
Towards Bidirectional Brain-computer Interfaces that Use
fNIRS and tDCS
Samuel W. Hincks
1
, Maya DeBellis
1
, Eun Youb Lee
1
, Ronna ten Brink
1
, Birger Moëll
2
and Robert J. K. Jacob
1
1
Tufts University, Medford, MA 02155, U.S.A.
2
Stockholm University, 11418 Stockholm, Sweden
Keywords:
BCI, Implicit Brain-computer Interface, fNIRS, Near-infrared Spectroscopy, tDCS, Transcranial Direct
Current Stimulation, Cognitive Workload, Bidirectional Brain-computer Interface, Entropic Brain-computer
Interface, N-back, ADHD, Attention.
Abstract:
We envision a future user interface that measures its user’s mental state and responds not only through a
display but also by sending output directly to the brain, leading to a primitive bidirectional brain-computer
interface. Previous interactive systems have measured brain state with functional near-infrared spectroscopy
(fNIRS) for communication from user to computer; we now explore transcranial direct-current stimulation
(tDCS) as a channel in the opposite direction. Our goal is to integrate this with brain measurements from
fNIRS, so that the stimulation parameters governing tDCS may be set dynamically to enhance user cognition
based on current mental state and task demands. To do this, the first step is to determine how long it takes for
tDCS to register cognitive effects and how long these effects last. We present an experiment that investigates
the temporal dimension of tDCS for this purpose. The findings suggest a long lag-time between the onset
of stimulation and any measurable cognitive effect, which may prohibit the effectiveness of tDCS in a brain-
adaptive application.
1 INTRODUCTION
Computers support several methods for communi-
cating with the user, but currently these output
methods are constrained by users’ sensory channels.
Non-invasive brain stimulation techniques, such as
transcranial direct-current stimulation (tDCS), might
transcend this limitation. Evidence in the psychology
literature suggests that tDCS can temporarily enhance
or emphasize aspects of user cognition (Brunoni and
Vanderhasselt, 2014) without imposing a health risk
(Bikson et al., 2016). tDCS delivers a weak (1 to 2
milliamp) electrical current to the exterior of the sub-
ject’s scalp through an electrode, taking the path to
the nearest cathode, which has been carefully placed
so that the current will enter and alter particular re-
gions of the subject’s brain. tDCS has been used to
treat depression (Nitsche et al., 2009), as well as en-
hance language learning (Flöel et al., 2008), working
memory (Fregni et al., 2005) and attention (Gladwin
et al., 2012). With the introduction of tDCS to the
standard output arsenal of HCI, an interactive system
may be able to judiciously enhance these abilities de-
pending on the circumstance and user state.
Our study is aimed at a future user interface that
uses brain measurement as input and responds not
only with the usual screen output but also by send-
ing output directly to the brain, suggesting a primitive
bidirectional brain-computer interface. Previous sys-
tems have measured brain state with fNIRS for com-
munication from user to computer (Afergan et al.,
2014a; Afergan et al., 2015; Solovey et al., 2012);
we now explore tDCS for the opposite direction in a
bidirectional brain-computer interface, with fNIRS or
another brain monitor as input, and tDCS as output.
For example, consider a brain-adaptive UAV system
(Afergan et al., 2014a). In this experiment, we trained
machine learning algorithms operating on data from
an fNIRS brain monitor to predict the user’s cogni-
tive workload as he or she was controlling the flight
paths of several simulated unmanned aerial vehicles
(UAV). The system added extra UAVs to the user’s
task when brain activity indicated that she was in a
state of low cognitive workload; and it removed some
in order to simplify the user’s task when workload in-
creased. The bidirectional version we propose would
Hincks, S., DeBellis, M., Lee, E., ten Brink, R., Moëll, B. and Jacob, R.
Towards Bidirectional Brain-computer Interfaces that Use fNIRS and tDCS.
DOI: 10.5220/0006380500570064
In Proceedings of the 4th International Conference on Physiological Computing Systems (PhyCS 2017), pages 57-64
ISBN: 978-989-758-268-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
57

apply tDCS stimulation briefly, precisely when the
measured workload increases and only for the du-
ration of the workload spike. For such a bidirec-
tional brain-computer interface to work in practice,
the lag time between stimulation and its result should
be short. However, much previous tDCS research,
especially experiments aimed at treating depression
(Shiozawa et al., 2014), have emphasized longer term
effects and longer stimulation periods, because inter-
activity was not the goal.
To proceed with an interactive system, the key
question is to determine how long it takes for tDCS
stimulation to register cognitive effects and how long
these effects last. We investigate that in this paper
with two experiments. At present, there are many un-
knowns regarding the relationship between settings of
the device and its associated cognitive effects, mak-
ing it difficult to gauge whether the device warrants
study and inclusion in next generation user inter-
faces. An alarming percentage of experiments (Hor-
vath et al., 2015) fail to elicit significant improve-
ments to user performance. The consensus is that re-
sults vary across person, possibly because each indi-
vidual has a different brain and unique rules for how
to conduct brain stimulation. However, deciding to
abandon tDCS for that reason is premature, because
the device has not yet been studied interactively. The
missing ingredient for effective tDCS may in fact be
a two-way digital ecosystem in which settings can be
dynamically adjusted based on their judged, subject-
to-subject effectiveness.
In this paper, we evaluate the feasibility of a bidi-
rectional brain-computer Interface. We present two
experiments aimed at estimating temporal properties
of tDCS by estimating performance changes in a visu-
ospatial n-back task over a 15 minute time-course. In
the first experiment, we compare 5 minutes of tDCS
stimulation to a placebo condition; and in the second
experiment, we compare 10 minutes of stimulation to
a placebo condition. We evaluate changes in reaction
time and accuracy for each minute of the experiment.
2 BACKGROUND
2.1 Transcranial Direct Current
Stimulation
While introducing tDCS brain stimulation into HCI
raises safety and ethical questions, research to date
has shown that when stimulation does not exceed 2
milliamps and lasts shorter than 40 minutes, there
have been no cases of irreversible injury caused by
tDCS in a sample of 33,200 sessions (Bikson et al.,
2016). Compared to other brain stimulation tech-
niques, tDCS is easy to use and potentially inexpen-
sive; it already supports a do-it-yourself community
(Fitz and Reiner, 2013). Although experiments typi-
cally use a more advanced setup, the basic device con-
sists of just two electrodes and a battery to energize
them. Direct current is then administered through a
saline-soaked sponge or rubber electrode with con-
ductive gel on the subject’s scalp. In a typical setup
(and the one used in this experiment), one electrode is
placed over the target of stimulation, initiating a path
for the current to take to a second electrode placed
somewhere nearby. The current is presumed to al-
ter the cortical excitability of the neurons it interacts
with, either depolarizing their membranes and mak-
ing the neurons more likely to fire in the case of an-
odal stimulation, or hyperpolarizing the membranes,
making the neurons less likely to fire in the case of
cathodal stimulation (Medeiros et al., 2012).
Given that working memory, compulsivity, and
attention are often impaired in individuals with
Attention-deficit/hyperactivity disorder (ADHD)
(Moëll et al., 2015), tDCS has been explored as treat-
ment for individuals with this condition (Cachoeira
et al., 2017). Experiments aimed to enhance working
memory typically administer anodal stimulation to
the left dorsolateral prefrontal cortex (dlPFC) at the
site F3 (in the International 10-20 system (Nitsche
et al., 2008)) and allow current to flow through a
reference electrode at a symmetrical location on the
brain’s right hemisphere at site F4 (Zaehle et al.,
2011) (see Figure 1). Many experiments have used
this montage to enhance performance at an n-back
test (Brunoni and Vanderhasselt, 2014). The present
experiment makes use of the same montage and
n-back paradigm, except we investigate shorter
stimulation periods and track performance on a
minute-by-minute basis in order to evaluate the usage
of tDCS in an interactive system.
2.2 Functional Near Infrared
Spectroscopy
fNIRS is a non-invasive neuroimaging technique,
which can be implemented cheaply since it consists
merely of light sources and detectors (Piper et al.,
2014). fNIRS depicts brain activation by shining
near-infrared light into the scalp and detecting the
amount that returns to the sensor, which changes
based on the relative concentration of oxygenated and
deoxygenated hemoglobin, the basic energy supply
for neurons. These measurements have been found to
fluctuate in response to the user’s cognitive workload
(Herff et al., 2014).
PhyCS 2017 - 4th International Conference on Physiological Computing Systems
58
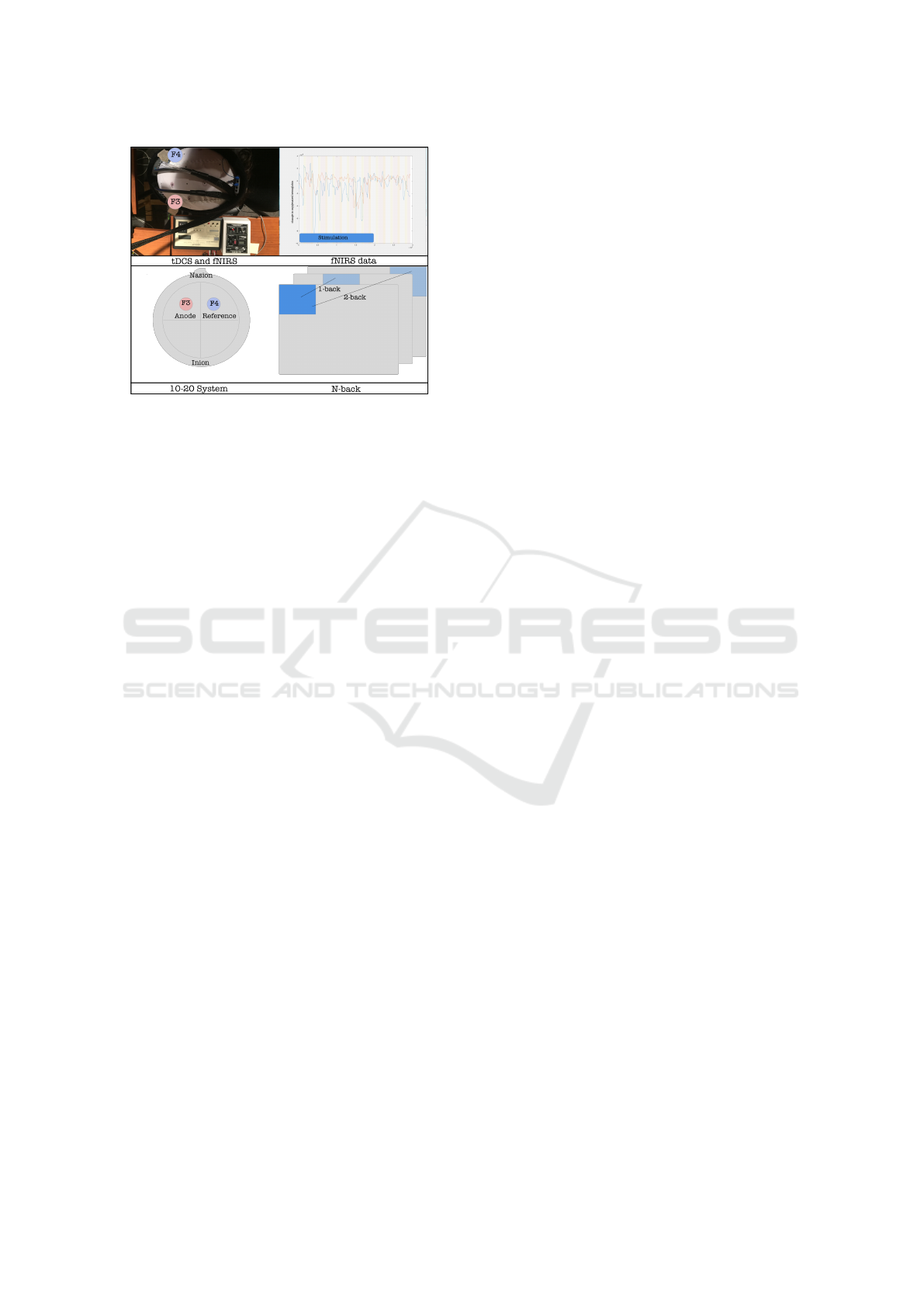
Figure 1: The n-back task, pictures of the device, and one
subject’s fNIRS activity.
2.3 N-Back
Cognitive workload is a basic index of the cerebral
strain a task poses on a user (Herff et al., 2014). We
focus on the workload in the prefrontal cortex, which
generally correlates with short-term memory work-
load. In experiments, this type of workload is typi-
cally induced using an n-back task. In the visuospa-
tial variety of this task (see Figure 1), the user tracks
a 3-by-3 grid where one box is colored. The colored
box changes every couple seconds, and the user’s job
is to indicate whether or not the colored box is in the
same location as it was n iterations ago. Higher val-
ues of n induce higher degrees of short term memory
workload. tDCS has been reported to improve n-back
performance (Brunoni and Vanderhasselt, 2014) and
fNIRS has been reported to differentiate brain sig-
nals pertaining to trials with higher or lower associ-
ated cognitive workload (Herff et al., 2014; Hincks
et al., 2016).
2.4 Implicit Brain-computer Interfaces
Implicit Brain-Computer Interfaces (Zander et al.,
2014; Treacy Solovey et al., 2015) listen to run-
ning classifications of the user’s state as measured
by portable brain sensors (such as EEG and fNIRS),
and update implicit system settings to the user’s cur-
rent needs. Using fNIRS, predictions of the user’s
cognitive workload have been applied to control cur-
sor selection expansion (Afergan et al., 2014b), mu-
sical scores (Yuksel et al., 2016), robot automation
(Solovey et al., 2012), and task difficulty (Afergan
et al., 2014a). Likewise, this measure could drive the
administration of tDCS.
3 EXPERIMENTS
3.1 Equipment
For measuring brain activity, we used the multichan-
nel frequency domain Imagent fNIRS device from ISS
Inc. (Champaign, IL) to acquire brain data. It uses
two probes, each with four light sources emitting light
at 830 and 690 nanometers, and detectors located
between 0.5 and 3.5 centimeters away from these
sources. Sampling frequency was set to 11.79hz.
For altering brain activity, we used Soterix 4x1
HD-tDCS multi-channel stimulation interface (model
4X1-C2) to pass electrical currents and the Soterix
tDCS-CT (model 1507-LTE) to control stimulation
and placebo according to a double-blind protocol.
3.2 Experiment 1
Nine undergraduate college students (5 female) par-
ticipated in the first experiment. They were mone-
tarily compensated and gave consent at the beginning
of the experiment. A university Institutional Review
Board approved the experiment. The experimenter
explained the visual n-back task (Figure 1) on a white-
board, and let the user practice two trials of the 1-
back and two trials of the 2-back. For the 1-back, the
user hit the left arrow key if the visual arrangement
matched the previous one and the right arrow key oth-
erwise, and for the 2-back they indicated whether or
not it matched what they saw 2 iterations ago. These
keys were marked with ‘YES’ and ‘NO’ with tape
on the keyboard. This task was implemented with
custom software for the purpose of recording reac-
tion time and dynamically labeling fNIRS data. Af-
ter these practice trials, the experimenter fit the user
with tDCS and fNIRS. This entailed first measuring
the size of the subject’s head and selecting between
four cap sizes, and then placing one gel-covered an-
odal electrode at site F3 and the other reference elec-
trode at site F4 (Nitsche et al., 2008), and then con-
necting the electrodes to the Soterix device (see Fig-
ure 1). Next, we placed the two fNIRS probes as near
as possible to those sites. (We do not report on any
fNIRS data in this paper for experiment one or two
because we were unable to discover stimulation de-
pendent patterns).
The subsequent experiment proceeded in two
phases. In the first phase, subjects alternated between
30 seconds of the 1-back and 30 seconds of the 2-
back, performing each task 7 times. This served as
practice as well as the opportunity to group partici-
pants by the separability of their fNIRS data. In the
second phase, the subject alternated between 40 sec-
Towards Bidirectional Brain-computer Interfaces that Use fNIRS and tDCS
59
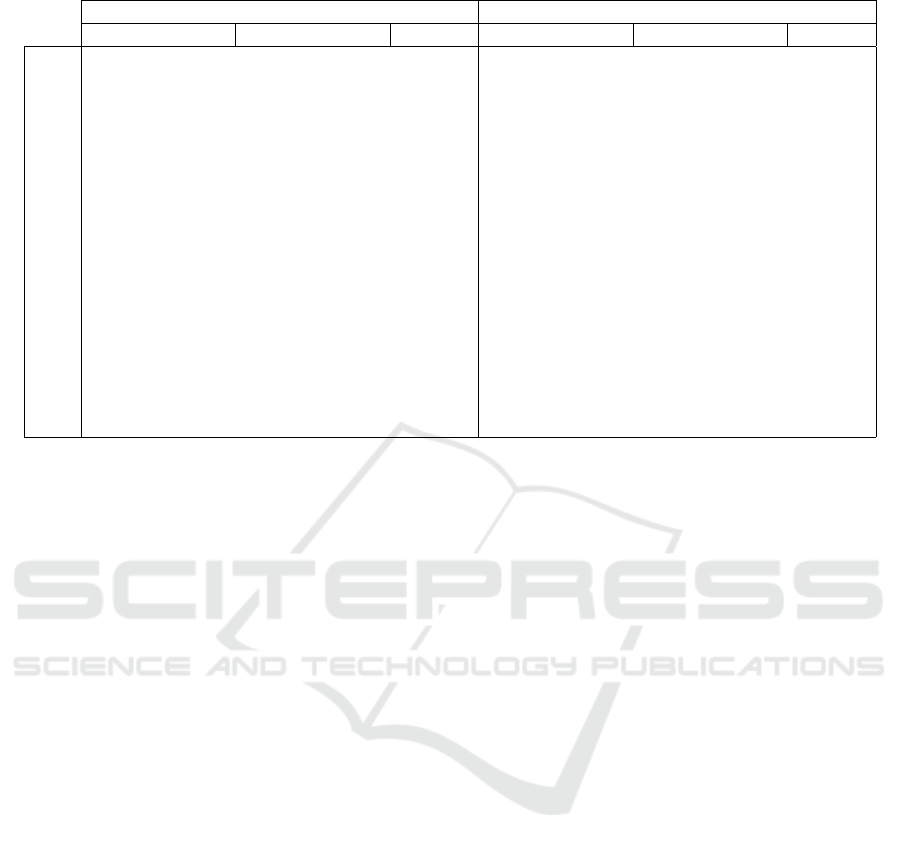
Table 1: Differences in N-Back Accuracy and Reaction Time for each Minute of Experiment 1.
Percent Accuracy Reaction Time (milliseconds)
Sham Real Sham Real
min mean std dev mean std dev p-value mean std dev mean std dev p-value
1 74 24 77 22 0.82909 930 247 808 209 0.4575
2 82 34 93 10 0.58809 701 216 852 250 0.36142
3 80 39 90 14 0.65056 770 261 880 41 0.43568
4 81 28 98 5 0.27549 808 310 811 40 0.98716
5 84 29 88 10 0.84721 651 189 832 166 0.17768
6 82 34 93 15 0.60227 744 201 728 167 0.90031
7 78 32 100 0 0.2236 681 224 691 96 0.9418
8 83 28 93 10 0.52828 669 198 718 149 0.69088
9 80 33 87 13 0.70177 631 218 699 146 0.61433
10 82 29 98 5 0.33798 655 230 651 73 0.97642
11 84 35 95 10 0.57964 637 182 723 109 0.43732
12 80 39 89 16 0.67354 658 159 763 93 0.28151
13 81 28 90 8 0.54059 666 273 620 81 0.75824
14 84 35 90 8 0.76657 590 168 704 149 0.32322
15 78 26 92 5 0.34072 624 201 715 187 0.50996
m 81 31 91 4 0.53000 694 209 746 52 0.65
onds of the 2-back and 20 seconds of rest, repeating
this 15 times for a total of fifteen minutes. In the n-
back task (for experiment 1 and 2), a new stimulus
appeared every 3 seconds, and accuracy and reaction
time for the 40 second task was therefore based on the
average of 13 responses.
We used a between subject design. Prior to the
experiment, the participant had been placed in two
groups: four in the real tDCS group and five in the
sham group, and neither experimenter nor subject
knew the groups. The real group received 2 milliamps
of anodal stimulation at site F3 for 5 minutes. The
sham group received 2 milliamps of stimulation only
for 30 seconds, a standard placebo, since subjects tend
to sense when the device turns on but forget about it
when it has been on for a while (Brunoni and Vander-
hasselt, 2014). Participants began the experiment in
parallel to onset stimulation. Afterwards, the exper-
imenters removed the equipment from the user and
debriefed them.
Results: We have summarized the results of the
first experiment in Table 1, and there were no sig-
nificant effects for the 5 minute stimulation, although
stimulated user’s trended towards better accuracy and
the control group trended towards faster speed, hint-
ing more at a speed-accuracy trade-off than cognitive
enhancement. Table 1 shows the mean and standard
deviation of the participants’ mean accuracy and re-
action time for each of the fifteen trials under both
sham and real conditions, as well as the probability
that these averages differed between sham and real
conditions in an independent t-test. Without a clear
indication that 5 minutes of stimulation exerted sig-
nificant improvements to user performance, we mod-
ified our design and conducted a second experiment.
3.3 Experiment 2
Fourteen college students (4 female) participated in
the second experiment. Based on the lack of sig-
nificant results in the first experiment, we increased
stimulation time from 5 to 10 minutes, and used a
within subject design so that all participants received
both real and sham stimulations. Participants alter-
nated whether or not they received real stimulation
first, and both experimenter and subject were blind to
this information. To allow time for both conditions,
we removed the initial fifteen minute practice period,
and participants alternated between 1-backs and 2-
backs, starting with the 1-back. For both real and
sham stimulation, participants thus completed 8 sets
of 40-second 1-back and 8 sets of 2-backs with a 20
second rest in between. In total, each condition lasted
sixteen minutes, separated by a five minute break. Be-
cause interference from hair prevented fNIRS mea-
surement in the first experiment, we placed the two
fNIRS probes on the user’s forehead. Apart from
these changes, the second experiment proceeded iden-
tically to the first.
Results: We have summarized the results of the
second experiment in Table 2, which is arranged iden-
tically to Table 1, and illustrate changes in accuracy
in Figure 2 and changes in reaction time in Figure
3. Overall, tDCS did not significantly improve ei-
ther n-back accuracy or reaction time after 10 minutes
of stimulation. However, there was a significant im-
PhyCS 2017 - 4th International Conference on Physiological Computing Systems
60
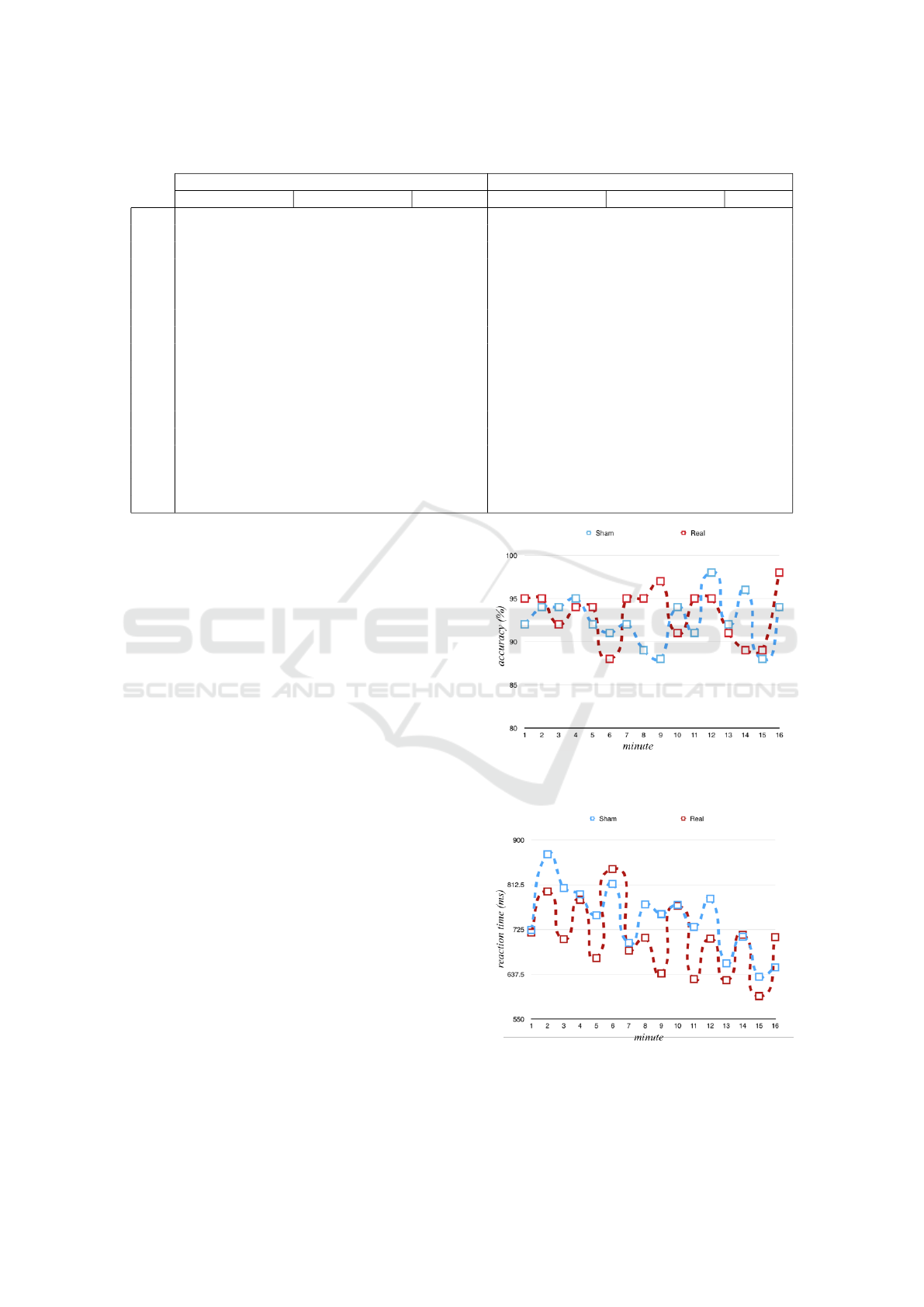
Table 2: Differences in N-Back Accuracy and Reaction Time for each Minute of Experiment 2.
Percent Accuracy Reaction Time (milliseconds)
Sham Real Sham Real
min mean std dev mean std dev p-value mean std dev mean std dev p-value
1 93 7 95 6 0.3028 716 167 719 219 0.9216
2 95 7 95 5 0.7184 851 260 799 203 0.2297
3 94 11 92 9 0.7229 799 193 706 222 0.0629
4 95 8 94 7 0.7001 770 260 783 341 0.8458
5 93 12 94 11 0.5162 731 208 669 205 0.1777
6 91 10 88 15 0.4098 793 249 843 355 0.7569
7 93 10 95 8 0.4657 684 157 684 154 0.7898
8 90 13 95 7 0.1775 751 278 709 217 0.2580
9 88 9 97 6 0.0034** 741 204 639 185 0.0323*
10 94 9 91 10 0.2456 749 230 771 313 0.9678
11 90 9 95 8 0.0631 720 177 628 234 0.0975
12 99 5 95 8 0.2519 762 299 707 277 0.2690
13 93 7 91 12 0.7966 649 157 626 193 0.5743
14 96 7 89 20 0.1930 701 211 715 270 0.9637
15 88 12 89 9 0.7260 624 144 595 155 0.2914
16 94 11 98 4 0.1944 640 198 710 314 0.4852
m 93 7 93 5 0.5049 746 185 706 223 0.3618
provement to n-back accuracy during the last minute
of stimulation. For minute 9-10, the mean accuracy
of the 1-back in the sham condition was 88% (std =
9) and the mean accuracy in the 10 minute stimula-
tion condition was 97% (s = 6) (N =13, p = 0.0034
in a paired sample t-test). At minute 9-10, improved
accuracy in the 1-back did not come at the expense of
speed. In fact, reaction times in the 10 minute stimu-
lation condition (m = 639 ms, s = 185 ms) were sig-
nificantly faster than reaction times in the sham con-
dition (m = 741 ms, std = 204 ms) (N =13, p = 0.0323
in a paired sample t-test).
Note that since 1 out of 20 tests should be signif-
icant with a threshold set to 0.05, it is hard to verify
whether variation has occurred due to chance or not.
If significance thresholds are modified according to a
Bonferroni correction, then the new threshold is 0.05
/ 16 = 0.003125 since there are 16 tests, and neither
accuracy nor reaction time are significantly better in
the stimulation condition than in the sham condition,
although accuracy at minute 9 misses Bonferonni cor-
rected significance by less than 0.0003. There are two
reasons why the results between minute nine and ten
could be regarded as more valid. First, significance
occurs at the very last minute of stimulation and not in
a more random minute during the ten stimulation min-
utes or five non-stimulation minutes. Second, the two
dependent variables exhibiting a statistically signif-
icant effect according to non-conservative statistical
thresholds refer to the same minute, which is improb-
able unless there was a true effect driving enhance-
ment at this minute, especially given the expectation
of a speed accuracy trade-off.
Figure 2: Changes in percent accuracy over time, recorded
at the end of each minute.
Figure 3: Changes in reaction time measured in millisec-
onds, recorded at the end of each minute.
Towards Bidirectional Brain-computer Interfaces that Use fNIRS and tDCS
61

4 DISCUSSION
According to these results, tDCS requires at least 9
minutes of stimulation in order to register an effect.
Whether or not effects escalate beyond 10 minutes
is an interesting investigation for future work. For
present purposes, the delayed response between stim-
ulation and effect implies that fNIRS-adaptive stim-
ulation using tDCS may not work effectively. In
the interactive application that motivated the design
of this experiment, a subject would perform a com-
puter task under the interrogation of fNIRS measure-
ment, and tDCS would apply stimulation to the user
when brain activation measures indicated that cogni-
tive workload had increased. The results indicate that
the user would need to wait at least 9 minutes before
enjoying a boost to cognition, and a brain-adaptive
deployment of the technology would therefore be ap-
plicable to tasks with a time span in this range. This
is feasible in practice, but less amenable to study in
an experimental setting.
It is not clear why it takes 9 minutes of stimula-
tion for behavioral effects to register nor whether this
limitation disappears given better settings to the de-
vice. Individual differences in skin texture, bone den-
sity, and brain structure may imply that standardized
stimulation protocols fail to appropriately customize
to any given subject. If that is the case, better set-
tings to device parameters such as intensity, polarity,
duration, and probe location could be discovered and
change based on simultaneous brain measurements
(McKendrick et al., 2015).
We envision a design in which fNIRS could mon-
itor the relative activation of the user’s task-positive
and task-negative networks, which oscillate in inverse
correlation to each other depending on whether or not
the user is sensorily immersed or in an introspective
mode of cognition (Raichle et al., 2001). The back-
and-forth activity of these networks could be moni-
tored; a bidirectional brain-computer interface might
discover how to stimulate the user’s brain in order
to maximize task-positive immersion and minimize
task-negative introspection when warranted. A first
step in this direction would be to evaluate whether
or not fNIRS detects short term neurobiological re-
actions to stimulation. We attempted such an investi-
gation in this experiment, but in the first experiment
hair prevented our device from appropriately measur-
ing the targeted F3 and F3 nodes. We note that other
fNIRS devices (such as Hitachi ETG 4000) can solve
this problem. In the second experiment, when probes
were placed approximately 3 inches from the site of
stimulation, we did not observe any obvious fNIRS
patterns separating the stimulation and real condi-
tions. However, we found no severe limitations pre-
venting the two devices from being used in concert.
Our target is an interactive system in which real-time
fNIRS measurements are used to modify the tDCS
stimulation parameters for better effectiveness. Our
experimental configurations and results present a first
step in support of such a bidirectional brain-computer
interface.
5 FUTURE WORK: ENTROPIC
BRAIN-COMPUTER
INTERFACING
Because of the lag-time between the onset of stimu-
lation and any measurable cognitive effect, research
into bidirectional brain-computer interfacing might
instead focus on stimulation modalities with a more
immediate impact on the user’s mental state. Re-
search suggests that listening to music with lyrics is
detrimental to performance on tasks that require con-
centration (Shih et al., 2012), but the conclusion is
unclear for music without lyrics, which may or may
not enhance cognition depending on task, user, and
song. As with tDCS, there likely exists some cog-
nitively enhancing stimulation procedure for a given
user although the exact procedure may vary person-
to-person and the necessary information to determine
which stimulation procedure to administer is encoded
as a physical constellation in the user’s brain.
The problems of cognitive enhancement via music
and electrical stimulation may have similar computa-
tional solutions, and should thus be studied in con-
cert. In both cases, variables controlling procedures
that output physical events to the brain (as current
or sound) need to be configured such that stimula-
tion is both safe and beneficial to the recipient. For
tDCS, these variables describe the location of electri-
cal probes applying current of a given intensity and
polarity for a duration of time. For music, the vari-
ables of interest govern a procedure for generating an
array of decibel amplitudes in the frequency domain.
The collective work of music theory describes
rules for producing harmonious sound, restricting the
large space of sound possibilities. For example, a
given sequence of sounds should be organized around
some tonic note (or frequency series) known as the
key of the song, and harmonious sounds are math-
ematically related to this fundamental frequency ac-
cording to some scale of intervals (e.g., the major or
minor scales). Engineers have encoded these rules
into digital audio workstations, enabling an opportu-
nity for auditory bidirectional brain-computer in-
PhyCS 2017 - 4th International Conference on Physiological Computing Systems
62

terfacing if the song is augmented with an interface
that allows it to branch between different versions de-
pending on implicit input from sensors measuring the
user’s physiology. For this research, we recommend
music production via the Web Audio API (Rogers,
2012) and open source javascript software that ex-
tends it (Choi and Berger, 2013; Mann, 2015) since
adaptive songs written using web tools can be played
in a browser and distributed online for use by anyone
with access to the Internet.
In (Hincks et al., 2017), we refer to information-
based bidirectional brain-computer interfacing as en-
tropic brain-computer interfacing to emphasize a use-
ful model of consciousness that relates subjective
measures of rich experience to fMRI-based measures
of system entropy (quantified as the difficulty to pre-
dict future states of the brain from previous states)
(Carhart-Harris et al., 2014). This model and the
larger enterprise of Bayesian cognitive neuroscience
suggests that a major goal of the brain is to optimally
compress and learn from sensory data. The brain
uses existing models to predict the content of sen-
sory signals, and propagates information which vio-
lates expectation up cognitive hierarchies where ex-
isting models are modified (Friston, 2010). Music -
or sound which obeys mathematical patterns - may
exist as a happy coincidence of the brain’s procliv-
ity to direct computation (and associated conscious
experience) towards stimuli which engages its predic-
tive machinery (Huron, 2006). By this reasoning, the
state of the brain and attention can be modulated by
manipulating the user-relative predictability of sound.
Several components of a system which adapts
sound to physiological measures of the brain could
be recycled to perform bidirectional brain-computer
interfacing in other modalities (e.g. electrical stim-
ulation). This generic brain optimization algorithm
hinges on non-invasive methods for detecting some
aspect of user cognition worth optimizing in all cases.
In (Hincks et al., 2017), we argue for two user dimen-
sions describing the direction (internal vs. external
origin) and intensity (high vs. low entropy) of atten-
tion and attempt to measure these states using fNIRS
and EEG. If these states are measured in real-time and
allowed jurisdiction over variables governing concur-
rent stimulation, a machine learning algorithm could
infer a relationship between state transitions and the
variables governing stimulation, so that the system
over time learned how to coerce desirable states.
ACKNOWLEDGEMENTS
We thank Tad Brunye, Erika Hussey, Leanne Hirsh-
field, Tomoki Shibata, Daniel Afergan, Beste Yuksel,
Remco Chang, Evan Peck, Angelo Sassaroli, and Ser-
gio Fantini who are students, alumnni, and professors
at Tufts University.
Research was sponsored by the U.S. Army Nat-
ick Soldier Research, Development and Engineer-
ing Center,and was accomplished under Cooperative
Agreement Number W911QY-15-2-0001. The views
and conclusions contained in this document are those
of the authors and should not be interpreted as rep-
resenting the official policies, either expressed or im-
plied, of the U.S. Army Natick Soldier Research, De-
velopment and Engineering Center, or the U.S. Gov-
ernment. The U.S. Government is authorized to re-
produce and distribute reprints for Government pur-
poses notwithstanding any copyright notation hereon.
REFERENCES
Afergan, D., Hincks, S. W., Shibata, T., and Jacob, R. J.
(2015). Phylter: a system for modulating notifications
in wearables using physiological sensing. In Inter-
national Conference on Augmented Cognition, pages
167–177. Springer.
Afergan, D., Peck, E. M., Solovey, E. T., Jenkins, A.,
Hincks, S. W., Brown, E. T., Chang, R., and Jacob,
R. J. (2014a). Dynamic difficulty using brain metrics
of workload. In Proceedings of the 32nd annual ACM
conference on Human factors in computing systems,
pages 3797–3806. ACM.
Afergan, D., Shibata, T., Hincks, S. W., Peck, E. M., Yuksel,
B. F., Chang, R., and Jacob, R. J. (2014b). Brain-
based target expansion. In Proceedings of the 27th
annual ACM symposium on User interface software
and technology, pages 583–593. ACM.
Bikson, M., Grossman, P., Thomas, C., Zannou, A. L.,
Jiang, J., Adnan, T., Mourdoukoutas, A. P., Kronberg,
G., Truong, D., Boggio, P., et al. (2016). Safety of
transcranial direct current stimulation: evidence based
update 2016. Brain Stimulation, 9(5):641–661.
Brunoni, A. R. and Vanderhasselt, M.-A. (2014). Working
memory improvement with non-invasive brain stimu-
lation of the dorsolateral prefrontal cortex: a system-
atic review and meta-analysis. Brain and cognition,
86:1–9.
Cachoeira, C. T., Leffa, D. T., Mittelstadt, S. D., Mendes,
L. S. T., Brunoni, A. R., Pinto, J. V., Blazius,
V., Machado, V., Bau, C. H. D., Rohde, L. A.,
et al. (2017). Positive effects of transcranial direct
current stimulation in adult patients with attention-
deficit/hyperactivity disorder–a pilot randomized con-
trolled study. Psychiatry Research, 247:28–32.
Carhart-Harris, R. L., Leech, R., Hellyer, P. J., Shanahan,
M., Feilding, A., Tagliazucchi, E., Chialvo, D. R., and
Towards Bidirectional Brain-computer Interfaces that Use fNIRS and tDCS
63

Nutt, D. (2014). The entropic brain: a theory of con-
scious states informed by neuroimaging research with
psychedelic drugs.
Choi, H. and Berger, J. (2013). Waax: Web audio api ex-
tension. In NIME, page 499–502.
Fitz, N. S. and Reiner, P. B. (2013). The challenge of craft-
ing policy for do-it-yourself brain stimulation. Jour-
nal of medical ethics, pages medethics–2013.
Flöel, A., Rösser, N., Michka, O., Knecht, S., and Breit-
enstein, C. (2008). Noninvasive brain stimulation im-
proves language learning. Journal of Cognitive Neu-
roscience, 20(8):1415–1422.
Fregni, F., Boggio, P. S., Nitsche, M., Bermpohl, F., An-
tal, A., Feredoes, E., Marcolin, M. A., Rigonatti, S. P.,
Silva, M. T., Paulus, W., et al. (2005). Anodal tran-
scranial direct current stimulation of prefrontal cortex
enhances working memory. Experimental brain re-
search, 166(1):23–30.
Friston, K. (2010). The free-energy principle: a uni-
fied brain theory? Nature Reviews Neuroscience,
11(2):127–138.
Gladwin, T. E., den Uyl, T. E., Fregni, F. F., and Wiers,
R. W. (2012). Enhancement of selective attention by
tdcs: interaction with interference in a sternberg task.
Neuroscience letters, 512(1):33–37.
Herff, C., Heger, D., Fortmann, O., Hennrich, J., Putze, F.,
and Schultz, T. (2014). Mental workload during n-
back task—quantified in the prefrontal cortex using
fnirs. Frontiers in human neuroscience, 7:935.
Hincks, S. W., Afergan, D., and Jacob, R. J. (2016). Us-
ing fnirs for real-time cognitive workload assessment.
In International Conference on Augmented Cognition,
pages 198–208. Springer.
Hincks, S. W., Bratt, S., Poudel, S., Phoha, V., Dennett,
D. C., Jacob, R. J. K., and Hirshfield, L. M. (2017).
Entropic brain-computer interfacing: Using fnirs and
eeg to measure attentional states in a bayesian frame-
work. In PhyCS.
Horvath, J. C., Forte, J. D., and Carter, O. (2015). Evidence
that transcranial direct current stimulation (tdcs) gen-
erates little-to-no reliable neurophysiologic effect be-
yond mep amplitude modulation in healthy human
subjects: a systematic review. Neuropsychologia,
66:213–236.
Huron, D. B. (2006). Sweet anticipation: Music and the
psychology of expectation. MIT press.
Mann, Y. (2015). Interactive music with tone.js. In Proceed-
ings of the 1st annual Web Audio Conference. Citeseer.
McKendrick, R., Parasuraman, R., and Ayaz, H. (2015).
Wearable functional near infrared spectroscopy (fnirs)
and transcranial direct current stimulation (tdcs):
expanding vistas for neurocognitive augmentation.
Frontiers in systems neuroscience, 9:27.
Medeiros, L. F., de Souza, I. C. C., Vidor, L. P., de Souza,
A., Deitos, A., Volz, M. S., Fregni, F., Caumo, W.,
and Torres, I. L. (2012). Neurobiological effects of
transcranial direct current stimulation: a review.
Moëll, B., Kollberg, L., Nasri, B., Lindefors, N., and
Kaldo, V. (2015). Living smart—a randomized con-
trolled trial of a guided online course teaching adults
with adhd or sub-clinical adhd to use smartphones to
structure their everyday life. Internet Interventions,
2(1):24–31.
Nitsche, M. A., Boggio, P. S., Fregni, F., and Pascual-
Leone, A. (2009). Treatment of depression with tran-
scranial direct current stimulation (tdcs): a review. Ex-
perimental neurology, 219(1):14–19.
Nitsche, M. A., Cohen, L. G., Wassermann, E. M., Priori,
A., Lang, N., Antal, A., Paulus, W., Hummel, F., Bog-
gio, P. S., Fregni, F., et al. (2008). Transcranial direct
current stimulation: state of the art 2008. Brain stim-
ulation, 1(3):206–223.
Piper, S. K., Krueger, A., Koch, S. P., Mehnert, J., Haber-
mehl, C., Steinbrink, J., Obrig, H., and Schmitz, C. H.
(2014). A wearable multi-channel fnirs system for
brain imaging in freely moving subjects. Neuroimage,
85:64–71.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers,
W. J., Gusnard, D. A., and Shulman, G. L. (2001). A
default mode of brain function. Proceedings of the
National Academy of Sciences, 98(2):676–682.
Rogers, C. (2012). Web audio api. s Draft [online specifi-
cation], Version, 1.
Shih, Y.-N., Huang, R.-H., and Chiang, H.-Y. (2012).
Background music: Effects on attention performance.
Work, 42(4):573–578.
Shiozawa, P., Fregni, F., Benseñor, I. M., Lotufo, P. A.,
Berlim, M. T., Daskalakis, J. Z., Cordeiro, Q., and
Brunoni, A. R. (2014). Transcranial direct current
stimulation for major depression: an updated system-
atic review and meta-analysis. International Journal
of Neuropsychopharmacology, 17(9):1443–1452.
Solovey, E., Schermerhorn, P., Scheutz, M., Sassaroli, A.,
Fantini, S., and Jacob, R. (2012). Brainput: enhanc-
ing interactive systems with streaming fnirs brain in-
put. In Proceedings of the SIGCHI conference on
Human Factors in Computing Systems, pages 2193–
2202. ACM.
Treacy Solovey, E., Afergan, D., Peck, E. M., Hincks, S. W.,
and Jacob, R. J. (2015). Designing implicit interfaces
for physiological computing: guidelines and lessons
learned using fnirs. ACM Transactions on Computer-
Human Interaction (TOCHI), 21(6):35.
Yuksel, B. F., Oleson, K. B., Harrison, L., Peck, E. M.,
Afergan, D., Chang, R., and Jacob, R. J. (2016). Learn
piano with bach: An adaptive learning interface that
adjusts task difficulty based on brain state. In Proceed-
ings of the 2016 CHI Conference on Human Factors
in Computing Systems, pages 5372–5384. ACM.
Zaehle, T., Sandmann, P., Thorne, J. D., Jäncke, L., and
Herrmann, C. S. (2011). Transcranial direct current
stimulation of the prefrontal cortex modulates work-
ing memory performance: combined behavioural and
electrophysiological evidence. BMC neuroscience,
12(1):1.
Zander, T. O., Brönstrup, J., Lorenz, R., and Krol, L. R.
(2014). Towards bci-based implicit control in human–
computer interaction. In Advances in Physiological
Computing, pages 67–90. Springer.
PhyCS 2017 - 4th International Conference on Physiological Computing Systems
64
