract, since this extract shown the highest
ability to dissolve calcium contain renal stone. A
hundred percent of calcium contain renal stone (1
gram of renal stone contain 10.29 mg calcium)
should be dissolve in 27% nano-simplicia of water
extract approximately. By this concentration, the
future research of animal study of anti-
nephrolithiasis of R. pinnata should be done.
Figure 2: The Dissolution Percentage of Calcium Nano-
simplicia R. pinnata water extract extrapolation
Calcium oxalate is the constituent component of
the process of formation of kidney stones in the
human body. Calcium oxalate stones are difficult to
dissolve in water and clog up the urinary system. By
the content of potassium ions and also high
magnesium, calcium oxalate can be broken down
and form new compounds that easily dissolve in
water (Girsang, 2016), (Permata, 2017). After
incubation with water extract of each treatments, the
levels of calcium in the water extract as a whole
increased. The highest dissolution was shown by a
20% nano-simplicia water extract, 80.55% calcium
of kidney stones decomposed within 4 hours of
incubation. But the fresh water extract of R. pinnata
leaves and water extract of R. Pinnata simplicia did
not show a significant difference that is below 5%.
However, there is little evidence to recommend
magnesium therapy in patients with renal stones.
4 CONCLUSIONS
R. pinnata water extract has the ability to dissolve
calcium containing renal stone allegedly due to their
potassium and magnesium contained in the water
extract. Nano-simplicia shown the highest
dissolution percentage, 80,55%. However, the future
research of animal study of anti-nephrolithiasis of R.
pinnata should be done.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
474

ACKNOWLEDGEMENTS
We are gratefully to Ministry of Higher Education
for financial support in order to carried out this
research.
REFERENCES
Alvin, Y. (2015). Analisis Kelarutan Kalsium Oksalat dan
Kalsium Karbonat Pada Infusa Daun Tempuyung
Segar (Sobchus arvesis L.) dan Sediaan Kapsul
Ekstrak Daun Tempuyung Secara Spektrofotometri
Serapan Atom. Skripsi. Medan: Fakultas Farmasi
Universitas Sumatera Utara. hal. 2.
Basavaraj, D.R., Biyani, C.S., Brawning, A.J., and
Cartledge, J.J. (2007). The Role of Urinary Kidney
Stone Inhibitors and Promoters in the Pathogenesis of
Calcium Containing Renal Stones. Eau-Ebu. 5: 126-
136.
Brown, S. B (1989). Manual Penyakit Ginjal.
Diterjemahkan oleh Moch. Sadikin dan Winarsi
Rudihorso. Jakarta: Gramedia Pustaka Utama. Hal.
228-231, 233-235, 324-325. Binarupa Aksara
Cunningham, P., Noble, H., Al-Modhefer, A-K., and
Walsh, I. (2016). Kidney stones: pathophysiology,
diagnosis and management. British Journal of
Nursing, 25(20), 1112-1116.
Depkes RI. (1995). Farmakope Indonesia. Edisi IV.
Jakarta: Depertemen Kesehatan RI. hal. 1126, 1213.
Desmars JF, and Tawashi R. (1973). Dissolution and
growth of calcium oxalate monohydrate. I. Effect of
magnesium and pH. Biochim Biophys Acta;313:256–
67.
Girsang, C.A. (2016). Pengaruh Infusa Keji Beling
(Sericocalyx cripus (l.) Bremeck) terhadap Kelarutan
Kalsium pada Batu Ginjal Secara In Vitro. Skripsi.
Medan: Fakultas Farmasi Universitas Sumatera Utara.
hal. 1-11.
Liebman M, and Costa G. (2000). Effects of calcium and
magnesium on urinary oxalate excretion after oxalate
loads. J Urol;163:1565–9.
Maharani, E.T., Mukaromah, A.H., and Susilo, J. (2012).
Analisis Kalium dan Prosentase Daya Larut Calsium
Oksalat oleh Kalium dalam Ait The Daun Sukun
(Artocarpus altilis). LPPM Unimus. ISBN: 978-6020-
18809-0-6: 196-202.
Muhgni, A.I. (2013). Uji Aktivitas Ekstrak Etanol 70%
Kulit Batang Kapuk Randu (Ceiba petandra (L.)
Gaertn) Sebagai Penghambat Batu Ginjal pada Tikus
Putih Jantan. Skripsi. Jakarta: UIN Syarif
Hidayatullah. hal.1-26.
Nessa, Helmi, A., and Husni, M. (2013). Efek Diuretik
dan Daya Larut Batu Ginjal Dari Ekstrak Etanol
Rambut Jagung (Zea mays L.). Prosiding Seminar
Nasional Perkembangan Terkini Sains Farmasi dan
Klinik III. ISSN: 2339-2592. hal.1-14.
Nirumand, M.C., Hajialyani, M., Rahimi, R., Farzael,
M.H., Zingue, S., Nabavi, S.M., and Bishayee, A.
(2018). Dietary Plants for the Prevention and
Management of Kidney Stones: Preclinical and
Clinical Evidence and Molecular Mechanisms.
International Journalof Molecular Sciences. 19: 765.
Nouvenne, A., Ticinesi, A., and Meschi, T. (2013).
Nephrolithiasis and Gastrointestinal Tract Diseases:
Can Diet Intervention Help? Practical
Gastroenterology. 116: 27-35.
Permata, Y.M., Angkat, L and Wahyuni, H.S. (2017).
Analisis Kadar Kalium dan Daya Larut Kalsium
Oksalat oleh Infusa Selada (Lactuca sativa L.) secara
Spektrofotometri Serapan Atom. Jurnal Farmasi
Galenika, 4(2):38-44.
Pramono, S. (1988). Buku Temu Risalah Temu
Ilmiah.
Yogyakarta: Pustaka Baru. Hal. 10-13, 21-29,
68-72.
Putra, M.M.A. and Fauzi, A. (2016). Nefrolitiasis.
Majority. 5(2): 69-73.
Vina, J.A., Siti, A., and Iqbal, M. (2010). Isolasi dan
karakterisasi senyawa turunan terpenoid dari fraksi n-
heksana Momordica charantia L. Jurnal Sains dan
Teknologi Kimia:l1(1): 88-93.
Worcester, E.M. and Coe, L.F. (2008). Nephrolithiasis.
PrimCare. 35(2): 369.
The Role of Rhaphidophora pinnata (L.f) Schott Water Extract on Dissolution of Calcium Containing Renal Stones
475
