
Using Frontal Brain Asymmetry to Control Sensory Treatment of
Anxiety and Depression
Tim J. C. Jacob
1
, Jeremy Warden-Smith
1
, Neil Kernot
2
and Malyka Galay Burgos
1
1
School of Biosciences, Cardiff University, Museum Avenue, Cardiff, U.K.
2
Chelker Technology, 16 Swadford Street, Skipton, North Yorkshire, U.K.
Keywords: Depression, Anxiety, EEG, Alphawaves, Frontal Asymmetry, Bright Light Therapy, Smell Stimulation.
Abstract: Anxiety and depression are increasingly common disorders. Globally, more than 350 million people of all
ages suffer from these illnesses. Depression and anxiety are treated with medication, psychotherapy, or
electroconvulsive therapy (ECT), either individually or in combination. Drugs and ECT are not cures and
often involve unpalatable adverse side-effects necessitating safer more sustainable alternatives. The
antidepressant properties of bright light are well established and aroma stimulation has been shown to improve
mood and reduce markers for anxiety and depression. A combinatory therapy of light and smell stimulation
has been shown to have a positive impact on mood, physiological markers for stress, anxiety and depression.
In particular, negative alphawave brain asymmetry, an objective marker for depression, is reduced by a 15min
stimulus treatment. The proposal outlined in this paper is that real-time frontal alpha asymmetry, recorded by
EEG, be used to control the frequency, duration and amplitude of the light and aroma signals to optimise the
effectiveness of the treatment. The object of this treatment is to rebalance the frontal asymmetry restoring a
frontal activity representative of a non-depressed, non-anxious state.
1 INTRODUCTION
It has been estimated that Common Mental Disorders
affect 1 in 6 British adults every week with over half
of these having a mixed anxiety and depressive
disorder (Deverill and King, 2009). In the USA, in
any given one-year period, 13 million to 14 million
people (which equates to approximately 6.6% if the
US population) experience depression (Kessler et al,
2003); globally, more than 350 million people of all
ages suffer from the illness (WHO, 2012) and the
annual incidence in UK is 36 per 1000 (NICE, 2004).
Depression has a heavy human cost including feelings
of sadness, worthlessness, isolation and an inability to
enjoy life. Depressed people are more likely to take
drugs, be off work or unemployed and kill
themselves. Depression damages individuals,
relationships and families. And it is on the rise
globally.
Electroconvulsive Therapy (ECT) is thought by
some to be one of the fastest ways to relieve the
symptoms of depression and the use of ECT is on the
rise in the UK (Guardian, 2017). It is generally given
when other treatments, e.g. drug therapy, have failed.
Neither ECT nor drug therapy are cures for
depression and both can have significant adverse
side-effects. In view of this it is important that new
safer ways are developed to combat this growing
problem.
Light and smell stimuli have both been used
independently in human studies to achieve positive
psychophysiological benefit. For example, light and
smell have been demonstrated to affect mood and
alleviate depression (for reviews see Oldham and
Ciraulo, 2014; Herz, 2009). Bright Light Therapy
(BLT) is an established treatment for seasonal
affective disorder (SAD) and other mood disorders
(Golden et al, 2005; Pail et al, 2011), having been
successfully used for over 20 years. It has also been
shown to be effective in other kinds of non-seasonal
depression (Naus et al., 2013; Niederhofer and von
Klitzing, 2012) and, in Major Depressive Disorder
(MDD) a randomised, placebo controlled trial
demonstrated that BLT was comparable to
antidepressant medication in effectiveness (Lieverse
et al, 2011). Smell has also been shown to have
effects on mood, stress, anxiety and depression
(Johnson, 2011; Herz, 2009; Ehrlichman & Bastone,
1992; Vernet-Maury et al, 1999; Alaoui-Ishmaili et
al, 1997).
84
Jacob, T., Warden-Smith, J., Kernot, N. and Burgos, M.
Using Frontal Brain Asymmetry to Control Sensory Treatment of Anxiety and Depression.
DOI: 10.5220/0006471000840088
In Proceedings of the 4th International Conference on Physiological Computing Systems (PhyCS 2017), pages 84-88
ISBN: 978-989-758-268-4
Copyright © 2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
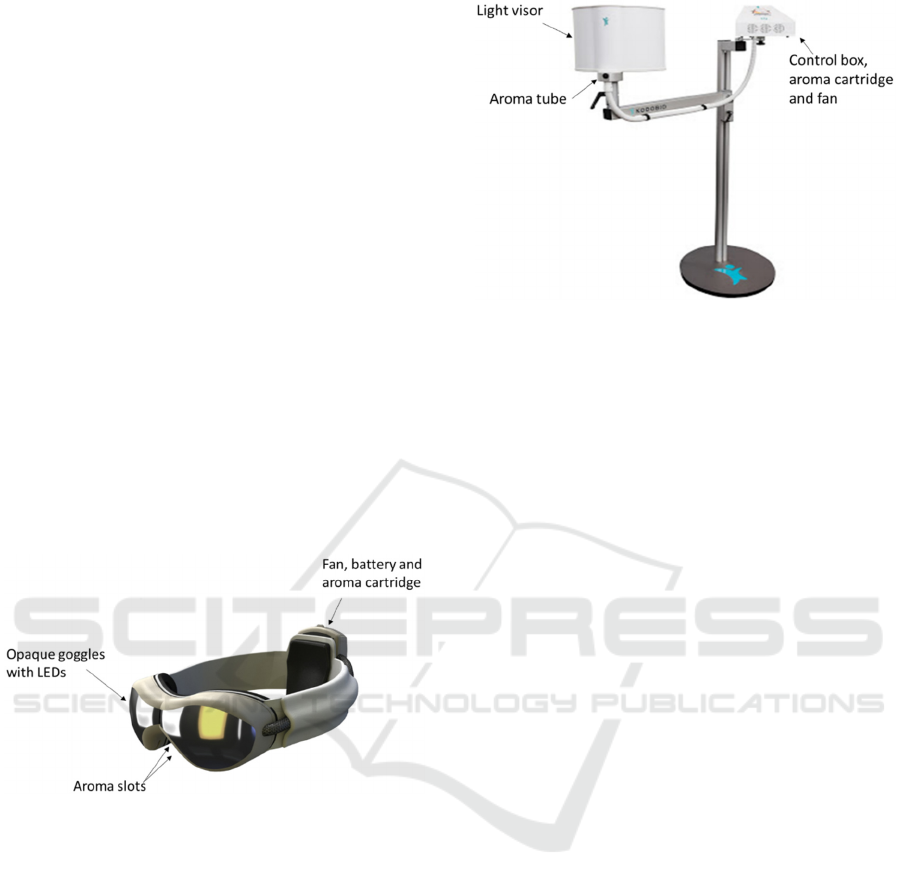
2 BACKGROUND
The concept for this Position paper originates from
two recent papers in which further details about the
subjects, methods and protocols can be found; Dong
and Jacob (2016), Warden-Smith et al. (2017).
2.1 Light and Smell Stimulation
We have designed devices (Figures 1 and 2) to deliver
an integrated stimulation protocol of fluctuating light
and smell with a 60s cycle (Figure 3). A 15min
stimulus session using lemon essential oil and
fluctuating light (0-2500 lux) has positive effects on
mood, lowers blood pressure and heart rate, increases
galvanic skin resistance and rebalances frontal
brainwave asymmetry and we have demonstrated that
this treatment is both anxiolytic and anti-depressive.
The results are presented in detail in Dong and Jacob
(2016) and Warden-Smith et al. (2017). For the
purposes of this present paper it is the possibility of
recording the EEG signal during the light and smell
stimulus protocol that is the prime consideration.
Figure 1: The light and smell stimulus delivery goggles.
The light source in the eyepieces of the goggles is
UV-free light stimulus emitting up to 2500 lux when
in close proximity (2-4 cm) to the eyes. Aroma
vapour of essential oil (lemon) evaporating from a
circular (5mm radius) absorbent cotton pad in a
cartridge in the rear fan pack is delivered to the
nostrils by air blown over the pad driven by an axial
fan (5v,100mA, 0.7 cu.ft/min (0.33l/s), Farnell,
Leeds, UK). Further details in Dong and Jacob
(2016).
Figure 2: Light and smell delivery device.
A free-standing device delivering the same light
and aroma stimuli as described in (a). The subject sits
or lies and the visor is moved to within 5cm of the
face. Odorised air is delivered from a control box
containing fans and an aroma cartridge via a tube to
exit at the base of the visor.
2.2 Frontal Alphawave Asymmetry
EEG alphawave power in the brain has been used as
an index of brain activity. Alphawaves have been
shown to be inversely correlated with brain activity
(Jones, 2007). The brain in its resting state tends to
produce alphawaves. Many studies have demonstra-
ted that a decrease in alphawave activity in the left
frontal hemisphere is associated with an appetitive
response, approach behaviour and positive experien-
ce. Low right frontal alphawave activity is associated
with negative mood and withdrawl behaviour. In
normal subjects, the balance between right and left
frontal alphawave activity (right-left) is positive. In
depression and anxiety the frontal alphawave
asymmetry (FA) becomes reversed (negative) and has
been used as an objective measure of these disorders
(Henriques and Davidson, 1991; Heller et al, 1997;
Davidson, 1998a and 1998b; Thibodeau et al, 2006;
reviewed in Harmon-Jones et al, 2010). FA can
predict future development of anxiety and depression
(Blackhart, Minnix and Kline, 2006) and this
asymmetry has been shown to be a moderately stable
individual difference in adults, irrespective of sex and
history of depression (Allen et al., 2004; Vuga et al.,
2005).
Using Frontal Brain Asymmetry to Control Sensory Treatment of Anxiety and Depression
85
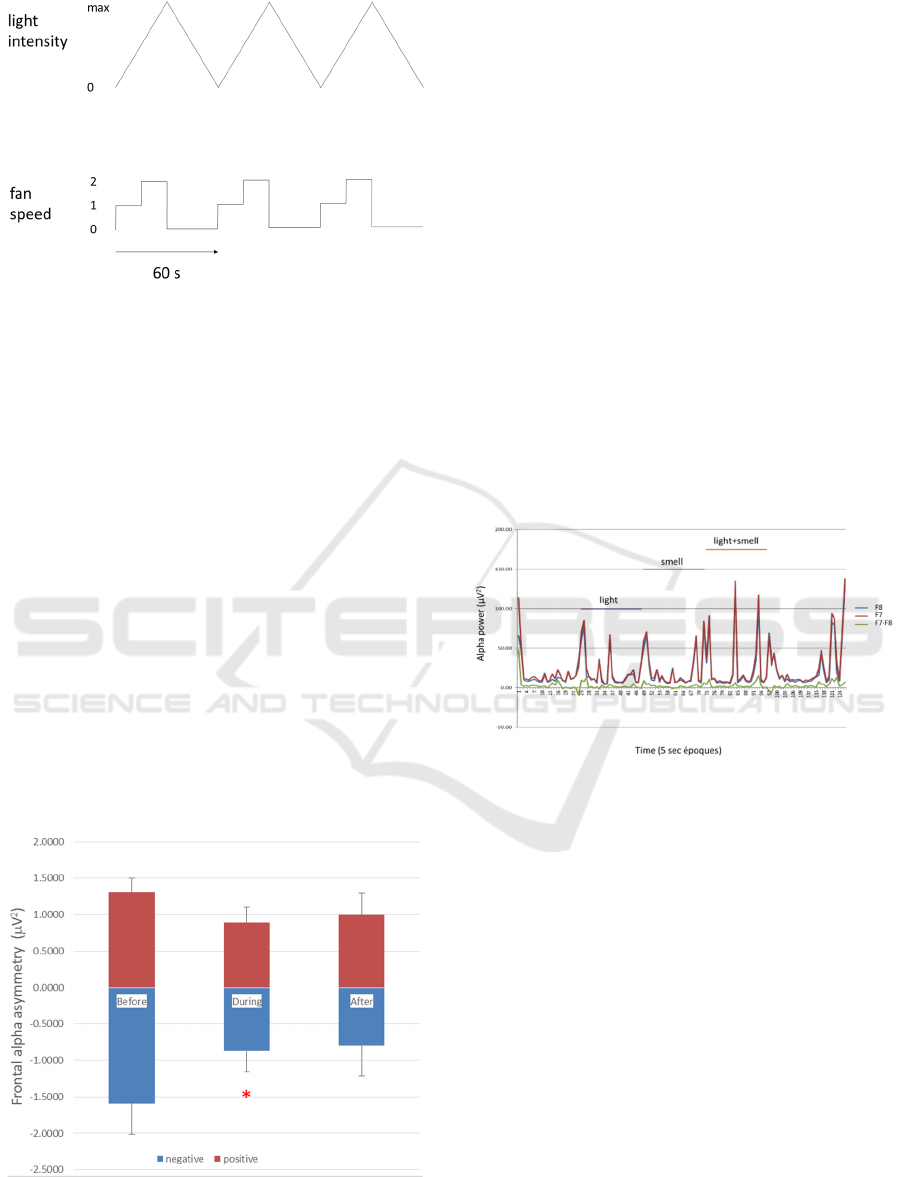
Figure 3: The light and smell stimulus protocol.
The light and smell stimulus protocol. Diffuse
full-spectrum white light (maximum 2500 lux) is
presented as a triangular wave starting from zero
light, rising to a maximum (2500 lux) linearly over
30s and then declining linearly to zero over 30s.
Simultaneously an airstream containing essential oil
vapour is delivered to the nostrils at two flow rates
(0.17 and 0.33 l/s) to coincide with the up ramp of the
light stimulus. Three cycles are illustrated. The reason
for delivering the stimuli in this manner is to
overcome olfactory adaptation/habituation.
We have demonstrated that 15min stimulation
with fluctuating light and smell stimuli (Fig.3) can
reduce negative FA in those subjects who experience
this asymmetry (see Fig.4; taken from Warden-Smith
et al., 2017). The blue bars in figure 4 indicate the
negative FA experienced by half (32 out of 64) of our
experimental population. During the light and smell
stimulation the negativity of the FA was reduced and
this was maintained after the 15min stimulus period
(at least for 5 mins).
Figure 4: EEG alphawave frontal asymmetry (FA).
The effect of 15min exposure to light and lemon
odour on alpha wave asymmetry. The subjects were
divided into positive (red bars, n=32) and negative FA
(blue bars, n=32) on the basis of their alpha wave
asymmetry (F8-F7). Alpha wave power is expressed
as the average ± standard error (bars) per 10s époque
for 2min before, during and 5min after stimulation.
*p<0.05. Taken from Warden-Smith et al. (2017).
2.3 Real Time Frontal Asymmetry
(FA)
Frontal alphawaves can be measured in the brain
using EEG electrodes and the FA can be determined
in real-time with software (e.g. SPIKE2, Cambridge
Electronic Design, UK) that, by power spectrum
analysis, calculates the power in the alpha frequency
band (8-12Hz) for left and right hemispheres (Fig.5).
These two signal outputs can then be subtracted to
give the FA (green line, Fig.5) which represents the
real-time frontal brain activity with a 5s delay for the
integration period.
Figure 5: Alphawave power in real time.
EEG recording of alphawaves (8-12Hz) from F7
(left frontal; red) and F8 (right frontal; blue) electrode
positions and the subtraction of the two (green). The
alpha power was determined by spectrum analysis
(SPIKE2 software, CED, Cambridge, UK) per 5s
époque. The y-axis is the alpha power (µV
2
per
epoque) and the x-axis represents time in 5s époques.
Light (2500 lux), smell (vanillin) and light+smell
were applied for 3min.
In figure 5 the difference signal between the alpha
activity in the two frontal hemispheres (FA) is given
by the green line. The FA varies with time and in
normal, healthy subjects is positive on balance
although it can include some negative episodes. The
reverse is true for subjects prone to anxiety and
depression, the balance is negative. How often this
FA signal shifts from positive to negative and vice
versa and what causes it to do so are unknown.
PhyCS 2017 - 4th International Conference on Physiological Computing Systems
86

3 PROPOSITION
The frontal asymmetry (FA) signal reflects brain
activity in the frontal lobes and is believed to convey
information about psychological state. FA can be
displayed in real-time and could itself be converted
into a feedback signal by transducing it into a tone or
colour thereby relaying the information about the sign
and magnitude of the FA directly back to the subject.
Positive FA is the normal, healthy state and the
desired goal of the light and smell stimulus is to shift
a negative FA pattern back to a positive pattern. We
have demonstrated that a negative FA can be reduced
by 15min light and smell treatment (Fig.4). What
might be achieved by providing a direct and
immediate feedback of the effectiveness of the
stimulation by using the FA signal to control the
stimulus protocol? This might be implemented using
a negative FA reading to increasing the intensity or
the frequency of the stimuli, for example by
delivering a pulse of bright light accompanied by a
pulse of odour when a negative FA period is detected.
3.1 Challenges
1. What are the short-term effects of different
stimulus protocols on the FA sign?
2. Do longer term effects result from short term
changes?
3. How can FA signal be used to control stimulus
protocol to optimise the outcome?
4 CONCLUSIONS
A fluctuating light and smell stimulus protocol has
been shown to have positive effects on mood and
stress-related physiological markers and, in addition,
rebalances frontal brainwave asymmetry towards a
healthy, normal pattern. Using a frontal brainwave
asymmetry feedback paradigm could radically
enhance the effectiveness of such therapy and offer a
real, effective, safe alternative to drugs and
electroconvulsive therapy as a treatment for
depression and anxiety.
REFERENCES
Alaoui-Ismaïli, O., Robin, O., Rada, H., Dittmar, A.,
Vernet-Maury, E. (1997) Basic emotions evoked by
odorants: comparison between autonomic responses
and self-evaluation. Physiol. Behav. 62, 713-720.
Allen, J. J., Urry, H. L., Hitt, S. K., & Coan, J. A. (2004)
The stability of resting frontal electroencephalographic
asymmetry in depression. Psychophysiology 41,
269-80.
Blackhart, G. C., Minnix, J. A., & Kline, J. P. (2006) Can
EEG asymmetry patterns predict future development of
anxiety and depression? A preliminary study. Biol.
Psychol. 72, 46–50.
Davidson, R. J. (1998a) Anterior electrophysiological
asymmetries, emotion, and depression: conceptual and
methodological conundrums. Psychophysiol. 35, 607–
614.
Davidson, R. J. (1998b) Cerebral Brain asymmetry. Chap
13 in Brain Asymmetry, eds. R J Davidson and K
Hugdahl. The MIT Press, Cambridge, MA, USA 1998.
Deverill C., King, M., Common mental disorders. In: Adult
Psychiatric Morbidity in England: Results of a
household survey, eds. S. McManus, T. Brugha, P.
Bebbington, and R. Jenkins, The NHS Information
Centre, pp.38-58, 2009
Dong, S. and Jacob, T.J.C. (2016) Combined non-adaptive
light and smell stimuli lowered blood pressure, reduced
heart rate and reduced negative affect. Physiol. Behav.
156, 94–105, doi:10.1016/j.physbeh.2016.01.013
Ehrlichman, H., & Bastone, L. (1992) Olfaction and
emotion. In: M. J. Serby, K. L. Chobor (eds.). Science
of olfaction. New York: Springer-Verlag. p. 410–438.
Guardian, (2017) https://www.theguardian.com/society/
2017/apr/17/electroconvulsive-therapy-on-rise-
england-ect-nhs
Golden, R. N., Gaynes, B. N., Ekstrom, R. D., Hamer, R.
M., Jacobsen, F. M., Suppes, T., Wisner, K. L., &
Nemeroff, C. B. (2005) The efficacy of light therapy in
the treatment of mood disorders: a review and meta-
analysis of the evidence. Am. J. Psychiatry 162, 656-
662.
Harmon-Jones, E. (2007) Asymmetrical Frontal Cortical
Activity, Affective Valence, and Motivational
Direction. Chap. 7 in Social Neuroscience: Integrating
Biological and Psychological Explanations of Social
Behavior. Eds. E Harmon-Jones, P. Winkelman. The
Guilford Press, New York, 2007, pp.138.
Harmon-Jones, E., Gable, P. A., Peterson, C. K. (2010)
The role of asymmetric frontal cortical activity in
emotion-related phenomena: A review and update.
Biological Psychology, 84, 451-462.
Heller, W., Nitschke, J. B., Etienne, M. A., & Miller, G. A.
(1997) Patterns of regional brain activity differentiate
types of anxiety. J. Abnorm. Psychol. 106, 376-385.
Henriques, J. B., & Davidson, R. J. (1991) Left frontal
hypoactivation in depression. J. Abnorm. Psychol. 100,
535-545.
Herz, R.S. (2009) Aromatherapy Facts and Fictions: A
Scientific Analysis of Olfactory Effects on Mood,
Physiology and Behavior. Int. J. Neuroscience 119,
263-290, doi: 10.1080/00207450802333953
Johnson, A. J. (2011) Cognitive facilitation following
intentional odor exposure.Sensors (Basel) 11, 5469-88,
doi: 10.3390/s110505469
Using Frontal Brain Asymmetry to Control Sensory Treatment of Anxiety and Depression
87

Kessler, R. C., Berglund, P., Demler, O., Jin, R., Koretz, D.,
Merikangas, K. R., Rush, A. J., Walters, E. E., & Wang,
P. S. 2003. National Comorbidity Survey Replication.
The epidemiology of major depressive disorder: results
from the National Comorbidity Survey Replication
(NCS-R). JAMA 289(23), 3095-3105.
Lieverse, R., Van Someren, E. J., Nielen, M. M., Uitdehaag,
B. M., Smit,J.H., & Hoogendijk, W. J. (2011) Bright
light treatment in elderly patients with nonseasonal
major depressive disorder: a randomized placebo-
controlled trial. Arch. Gen. Psychiatry 68, 61-70, doi:
10.1001/archgenpsychiatry.2010.183
Naus, T., Burger, A., Malkoc, A., Molendijk, M., &
Haffmans, J. (2013) Is there a difference in clinical
efficacy of bright light therapy for different types of
depression? A pilot study. J. Affect. Disord. 151, 1135-
7, doi: 10.1016/j.jad.2013.07.017
Niederhofer, H., & von Klitzing, K. (2012) Bright light
treatment as mono-therapy of non-seasonal depression
for 28 adolescents. Int. J. Psychiatry Clin. Pract. 16,
233-7, doi: 10.3109/13651501.2011.625123
NICE, 2004. Costing Clinical Guidelines:Depression
(England and Wales), http://www.nccmh.org.uk/
downloads/DCHP/CG23CostReport.pdf
Oldham, M.A., & Ciraulo, D.A. (2014) Bright light therapy
for depression: a review of its effects on chronobiology
and the autonomic nervous system. Chronobiol. Int. 31,
305-19, doi: 10.3109/07420528.2013.833935
Pail, G., Huf, W., Pjrek, E., Winkler, D., Willeit, M.,
Praschak-Rieder, N., & Kasper, S. (2011) Bright-light
therapy in the treatment of mood disorders.
Neuropsychobiol. 64,152-162.
Thibodeau, R., Jorgensen, R.S., & Kim, S. (2006)
Depression, anxiety, and resting frontal EEG
asymmetry: a meta-analytic review. J. Abnorm.
Psychol.115, 715-29.
Vernet-Maury. E., Alaoui-Ismaïli, O., Dittmar, A.,
Delhomme, G., & Chanel, J. (1999) Basic emotions
induced by odorants: a new approach based on
autonomic pattern results. J. Auton. Nerv. Syst. 75,
176-183.
Vuga, M., Fox, N.A., Cohn, J.F., George, C.J., Levenstein,
R.M., & Kovacs, M. (2005) Long-term stability of
frontal electroencephalographic asymmetry in adults
with a history of depression and controls. Int. J.
Psychophysiol. 59,107-115.
Warden-Smith, J., Paul, L., Olukogbon, K., Bointon, E.S.,
Cole, R.H., John, R.R., Dong, S. and Jacob, T.J.C.
(2017) Light and smell stimulus protocol reduced
negative frontal EEG asymmetry and improved mood.
Open Life Sci., 12: 51–61, doi: 10.1515/biol-2017-
0006
WHO. 2012. Fact sheet N°369, http://www.who.int/
mediacentre/factsheets/fs369/en/index.html
PhyCS 2017 - 4th International Conference on Physiological Computing Systems
88
