
Maximising Patient Throughput using Discrete-event Simulation
Fraser L. Greenroyd
1,2
, Rebecca Hayward
2
, Andrew Price
1
, Peter Demian
1
and Shrikant Sharma
2
1
Centre for Innovative and Collaborative Construction Engineering, School of Civil and Building Engineering,
Loughborough University, Loughborouogh, U.K.
2
BuroHappold Engineering, Bath, U.K.
Keywords: Hospital Operations, Patient Scheduling, Discrete-event Simulation, Healthcare Delivery, Outpatient
Operations.
Abstract: As the National Health Service (NHS) of England continues to face tighter cost saving and utilisation
government set targets, finding the optimum between costs, patient waiting times, utilisation of resources,
and user satisfaction is increasingly challenging. Patient scheduling is a subject which has been extensively
covered in the literature, with many previous studies offering solutions to optimise the patient schedule for a
given metric. However, few analyse a large range of metrics pertinent to the NHS. The tool presented in this
paper provides a discrete-event simulation tool for analysing a range of patient schedules across nine
metrics, including: patient waiting, clinic room utilisation, waiting room utilisation, staff hub utilisation,
clinician utilisation, patient facing time, clinic over-run, post-clinic waiting, and post-clinic patients still
being examined. This allows clinic managers to analyse a number of scheduling solutions to find the
optimum schedule for their department by comparing the metrics and selecting their preferred schedule.
Also provided is an analysis of the impact of variations in appointment durations and their impact on how a
simulation tool provides results. This analysis highlights the need for multiple simulation runs to reduce the
impact of non-representative results from the final schedule analysis.
1 INTRODUCTION
The National Health Service (NHS) of England,
despite being viewed as one of the best health
systems in the Western world (Davis et al., 2014), is
facing some of the toughest challenges since its
inception in 1948 (NHS England, 2013). Since 2009
these challenges have been focussed heavily on cost
efficiencies and reducing overall operating costs
(Nicholson, 2009; Carter, 2016). The Department of
Health in England has taken steps towards making
cost savings in NHS facilities by removing
unwarranted variations, with a view that this will
save the NHS £5bn per annum by 2020 (Carter,
2016). The report by Lord Carter of Coles (2016)
estimates that £3bn of efficiency savings can come
from a combined optimised use of clinical staff
along with better estates and facilities’ management.
A review of the healthcare estates of the NHS in
England revealed that as much as 16% of occupied
floor area (m
2
) is either unsuitable for use, under-
utilised or not used at all (Health and Social Care
Information Centre, 2015). Of the floor area
available, 4.4% is reported as being under-utilised or
unused completely. It was recommended to the NHS
that the amount of unoccupied or underused space
should not exceed 2.5% (Carter, 2016) by April
2017.
However, the optimisation of space usage is not
the only concern the NHS has to consider. The
utilisation of clinical staff is highlighted as the
biggest area (£2bn per annum) of potential cost
savings through an optimised use of the clinical
workforce (Carter, 2016). This is further combined
with continued work towards improving patient
satisfaction (Nicholson, 2009; NHS England, 2014)
through a reduction in waiting times and crowding
(Bernstein et al., 2009). Similarly, changing
demographics gives rise to a changing NHS as the
needs of the population put a varying amount of
pressure upon the health service (Department of
Health, 2013).
There is a fine balance between the metrics by
which health providers are measured. Finding the
optimum between waiting times, clinician
utilisation, space utilisation and patient satisfaction
is increasingly challenging. There has been much
204
Greenroyd, F., Hayward, R., Price, A., Demian, P. and Sharma, S.
Maximising Patient Throughput using Discrete-event Simulation.
DOI: 10.5220/0006400002040214
In Proceedings of the 7th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH 2017), pages 204-214
ISBN: 978-989-758-265-3
Copyright © 2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
work in both academia and industry to analyse
existing situations and provide solutions to improve
the efficiency and effectiveness of care in healthcare
facilities (Gunal and Pidd, 2006; Marcario, 2006;
Maviglia et al., 2007; Hendrich et al., 2008;
Bernstein et al., 2009; Dexter and Epstein, 2009;
Reynolds et al., 2011; Greenroyd et al., 2016).
It can be argued that at the core of these concerns
is the scheduling of patient appointments, with much
research available on systems to aid appointment
scheduling (Fetter and Thompson, 1965; Kuljis, Paul
and Chen, 2001; Harper and Gamlin, 2003; Gunal
and Pidd, 2010). It can be difficult to successfully
balance utilisation and satisfaction if the patient
scheduling is not optimised for the current clinic
setup. There are many factors which can impact the
effectiveness of the patient schedule including no-
shows, arrival patterns (i.e. a patient arriving either
early, on-time, or late for an appointment) and
appointment duration variations. A study into
operating theatre tardiness found that for every
minute a surgery started late, the department’s
staffing was increased by 1.1 minutes for an 8-hour
surgery day (Dexter and Epstein, 2009), thus
negatively affecting the department’s performance
and efficiency.
The primary challenge with scheduling is the
uncertainty in appointment durations, with high
variations in appointment durations viewed as a key
cause for clinical delays (Huang and Kammerdiner,
2013), increasing waiting times and clinic over-run.
There have been attempts in the literature to tackle
these concerns by accommodating variations into
scheduling, with the implementation of decision
trees (Huang and Kammerdiner, 2013), or by using
discrete-event simulation to compare scheduling
techniques (Lee et al., 2013).
2 RELATED WORK
The use of discrete-event simulation to model
hospital departments is well documented in the
literature (Jun, Jacobson and Swisher, 1999;
Anderson and Merode, 2007; Gunal and Pidd,
2010). Studies include making strategic decisions for
various departments (Ballard and Kuhl, 2006;
Denton et al., 2006; Vanberkel and Blake, 2007;
Leskovar et al., 2011); estimating capacity levels
and measuring waiting times (Werker et al., 2009);
analysing patient flows (Brenner et al., 2010; Zeng
et al., 2012); measuring policy impact (Fletcher et
al., 2007); and simulating patient scheduling and
utilisations (Harper and Gamlin, 2003; Werker et al.,
2009; Lee et al., 2013; Quevedo and Chapilliquén,
2014). It has been argued that the extensive use of
process modelling is limited in healthcare compared
with other industries (Harper and Pitt, 2004) due to
the complexity of the processes and the vast
amounts of data required to provide accurate models
(Antonacci et al., 2016).
Those that have used discrete-event simulation to
analyse patient scheduling do so in an attempt to
resolve issues such as reducing waiting times
(Harper and Gamlin, 2003), reduce planning time for
schedules (Werker et al., 2009) or compare
scheduling models (Lee et al., 2013). With the
exception of Lee et al. (2013) there are few studies
which measure the performance of scheduling
models against a range of metrics. Typically, studies
have focused on one key metric, while Lee et al.
(2013) evaluated four metrics including clinic
overtime, waiting times, unmet demand, and use of
appointment slots, but did not measure such metrics
as clinic room utilisation or clinician utilisation.
These tools are typically built to analyse and
solve specific scenarios at specific facilities.
However, there are some generic models produced
using techniques such as Business Process
Modelling and Notation (BPMN) to build accessible
simulation models for optimising healthcare
processes (Rolón et al., 2008; Antonacci et al.,
2016). BPMN requires users to understand the
notation used, which may make the approach
prohibitive to healthcare estates managers or
department managers.
This paper introduces a new tool with modifiable
inputs offering a reusable simulation model for
optimising patient scheduling. This tool gives the
ability to balance performance for a range of metrics
applicable to the NHS, including waiting times,
clinic utilisation, waiting room utilisation, clinician
utilisation, and clinic over-run.
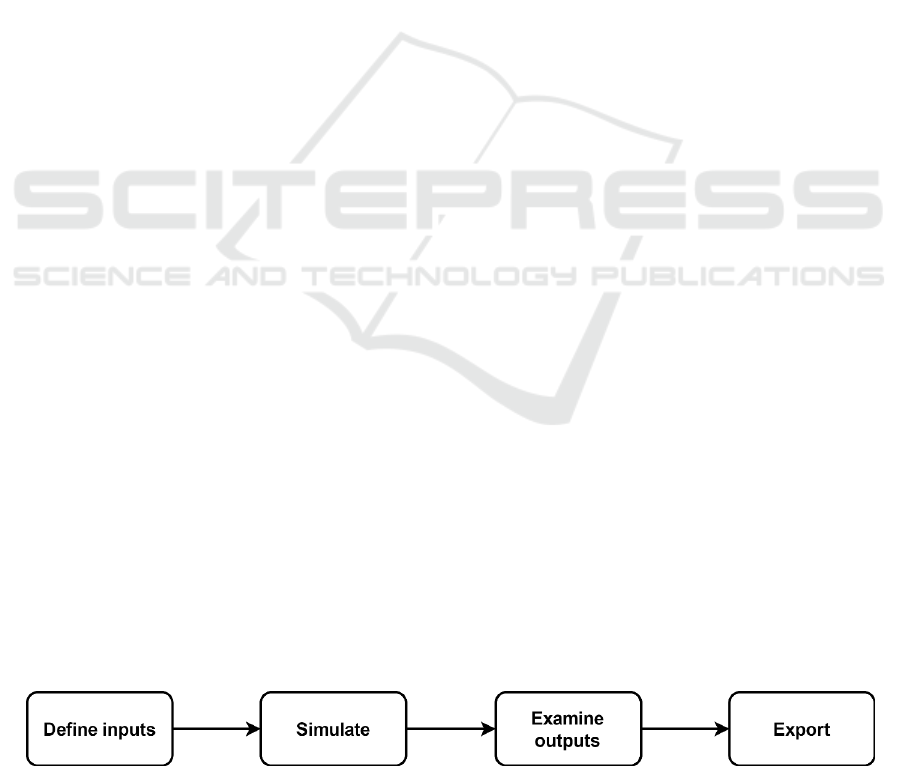
Figure 1: Flowchart of using the tool.
Maximising Patient Throughput using Discrete-event Simulation
205

3 METHODOLOGY
The tool presented in this paper was built in
response to increased demand for outpatient services
at a NHS hospital in the UK. The NHS Trust
designed a new cancer treatment centre with specific
space allocated for outpatient services. However,
since the design and construction of this facility
began, demand for the outpatient services at the
existing facility has risen to a level higher than
anticipated. The Trust wished to produce optimal
patient schedules based on a variety of clinic
scenarios, such as number of rooms or clinicians
available while operating within a set of
performance targets for key metrics, including:
patient waiting times, clinician utilisation, clinic
utilisation, waiting room utilisation, and clinic over-
run. The purpose of the tool presented here was,
therefore, to identify appropriate levels of activity
(e.g. number of daily attendances) that the outpatient
department could accommodate to maximise the
utilisation of the clinic rooms and clinicians whilst
maintaining a positive patient and clinician
experience.
The Trust were operating two clinic models in
the outpatient department; a dedicated clinic model
where clinicians stay in one room for the clinical
day; and a hub and spoke model where clinicians use
a central hub to complete admin work (e.g. patient
notes). These have a smaller number of clinic rooms
to consult with patients (i.e. clinicians use any free
clinic room). These clinic models can operate in
parallel during a day with variable numbers of
clinicians and rooms across two floors of the
outpatient department. This paper details the
development, inputs, simulation and outputs of the
tool developed to aid clinic planning for the Trust.
Figure 1 shows the process users take using the tool
presented here.
3.1 Inputs
Two factors which negatively impact patient
scheduling, and hospital performance, are variations
in the appointment durations (i.e. the time a patient
spends with a physician) and arrival times (i.e.
whether a patient arrives early or late for their
appointment). For appointment duration variation,
an analysis of anonymised historical appointment
data was performed to identify the variation.
Historical data were provided for a five month
period between July and November 2015 for a range
of outpatient appointment types and included the
arrival time of the patient, totalling 4,945 data
points. Of this, some appointments were excluded
from analysis where the duration was less than five
minutes or greater than 90 minutes (278), or where
the appointment data were incomplete (919), for
example, if it did not specify the activity undertaken.
This was done to remove appointments which were
logged after they occurred (i.e. typically resulting in
very short appointment durations) and ones which
could not be specifically linked to the outpatient
services of this department. This gave a remaining
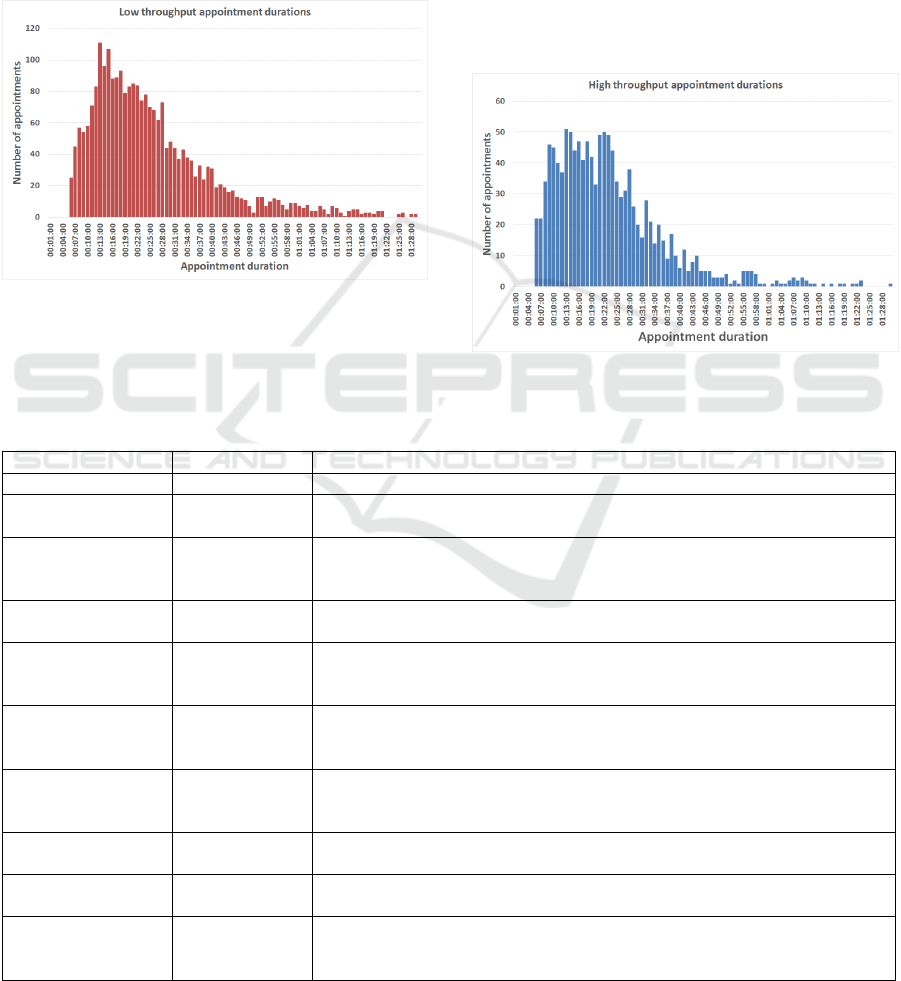
total of 3,748 data points for analysis. An average
(mean) appointment duration for low throughput
(24mins) and high throughput (22mins) clinic
models were extracted, along with the value
representing one standard deviation from this mean
appointment duration. Figure 2 shows the
distribution of appointment durations for the low
throughput model and Figure 3 shows the
distribution for the high throughput model from the
historical data.
The tool uses a number of inputs that define the
clinic day to be analysed, ranging from the number
of rooms and clinicians available, to the arrival
profile of patients. The inputs are modifiable by the
user at runtime, allowing them to simulate a variety
of scenarios. For example, users can simulate and
compare between 24 clinic rooms and 36 clinic
rooms with ease. Previous academic discussion has
noted that tools for this type of modelling are better
understood by users if the inputs have sensible
default values from the outset (Fletcher et al., 2007;
Gunal and Pidd, 2010). In acknowledgement of this,
the tool was developed with default values for each
input derived from discussions with the Trust and
analysis of the historical data. The inputs and their
default values are given in Tables 1 through 4.
The inputs provide a comprehensive analysis
model which evaluates the range of metrics defined
by the Trust. Of these inputs, some are related to the
clinician working practices and protocols. An
example of this is the clinician write-up period after
each appointment has been completed. This is the
time in which clinicians enter details of the patient’s
visit into their electronic records system, order
follow-up tests, and organise referrals as necessary.
For the dedicated clinic model, this occurs in the
same room as the appointment undertaken by the
clinician who does not leave, and so this time is
taken into account in the room turnaround (the time
taken for the room to be prepared for the next
patient). However, for the Hub/Spoke model, this
write-up time is conducted at the staff hub, allowing
the room to be freed up quicker for the next patient
to be seen by another clinician.
SIMULTECH 2017 - 7th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
206
3.2 Queues
Queueing is relatively simple in the dedicated
model, with patients arriving at the waiting area and
then waiting for a room to be free following the
room turnaround and the clinician write-up period.
However, for the Hub/Spoke model queuing is
slightly different, with there being a need for both a
room to be empty, and a clinician to be free. The
logic flow for this queue is presented in Figure 4.
Figure 2: Distribution of low throughput appointment
durations.
3.3 Patient Numbers
The objective of the tool is to produce an optimal
patient schedule that allows the department to
examine as many patients as possible in a given day
while keeping within the agreed target for a range of
metrics. As such, the tool does not take in a single
figure for the number of patients, but rather a range
and step size. This range is analysed, increasing by
the step size for each simulation run. This provides
the users output for each metric for the range of
patients, allowing them to compare schedules and
choose the optimal with ease.
Figure 3: Distribution of high throughput appointment
duration.
Table 1: Scenario inputs and default values.
Input
Default value
Description
Clinic hours
10 hours
How many hours does the clinic wish to run for?
Appointments per
day (min)
150
What is the smallest number of patients to simulate?
Appointments per
day (max)
500
What is the largest number of patients to simulate? Setting the maximum to
the same value as the minimum will result in a simulation run of just that
number of patients, regardless of step size.
Appointment step
size
50
What are the steps of patients to simulate? For the default values the patient
numbers simulated are: 150, 200, 250, 300, 350, 400, 450, 500.
Booking interval
15 minutes
What is the minimum amount of time between appointment slots. For
example, if a clinic starts at 9am, patients are given appointments at 9am,
9:15, 9:30, 9:45, etc.
Arrival profile –
percentage of early
arrivals
70%
How many patients will turn up early for their appointment.
Arrival profile –
percentage of late
arrivals
30%
How many patients will turn up late for their appointment.
Arrival profile –
minutes early
10 minutes
How early will patients turn up for their appointment. E.G. for a 9:15
appointment a patient will arrive at 9:05.
Arrival profile –
minutes late
9 minutes
How late will patients turn up for their appointment. E.G. for a 9:15
appointment a patient will arrive at 9:24.
Arrival profile – for
each clinic hour
Defaults as
above for
arrival profile
The user is given the option to define the arrival profile for each individual
hour of clinic operation for greater control of the profile.
Maximising Patient Throughput using Discrete-event Simulation
207
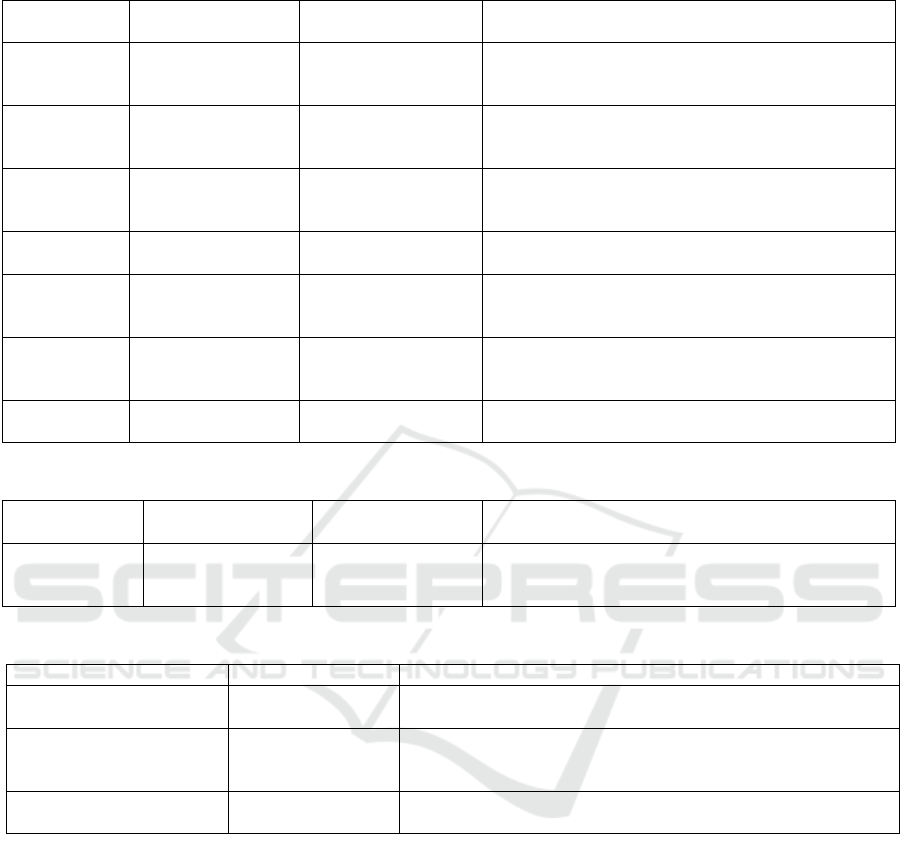
Table 2: Clinic inputs and default values.
Input
Default value
(low throughput)
Default value
(high throughput)
Description
Appointment
duration
24 minutes
22 minutes
The average appointment duration for each patient
type. The default value represents the average from
the analysis of historical data.
Standard
deviation
14 minutes
12 minutes
The time that represents one standard deviation from
the average, calculated from the analysis of historical
data.
Percentage of
patients
attending
50%
50%
Of the patients being simulated, what is the
percentage split between the types the department
caters for?
Clinic model
Hub/Spoke
Dedicated
What clinic model are those types using? Boolean
between Hub/Spoke and Dedicated.
Number of
clinic rooms
12 rooms
12 rooms
How many clinic rooms are available to each clinic. If
both clinics are running a Hub/Spoke model then
these values are combined.
Number of
clinicians
13 people
12 people
How many clinicians are working each type. For
Dedicated clinics, the number of clinicians matches
the number of rooms.
Clinician
write up time
20 minutes
10 minutes
How long do clinicians spend after the appointment
writing up notes or conducting follow-up actions?
Table 3: Clinic model inputs and default values.
Input
Default value
(Hub/Spoke)
Default value
(Dedicated)
Description
Turnaround
time
5 minutes
2 minutes
How long does it take to get the room prepared for the
next patient. This is time the room cannot be used for
appointment activity.
Table 4: Acceptance criteria inputs and default values.
3.4 Arrival Profiles
Another variance which can impact on a clinic’s
operational efficiency is the arrival times of patients
with appointments. Patients rarely turn up for an
appointment at the time of that appointment. Rather
they turn up early, to ensure they make it, or are late
for a variety of reasons. For this, the arrival profile
can be defined by the user as a uniform profile, or
define an arrival profile for each hour of the clinic’s
operations. This means that if users spot a trend in
patients arriving late in, for example, the afternoon,
this can be built into the simulation model to analyse
the impact of this.
Patients that arrive early will be registered in the
model from their arrival time, and will be placed in
the queue to be seen based on their arrival. Patients
that arrive ahead of their appointment timeslot
earlier in the model may have the opportunity to
begin their appointment prior to the scheduled
appointment timeslot if a room and a clinician are
free when they arrive and no other patients are in the
queue ahead of them. If a room or clinician is not
free however then they join the queue to be seen
when the resources are available.
Patients that arrive late are processed depending
on how late they arrive. For the Trust, the policy is
for patients that arrive within 15 minutes of their
appointment timeslot to be seen before patients with
later appointments already in the queue. Effectively
this allows late patients up to 15 minutes grace to
jump the queue before enduring an unknown wait to
Input
Default value
Description
Maximum waiting time
30 minutes
How long is an acceptable wait time for patients from their arrival
time to the time they are seen.
Waiting room capacity
68 people
What is the capacity of the waiting area for patients to sit in?
Patients that arrive early for their appointment take up a seat in
the waiting area from their arrival time.
Staff hub capacity
32 people
What is the capacity of the staff hub for Hub/Spoke clinicians to
work in.
SIMULTECH 2017 - 7th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
208
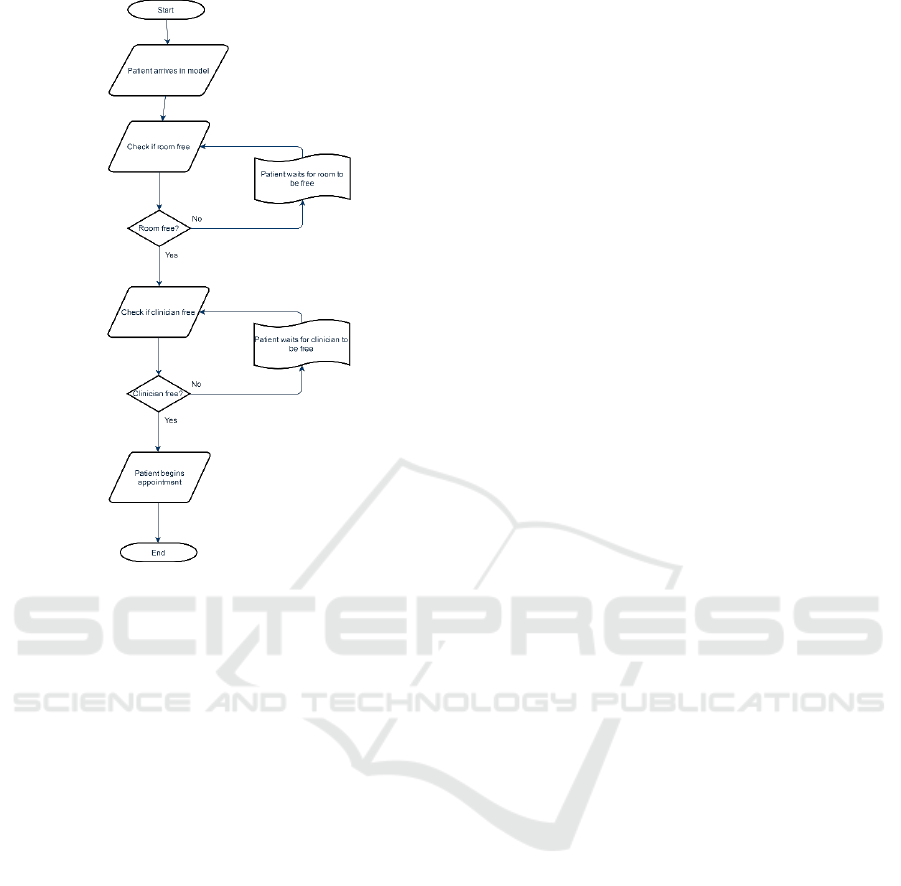
Figure 4: Logic diagram of patients using Hub/Spoke
clinics.
be seen. For example, if patient A has an
appointment at 9:30 and arrives at 9:20, while
patient B has an appointment at 9:15 but arrives at
9:24, patient B will be seen before patient A.
However, if patient B arrives at 9:31, then patient A
will be seen before patient B.
3.5 Simulation
The simulation is provided by the discrete-event
simulation (DES) tool SmartProcessAnalyser,
developed by BuroHappold, which builds a set of
clinic rooms based on the inputs provided by the
user and produces a simulation model for the first
grouping of patients. This model is then executed
and analysis results exported to a spreadsheet file
before a new simulation model is generated for the
next simulation run.
3.6 Multiple Analysis
The results of a single simulation can be misleading
with the variance in appointment durations and
arrival times providing a different result each
simulation run. Though the tool uses a random
function to generate the patient appointment
duration and arrival time, it is feasible to have a
‘near-perfect’ day simulated where appointments are
relatively quick and so it might appear that the clinic
could handle more patients than simulated with a
quick finish. Equally it is possible to have a ‘near
disastrous’ day with long appointments which may
give the impression that the clinic could only handle
a small number of patients.
To account for this, an analysis was performed to
determine how many simulation runs would be
optimal to obtain a valid prediction of the average,
rather than allowing a ‘near-perfect’ or ‘near-
disastrous’ day to provide misleading results. The
output of this recommended 40 simulation runs be
performed for each set of inputs to account for the
variation between each simulation run and prevent
outliers from providing misleading outputs. Table 5
outlines the results of this analysis. The simulation
model executes the analysis for a single grouping for
a given number of runs before moving onto the next
grouping. For example, a simulation of 50-100
patients with a step size of 10 for 20 runs will
analyse 20 scenarios of 50 patients before moving
on to analyse 20 scenarios of 60 patients, and so on.
A similar technique was seen in the work of Harper
& Gamlin (2003) which ran a DES model for an Ear
Nose and Throat (END) department 40 times to
prevent outliers skewing the results.
The multiple run analysis was performed using
default values for the inputs, with the exception of
patient numbers which were set at 500 patients. For
each set of runs, the average results from that
simulation were taken and measured for their
variance and confidence level around the mean
(measured at 95% confidence of the mean). For the
single run analysis, the average values are also the
only values that are exported, as opposed to multiple
runs where the average value is the average of all
values in that simulation. For example, a five run
simulation shows the average of each of the five
results on the graph outputs. Each simulation was
run 20 times, giving 20 samples for each ‘run’ being
analysed, ranging from 20 simulation results (20
times 1 run) to 1000 simulation results (20 times 50
runs) in the analysis. The results shown in Table 5
show that the variance is reduced for each metric as
the more simulation runs are performed, until 50
runs is reached. At this point, some metrics variance
increases while others stay the same, suggesting that
40-50 runs per simulation is likely to provide a more
reliable result than 1 run per simulation.
Maximising Patient Throughput using Discrete-event Simulation
209
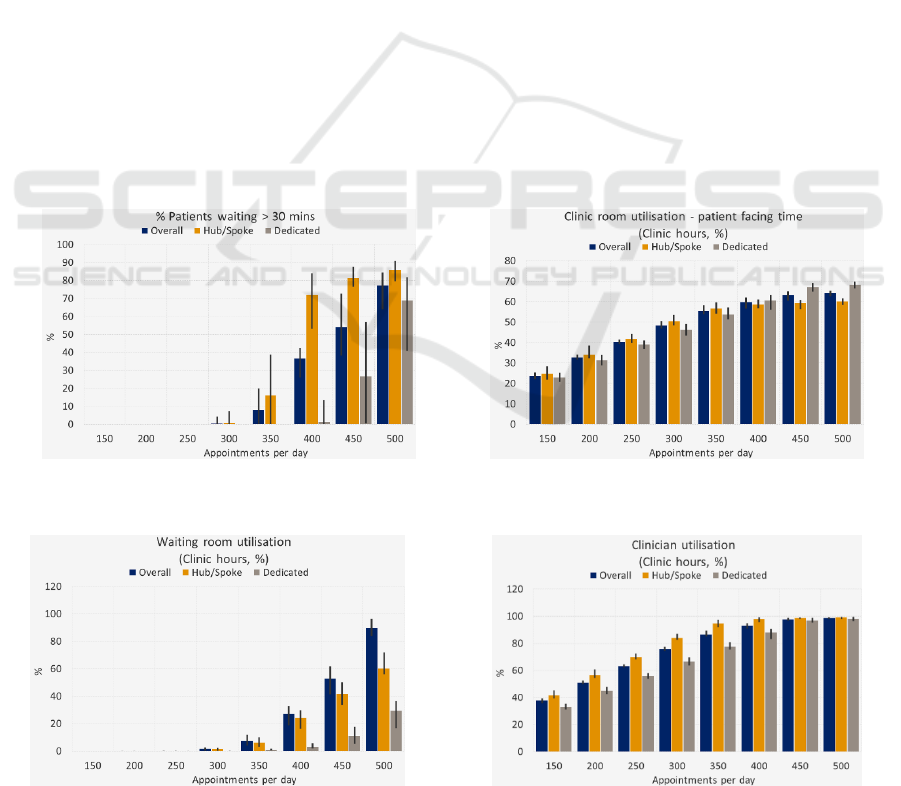
3.7 Analysis Results
The simulation exports the result of the analysis to a
spreadsheet file that can be examined in full by the
user and includes graphs that highlight the core
results of the simulations. This includes the
individual result for each simulation run for the user
to inspect if they so wish. The metrics included in
this tool are shown in Table 6. Each graph shows the
number of simulated patients along the X-Axis, and
for each patient grouping the average result after all
of the simulation runs. Also given are error bars
denoting the minimum result and maximum result of
all runs. Figures 5 through 8 provide examples of the
graph outputs following simulations using default
values.
The spreadsheet of results allows users to
explore the simulation results in detail. For each
metric an overall value (average of both clinic
models) is provided, as well as the result for each
clinic model. This is given for each simulation run.
For the room utilisation metrics, a detailed output of
the utilisation for each clinic type (and average of
overall) for each simulated minute is provided. This
is broken into the three states the room may be in,
idle (empty room ready for a patient), occupied
(with a patient), and being turned around (prepared
for the next patient). This allows users to analyse
periods of a simulated day when utilisations may be
lower than anticipated.
3.8 Result Interpretation
The tool provides the graph outputs on the user
interface (UI) for the user to work with as soon as all
of the simulations runs are completed, with the
detailed spreadsheet of data available to export.
However, the tool does not interpret the results to
make any recommendations of the best schedule to
adopt for the clinic. Rather, this interpretation is left
to the user, who can apply their experience and
knowledge to weight between each metric and select
the optimal patient schedule. For example, as
government focus shifts towards maximising
utilisation of space, it may become acceptable to
have a percentage of patients waiting more than a
given amount of time if the utilisation is increased.
Such trade-off decisions are left to the users, with
the tool providing no bias.
The error bars in the graphs shown in Figures
5 through 8 show the minimum and maximum
value of the results, with the columns denoting
the average result.
Figure 5: Percentage of patients waiting more than the
acceptance criteria.
Figure 6: Clinic room utilisation.
Figure 7: Waiting room utilisation.
Figure 8: Clinician utilisation.
SIMULTECH 2017 - 7th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
210
Table 5: Analysis results for multiple runs comparison (confidence of the mean measured at 95%).
Metric 1
Metric 2
Metric 6
Metric 7
Runs
Avg.
Var.
+/-
Avg.
Var.
+/-
Avg.
Var.
+/-
Avg.
Var.
+/-
1
76.5
16.3
2.5
63.8
0.8
0.6
2358.7
24824.7
97.7
266.8
566.6
14.8
5
75.2
1.8
1.3
63.6
0.1
0.3
2276.4
13711.6
56.4
261.3
63.0
5.5
10
76.3
2.2
0.9
63.8
0.2
0.3
2303.9
6737.0
50.9
261.4
91.8
5.9
20
75.9
1.0
0.6
63.8
0.2
0.3
2314
1044.2
20.0
262.9
26.1
3.7
30
75.5
2.1
0.9
64.0
0.0
0.0
2308.1
724.5
16.7
262.4
8.3
1.8
40
75.9
1.0
0.6
64.0
0.0
0.0
2313.4
461.8
13.3
264.4
4.9
1.4
50
76.0
0.9
0.6
64.0
0.0
0.0
2316.1
1545.0
24.4
263.9
16.1
2.5
Table 6 - List of outputs. * denotes a metric presented as a graph on the UI.
Metric 1*
Patients waiting >
x minutes
The percentage of patients whose waiting time exceeded the maximum
waiting time.
Metric 2*
Clinic room
utilisation
Clinic room demand as a percentage of capacity where demand is equal to
the total time spent by patients in rooms (for the given clinic hours) plus
the turnaround time (for the given clinic hours) plus the write-up time
(dedicated clinics only during clinic hours). Capacity is equal to the
number of rooms multiplied by the clinic hours.
Metric 3*
Waiting room
utilisation
Waiting area demand as a percentage of its capacity where demand is
equal to the total time spent by patient waiting for clinics (for the given
clinic hours) and capacity is the room capacity multiplied by the clinic
hours.
Metric 4*
Staff hub
utilisation
Staff hub demand as a percentage of its capacity where demand is the total
write-up time spent by Hub/Spoke clinicians in the hub (during clinic
hours only) and capacity is the hub capacity multiplied by the clinic hours.
Metric 5*
Clinician
utilisation
Clinician demand as a percentage of clinician capacity where demand is
equal to the total time spent with a patient or writing-up and capacity is
the number of clinicians multiplied by the clinic hours.
Metric 6*
Total patient
facing time (post
clinic)
The total amount of time spent by patients in appointments after the end of
the clinic hours. For patients whose appointment began prior to the end of
clinic hours, only the portion that occurred after the end of clinic hours is
included. For example, if patient A begins their appointment at 16:50 and
finishes at 17:15, with clinic hours finishing at 17:00, this would give a
patient facing time of 15 minutes. This is summed for all patients, so if
patient B has an appointment from 16:55 to 17:10, the total patient facing
time for A & B would be 25 minutes.
Metric 7
Clinic over-run
(mins)
The total amount of time after clinic hours until all patients have
completed the model. In the above example for metric 6, the clinic over-
run would be 15 minutes.
Metric 8
Patients waiting
(post clinic)
This is the number of patients who are still waiting to be seen at the end of
clinic hours.
Metric 9
Patients being
seen (post clinic)
This is the number of patients that are in appointments at the end of clinic
hours.
4 LIMITATIONS
Although the tool takes into account the variance in
appointment durations and arrival patterns, it does
not take into account other variances related to the
clinicians and facility which may impact on an
appointment schedule. For example, there is only a
fixed input for the length of time clinicians will
spend ‘writing up’ following an appointment.
However, this could be subject to variance
depending on the appointment, as follow-up tests
may need to be ordered, or subsequent appointments
Maximising Patient Throughput using Discrete-event Simulation
211

scheduled. This variance was unable to be captured
from the appointment history data used to calculate
the variance in appointment durations, and so the
default values for these inputs came from
discussions with experienced clinicians. However,
because the input is accessible to the user of the tool
the write-up time can be modified if the write-up
process or time changes.
Similarly, there is no variance accounted for in
the room turnaround times for each clinic type. The
clinic turnaround is the time it takes to prepare the
room for the next patient, which may include
changing bedsheets, replenishing equipment such as
gloves and needles, and removing expended
equipment. This variance was also unable to be
captured from historical data, though it is also less
likely to have as much variance as appointments and
clinician work. Turning a room round for the next
patient typically follows a given process for hygiene
and sanitary reasons and has a fixed protocol to be
followed. Thus, the impact of a variance in
turnaround times is likely to be negligible. However,
as with the clinician write-up input, this input is
exposed to the user to be modified as they see fit.
As the tool has been built for the outpatient
operations defined by the Trust, it follows a linear
unchanging clinic pathway for patients from arrival
to appointment to leaving the model. It does not take
into account other potential activities such as blood
work prior to or after the examination. However, the
underlying simulation engine allows for easy
adaptation at a later stage to include further
activities, both clinical and non-clinical, should
future scenarios warrant it. Full implementation
could allow users to build their own clinic pathway
for a patient, however, at present this is not a
function of the tool presented here.
5 DISCUSSION
This paper has presented a clinic planning tool that
is generalisable to any outpatient clinic wishing to
run dedicated, Hub/Spoke, or a mixture of both
clinic types, as well as incorporating variance in
patient appointment durations and arrival times for a
more accurate simulation. The variance for
appointment durations and arrival times was
calculated from 3,748 historical appointments.
However, the generic inputs are open to users of the
tool and thus allow for any Trust to adopt the tool to
produce their own simulation results with ease. This
allows the tool to be reused and prevents it being a
solution for one specific problem. Rather, this tool
can be used to tackle a range of appointment
scheduling problems provided sensible inputs are
given, saving a Trust the time and development cost
of developing their own tool.
With increasing budget restraints on the NHS, a
reusable tool that can be utilised by any hospital or
department is of benefit to the healthcare industry.
Its generic modelling inputs, not constrained by
spatial requirements, allow for reuse and easy
adoption by other healthcare providers. However, its
adaptability through the use of the extendable DES
engine also ensures the tool can evolve with policy
changes and continue to provide optimal patient
schedules.
6 CONCLUSIONS
The variance in appointment durations plays a large
part in the efficiency and operation of a clinic.
Though there may be attempts to standardise
appointment durations, variations will undoubtedly
occur as individual health concerns cannot always be
feasibly addressed in a strict appointment window.
As such, it is better to accept the variance and plan
with it, rather than to plan for no variance and
wonder why clinics are over-running every day and
clinicians are suffering from being overworked. This
tool assists with this, taking the variance and
randomness in appointment durations and building
this into the simulation model from the start. The use
of a generic input set-up to define the clinic model
allows the tool to be applicable to any department
utilising the given clinic models to find an optimal
appointment schedule.
The variance in outputs generated by each
individual simulation run has been highlighted as a
danger of incorporating variance in appointment
durations. This shows the need for multiple
simulation runs to be performed on a DES model to
reduce overall impact of outliers from producing
non-representative results and improve the
confidence in the outputs. The inclusion of clinician
resources for the clinic also allows for future
planning to be undertaken, by seeing the impact of
clinician changes (holiday, sickness, etc.) on the
system.
Finally, a range of metrics has been included in
the tool, providing a comprehensive analysis to the
user. The metrics offer output based on current
targets and guidelines for the NHS. These can be
easily adapted or added to as targets and guidelines
change. The end result of using the tool is the user’s
ability to produce an appointment schedule which
SIMULTECH 2017 - 7th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
212

will allow for seeing the maximum number of
patients possible in a day without negatively
impacting clinic utilisation, clinician utilisation,
clinic over-run, or patient waiting times.
ACKNOWLEDGEMENTS
We would like to thank the Engineering and
Physical Sciences Research Council, and Centre for
Innovative and Collaborative Construction
Engineering at Loughborough University for
provision of a grant (number EPG037272) to
undertake this research project in collaboration with
BuroHappold Engineering Ltd.
REFERENCES
Anderson, J. G. and Merode, G. G. (2007) ‘Special issue
on health care simulation’, Health Care Management
Science, 10(4), pp. 309–310. doi: 10.1007/s10729-
007-9031-x.
Antonacci, G., Calabrese, A., D’Ambrogio, A., Giglio, A.,
Intrigila, B. and Ghiron, N. L. (2016) ‘A BPMN-based
automated approach for the analysis of healthcare
processes’, in Proceedings - 25th IEEE International
Conference on Enabling Technologies: Infrastructure
for Collaborative Enterprises, WETICE 2016, pp.
124–129. doi: 10.1109/WETICE.2016.35.
Ballard, S. M. and Kuhl, M. E. (2006) ‘The use of
simulation to determine maximum capacity in the
surgical suite operating room’, in Proceedings -
Winter Simulation Conference, pp. 433–438. doi:
10.1109/WSC.2006.323112.
Bernstein, S., Aronsky, D., Duseja, R., Epstien, S.,
Handel, D., Hwang, U., McCarthy, M., McConnell, J.,
Pines, J., Rathlev, N., Schafermeyer, R., Zwemer, F.,
Schull, M. and Asplin, B. (2009) ‘The effect of
emergency department crowding on clinically oriented
outcomes’, Academic Emergency Medicine, 16(1), pp.
1–10. doi: 10.1111/j.1553-2712.2008.00295.x.
Brenner, S., Zeng, Z., Liu, Y., Wang, J., Li, J. and
Howard, P. K. (2010) ‘Modeling and analysis of the
emergency department at university of Kentucky
Chandler Hospital using simulations’, Journal of
Emergency Nursing, 36(4), pp. 303–310. doi:
10.1016/j.jen.2009.07.018.
Carter, P. (2016) Operational productivity and
performance in English NHS acute hospitals:
Unwarranted variations. London.
Davis, K., Stremikis, K., Squires, D. and Schoen, C.
(2014) ‘Mirror, mirror on the wall, 2014 update: how
the US health care system compares internationally’,
New York (NY): Commonwealth Fund, 16.
Denton, B., Rahman, A., Nelson, H. and Bailey, A. (2006)
‘Simulation of a multiple operating room surgical
suite’, in Proceedings of the 38th conference on
Winter simulation, pp. 414–424.
Department of Health (2013) The NHS Constitution.
London.
Dexter, F. and Epstein, R. (2009) ‘Typical savings from
each minute reduction in tardy first case of the day
starts’, Anesthesia & Analgesia, 108(4), pp. 1262–7.
doi: 10.1213/ane.0b013e31819775cd.
Fetter, R. B. and Thompson, J. D. (1965) ‘The Simulation
of Hospital Systems’, Operations Research, 13(5), pp.
689–711. doi: 10.1287/opre.13.5.689.
Fletcher, A., Halsall, D., Huxham, S. and Worthington, D.
(2007) ‘The DH Accident and Emergency Department
model: a national generic model used locally’, Journal
of the Operational Research Society, 58(12), pp.
1554–1562. doi: 10.1057/palgrave.jors.2602344.
Greenroyd, F. L., Hayward, R., Price, A., Demian, P. and
Sharma, S. (2016) ‘Using Evidence-Based Design to
Improve Pharmacy Department Efficiency’, HERD:
Health Environments Research & Design Journal,
10(1), pp. 130–143. doi: 10.1177/1937586716628276.
Gunal, M. and Pidd, M. (2006) ‘Understanding accident
and emergency department performance using
simulation’, Simulation Conference, 2006. WSC 06.
Proceedings of the Winter, pp. 446–452.
Gunal, M. and Pidd, M. (2010) ‘Discrete Event Simulation
for Performance Modelling in Healthcare: A Review
of the Literature.’, Journal of Simulation, 4(1), pp. 42–
51.
Harper, P. R. and Gamlin, H. M. (2003) ‘Reduced
outpatient waiting times with improved appointment
scheduling: A simulation modelling approach’, OR
Spectrum, 25(2), pp. 207–222. doi: 10.1007/s00291-
003-0122-x.
Harper, P. R. and Pitt, M. A. (2004) ‘On the challenges of
healthcare modelling and a proposed project life cycle
for successful implementation’, Journal of the
Operational Research Society, 55(6), pp. 657–661.
doi: 10.1057/palgrave.jors.2601719.
Health and Social Care Information Centre (2015) Estates
Returns Information Collection: England 2014 to
2015. London.
Hendrich, A., Chow, M., Skierczynski, B. and Lu, Z.
(2008) ‘A 36-hospital time and motion study: How do
medical-surgical nurses spend their time?’, The
Permanente Journal, 12(3), pp. 25–34.
Huang, Y.-L. and Kammerdiner, A. (2013) ‘Reduction of
service time variation in patient visit groups using
decision tree method for an effective scheduling’,
International Journal of Healthcare Technology and
Management, 14(1–2), pp. 3–21. doi:
10.1504/IJHTM.2013.055081.
Jun, J., Jacobson, S. and Swisher, J. (1999) ‘Application of
discrete - event simulation in health care clinics: A
survey’, Journal of the Operational Research Society,
50(2), pp. 109–123. doi:
10.1057/palgrave.jors.2600669.
Kuljis, J., Paul, R. J. and Chen, C. (2001) ‘Visualization
and Simulation: Two Sides of the Same Coin?’,
Maximising Patient Throughput using Discrete-event Simulation
213

Simulation, 77(3/4), pp. 141–152. doi:
10.1177/003754970107700306.
Lee, S., Min, D., Ryu, J. -h. and Yih, Y. (2013) ‘A
simulation study of appointment scheduling in
outpatient clinics: Open access and overbooking’,
Simulation, 89(12), pp. 1459–1473. doi:
10.1177/0037549713505332.
Leskovar, R., Accetto, R., Baggia, A., Lazarevič, Z.,
Vukovič, G. and Požun, P. (2011) ‘Discrete event
simulation of administrative and medical processes’,
Zdravniski Vestnik, 80(5).
Marcario, A. (2006) ‘Are your hospital operating rooms
“efficient”’, Anesthesiology, (2), pp. 233–243.
Maviglia, S. M., Yoo, J. Y., Franz, C., Featherstone, E.,
Churchill, W., Bates, D. W., Gandhi, T. K. and Poon,
E. G. (2007) ‘Cost-benefit analysis of a hospital
pharmacy bar code solution.’, Archives of internal
medicine, 167, pp. 788–794. doi:
10.1001/archinte.167.8.788.
NHS England (2013) ‘The NHS belongs to the people: a
call to action’, NHS England, London.
NHS England (2014) Five year forward view. London.
Nicholson, D. (2009) ‘The Year: NHS chief executive’s
annual report 2008/09’, London: Department of
Health, p. 47.
Quevedo, V. and Chapilliquén, J. (2014) ‘Modeling a
Public Hospital Outpatient Clinic in Peru Using
Discrete Simulation’.
Reynolds, M., Vasilakis, C., McLeod, M., Barber, N.,
Mounsey, A., Newton, S., Jacklin, A. and Franklin, B.
D. (2011) ‘Using discrete event simulation to design a
more efficient hospital pharmacy for outpatients’,
Health Care Management Science, 14, pp. 223–236.
doi: 10.1007/s10729-011-9151-1.
Rolón, E., García, F., Ruiz, F., Piattini, M., Calahorra, L.,
García, M. and Martin, R. (2008) ‘Process Modeling
of the Health Sector Using BPMN: A Case Study’, in
Proceedings of the first international conference on
health informatics (HEALTHINF), pp. 173–178.
Vanberkel, P. T. and Blake, J. T. (2007) ‘A
comprehensive simulation for wait time reduction and
capacity planning applied in general surgery’, Health
Care Management Science, 10(4), pp. 373–385. doi:
10.1007/s10729-007-9035-6.
Werker, G., Sauré, A., French, J. and Shechter, S. (2009)
‘The use of discrete-event simulation modelling to
improve radiation therapy planning processes’,
Radiotherapy and Oncology. Elsevier Ireland Ltd,
92(1), pp. 76–82. doi: 10.1016/j.radonc.2009.03.012.
Zeng, Z., Ma, X., Hu, Y., Li, J. and Bryant, D. (2012) ‘A
Simulation Study to Improve Quality of Care in the
Emergency Department of a Community Hospital’,
Journal of Emergency Nursing. Emergency Nurses
Association, 38(4), pp. 322–328. doi:
10.1016/j.jen.2011.03.005.
SIMULTECH 2017 - 7th International Conference on Simulation and Modeling Methodologies, Technologies and Applications
214
