
An Approach to Developing Electronic Textbook for Chemical
Experiment
Taking Walden’s Inversion as an Example
Akira Ikuo,
Hayato
Nieda, Nozomi Nishitani,
Yusuke Yoshinaga and Haruo Ogawa
Department of Chemistry, Tokyo Gakugei University, Tokyo 184-8501, Japan
Keywords: CG, Visualization, Walden’s Inversion, Structure Change, Electronic Textbook, Chemical Experiment.
Abstract: We are developing electronic textbook for of basic chemistry-experiment for university students in which
reaction mechanisms are shown by computer graphics (CG). The CGs of chemical reactions was made
based on the empirical molecular orbital calculations. The CGs include following reactions as a model of
Walden’s inversion where drastic change in structure takes place, such as, formation of 2-butyl alcohol and
1-butyl bromide. The CGs could simultaneously demonstrates the nature of the reaction such as structural
change by the space-filling model and by the ball-and-stick model in addition to providing image of energy
change by the reaction profile. The electronic textbook also displays picture of apparatus and flow-chart of
small-scale experiment. Result of preliminary study on effectiveness of the CG is included.
1 INTRODUCTION
Understanding the observation of the reaction,
chemists try to explain observations by using
molecular models. Observation and molecular
models are then described by chemical equation.
Student’s difficulties and misconceptions in
chemistry are from inadequate or inaccurate models
at the molecular level (Kleinman, 1987). A
molecular structure visualized by the computer
graphics (CG) provides a deeper understanding of
molecule (Tuvi-Arad, 2006).
It is our aim to produce a CG teaching material
based on quantum chemistry calculations, which
provides realizable images of the nature of reaction
(Ikuo, 2006 and 2009). Molecular level animations
combined with video clips of macroscopic
phenomena enabled students to predict the outcome
better (Velazquez-Marcano, 2004). If the CG is
combined with textbook of chemical experiments of
student’s laboratory, students can observe the
reaction from the three thinking levels (Gilbert, 2009
and Tasker, 2010), namely, phenomena in the
observable level and the CG in the molecular level,
and chemical equation in the symbolic level. Our
ultimate goal is to produce an electronic textbook of
chemical experiment, which integrates these three
thinking levels.
Electronic textbook has several advantages over
paper textbook. For example, realistic image can be
shown by photograph or 3-dimensional CG, and
movie. These images could be, photograph of
experimental apparatus, CG of molecular structure
and CG movie of reaction mechanism. In addition,
programmable capability (for example. Singhose,
2013), hyper-link, and networking features provide
inter-active operation. Many electronic textbooks of
chemistry are found but most of them are very
similar to the paper book, and very few are related to
the chemical experiment (Morvant, 2013).
Moreover, combination of the CG movie of reaction
and experiment are not seen.
Walden’s inversion is one of typical reactions in
organic chemistry, and the reaction is often adopted
in teaching material on the curriculum of the
university, including some appropriate schemes,
which are trying to show drastic change in structure
(McMurry, 2001). The schemes should be developed
for student to acquire more realizable images of the
nature of the reaction. Reaction of hydroxide and
chloromethane is a typical example of the
Nucleophilic Substitution in the 2nd order reaction.
Carbon atom at the centre to which halogen attaches
is attacked by the nucleophile, hydroxide, from a
position 180 degrees from chlorine and then methyl
alcohol forms. We reported CG visualization of the
416
Ikuo, A., Nieda, H., Nishitani, N., Yoshinaga, Y. and Ogawa, H.
An Approach to Developing Electronic Textbook for Chemical Experiment - Taking Walden’s Inversion as an Example.
In Proceedings of the 8th International Conference on Computer Supported Education (CSEDU 2016) - Volume 2, pages 416-420
ISBN: 978-989-758-179-3
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
reaction as a simple model of Walden’s inversion
and the produced CG in the tablet PC effectively
provide information about the nature of the reaction,
such as drastic structural change (Ikuo, 2012).
This paper introduces our works of CG
visualization of formation of 2-butyl alcohol and 1-
butyl bromide for realizing certain images of the
reaction mechanism of Walden’s inversion, which is
aiming at development of the electronic textbook for
chemical experiment of student’s laboratory at the
university and trying to integrates the observable
level experiment and the molecular world of the
Walden’s inversion.
2 METHOD
2.1 Developing Experimental Program
and Textbook
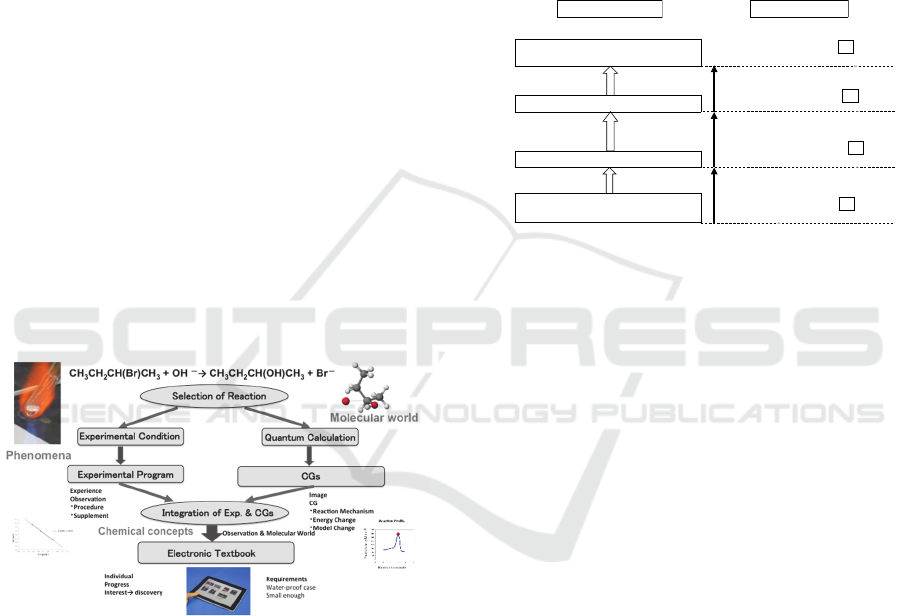
Flow chart adopting a policy (Ikuo, 2015) of
developing an electronic textbook for chemical
experiment is shown in the Scheme 1. Reaction was
selected based on importance in fundamental
chemistry. To exhibit phenomena (left side),
experimental condition was optimized for college
level small-scale chemistry-experiment.
Scheme 1: Flow chart of developing experimental
program and textbook.
The experimental program is made based on the
above-mentioned policy. The attainment targets and
contents of an experimental program are shown in
the Scheme 2. The reaction to which methyl alcohol
is generated from methyl chloride is studied with
CG teaching material as a typical example of the
nucleophilic substitution in the 2nd order reaction. A
learner is expected to grasp a three-dimensional
image of Walden’s inversion in STEP1. The
experiment that forms 1-butyl bromide from 1-butyl
alcohol with simple experiment apparatus is
conducted and the reactant and the product are
confirmed by the infrared spectroscopy. A learner
studies an actual reaction of Walden's inversion by
this unit in STEP2. In addition, the reaction that
forms 1-butyl bromide done by the experiment is
studied with CG teaching material to obtain
molecular image of the reaction. A learner is
expected to acquire an actual image of the reaction
in STEP3. The reaction to which 2-butyl alcohol is
generated from 2-butyl bromide and the nucleophilic
substitution is studied with CG and a learner is
expected to integrate observation and molecular
world of Walden’s inversion in STEP4.
Scheme 2: The attainment target and contents of an
experimental program.
2.2 Creating CG based on Quantum
Chemistry Calculation
Structures of intermediates on reaction were
calculated as follows: the semi-empirical molecular
orbital calculation software MOPAC (Stewart, 1989)
with PM5 Hamiltonian in the CAChe Work System
for Windows (Former name of SCIGRESS, ver.
6.01, FUJITSU, Inc.) was used in all of calculations
for optimization of geometry by the Eigenvector
following method, for search of transition state by
use of the program with saddle point search, and for
search of the reaction path from the reactants to the
products via the transition state by the intrinsic
reaction coordinate (IRC) calculation (Fukui, 1970).
A single absorption peak in the imaginary region
was confirmed by -134.36 cm
-1
in the reaction of 1-
butyl bromide formation and -327.28 cm
-1
in the
reaction of 2-butyl alcohol formation. Structure
changes of intermediate at the transition state were
confirmed. The structures of the initial state, the
transition state and the final state were obtained by
the IRC calculation as shown in Figure 1 and 2.
The Gibbs energies and the inter-atomic distances
obtained by the calculation were in good agreement
with the literature values. Energy changes during
reactions were confirmed. Therefore, it was
concluded that the reaction path and the molecular
Actual reaction of Walden's inversion
Grasp a thr ee-dimensional image of
Walden’s inversion
I ntegrate observation and molecular
world of Walden’s i n v er si on
Acquire actual image of the reaction
Attainment target
Contents of study
For mati on M et hyl alcohol
(Reaction of methyl chloride and nucleophilic substituent)
CG
CG
CG
Exp
STEP 1
STEP 2
STEP 3
STEP 4
Formation 2-Butyl alcohol
(Reaction of 2-butyl alcohol and nucleophilic substituent)
Formation 1-Butyl bromide
(Reaction of 1-butyl alcohol and nucleophilic substituent)
Formation 1-Butyl bromide and the
product are confir med by I R
(Experiment of form 1- butyl bromide from 1-butyl alcohol)
An Approach to Developing Electronic Textbook for Chemical Experiment - Taking Walden’s Inversion as an Example
417
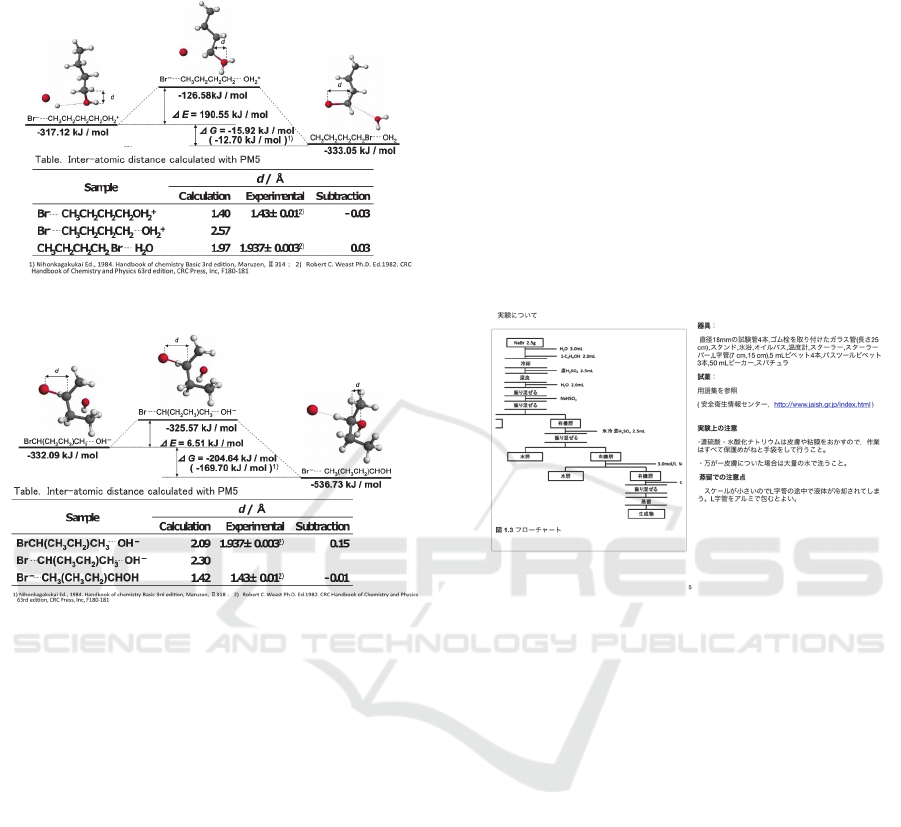
geometry obtained by the calculation were
appropriate for making the CG teaching material.
Figure 1: Results of formation 1-butyl bromide.
Figure 2: Results of formation 2-butyl alcohol.
2.3 Creating CG Teaching Material
and Electronic Textbook
A movie of the reaction path was produced by the
software DIRECTOR (ver. 8.5.1J, Macromedia,
Inc.) following the display of the bond order of the
structure of the reactants in each reaction stage,
which was drawn by the CAChe. The obtained CG
of the ball-and-stick and space filling models were
combined with reaction profile of the corresponding
reaction stage. It was confirmed that the drawn CGs
of the molecular models of reactants moves
smoothly. The ball, which indicates progress of the
reaction, was arranged on the reaction profile and
simultaneous movements of the ball and the
reactants were confirmed. Rotational models were
made with PyMOL (ver. 1.7, Schrödinger). Created
movie file was converted to the Quick Time movie
for iPad by the Quick Time PRO (ver. 7.66, Apple,
Inc.). Electric textbook was produced with iBooks
Author (ver. 2.4, Apple, Inc.) and was saved to iPad
(Apple, Inc.) by using the iTunes (ver. 12.3, Apple,
Inc.).
3 RESULTS AND DISCUSSION
3.1 Feature of Electronic Textbook
CG teaching materials of the Walden’s inversion
were combined with chemical experiments of
student’s laboratory for the purpose of making
electronic textbook of basic chemistry to provide
experiment at the observable-level, CG visualization
at the molecular-level, and chemical equation at the
symbolic-level.
The electronic textbook was inserted with images
of experimental procedure of the flow chart (Figure
3) and slides of photographs (Figure 4), which can
be enlarged by students touch.
Figure 3: Enlargeable flow chart of experimental
procedure.
CG teaching materials of reaction profiles in
both models, the space filling and the ball-and-stick,
were also inserted (Figure 5). The CG shows the
reaction profile, which demonstrates the degree of
the reaction progress by the ball indicating the
potential energy vs. the reaction coordinate. When
student touches the CG teaching material in the
tablet computer, the teaching material appears to
show image of the structural change during the
reaction. If student touches the material again, the
Quick Time control bar appears and the red ball on
the profile can move by student’s choice. Student
can manipulate the reaction back and forth until they
obtain the image of the reaction. A student is
expected to obtain the image of an umbrella reverse
like motion in Walden’s inversion.
If student wants to watch the model from different
angle, one could rotate the model (Figure 6) by
touching the CG.
CSEDU 2016 - 8th International Conference on Computer Supported Education
418

Figure 4: Slide Picture of apparatus.
Figure 5: CG teaching materials.
Figure 6: Rotational models.
3.2 Practice of Teaching Material
The CG Teaching material was practiced on 5
students, the second and third year students of
teacher training course at Tokyo Gakugei
University. Teaching material used for the trial was
the CG movie shown by the tablet computer. Pre and
Post survey about image of Walden’s inversion
reaction were conducted.
After watching CG teaching material, student
image of the structure change was improved.
Students described their comments in the free
description section of the questionnaire, such as,
“With image, it was easier for me to understand the
way of inversion,” and “I could see the reaction
mechanism and progress by the movie.” These
comments suggested that the teaching material was
able to provide image of the inversion. Another
student commented “It was good because I could
rotate the molecule.” This comment suggested that
the rotational model helped student to obtain
structure of molecule.
Although more study need to be done on the
effectiveness of the electronic textbook, students
were able to obtain image of the Walden’s inversion.
4 CONCLUSIONS
We developed computer graphics teaching material
for university student, concerning reaction with
drastic change of the structure of reactants in
following reaction as example of Walden’s
inversion; formation of 2-butyl alcohol and 1-butyl
bromide. The CGs could demonstrate the drastic
change of the structure and the reaction profile can
provide image of energy change during the reaction.
The textbook could display picture of apparatus and
flow-chart of experiment in addition to CGs.
Preliminary study on effectiveness of the CG
suggested that students were able to obtain image of
the Walden’s inversion. The developed electronic
textbook in the tablet could be used to integrate the
observable level experiment and the molecular
world.
ACKNOWLEDGEMENTS
This work was supported by JSPS KAKENHI Grant
Numbers 25350188, 26350227.
REFERENCES
Fukui, K., 1970. A Formulation of the Reaction
Coordinate, J. Phys. Chem., 74, 4161-4163.
Gilbert, J. K., Treagust, D. F., 2009. in Gilbert, J. K.,
Treagust, D. (eds.), “Models and Modelling in Science
Education Vol. 4 Multiple Representations in
Chemical Education”, Springer, 333-350.
Ikuo, A., Yoshinaga, Y., Ogawa, H., 2015. Development
of Electronic Textbook for Chemical Experiment -
An Approach to Developing Electronic Textbook for Chemical Experiment - Taking Walden’s Inversion as an Example
419

Taking esterification as an example -, Proc. CSEDU
2015, Vol.2, 553-557.
Ikuo, A., Ikarashi, Y., Shishido, T. and Ogawa, H., 2006.
User-friendly CG visualization with animation of
chemical reaction: esterification of acetic acid and
ethyl alcohol and survey of textbooks of high school
chemistry, Journal of Science Education in Japan, 30
(4), 210-215.
Ikuo, A., Nagashima H., Yoshinaga Y., and Ogawa H.,
2009. Calculation of potential energy in the reaction of
“I + H2 → HI + H” and its visualization, The
Chemical Education Journal (CEJ), Registration #13-
2.
Ikuo, A., Nishitani, N., Yoshinaga, Y., and Ogawa, H.
2012. Development of teaching material in tablet PC
based on computer graphics by quantum chemistry
calculation – Walden’s inversion -, Proc. The 20th
Intern. Conf. on Computers in Education (ICCE), pp.
418-423.
Kleinman, R. W., Griffin, H. C., Kerner, N. K., 1987. J.
Chem. Edu., 64, 766-770.
Morvant, C. M, Halterman, R.L., 2013. “Organic
Chemistry Laboratory Manual”, iBooks Store.
McMurry, J., 2001. “Organic Chemistry”5
th
ed., Tokyo
Kagaku Dojin, pp.367-381.
Nihonkagakukai Ed., 1984. Handbook of chemistry Basic
3rd edition, Maruzen, 314.
Singhose, W., Donnell, J., 2013. “Introductory
Mechanical Design Tools”, iBooks Store.
Stewart, J. J. P., 1989. Optimization of parameters for
semi empirical methods I. Method, J Comp. Chem., 10
(2), 209–220.
Tasker, R., Dalton, R., 2010. in Gilbert, J. K., Reiner, M.,
Nakhleh, M. (Eds.), “Models and Modelling in Science
Education Vol. 3 Visualization: Theory and Practice
in Science Education”, Springer, 103-131.
Tuvi-Arad, I. and Blonder, R., 2006. Continuous
symmetry and chemistry teachers: learning advanced
chemistry content through novel visualization tools,
Chem. Educ. Res. and Pract., 11(1), 48-58.
Velazquez-Marcano, A., Williamson, V. M., Ashkenazi,
G., Tasker, R. F., and Williamson, K. C., 2004. The
use of video demonstrations and particulate animation
in general chemistry, J. Sci. Educ. and Tech., 13(3),
315-323.
Weast, Robert, C. Ph.D. Ed.1982. CRC Handbook of
Chemistry and Physics 63rd edition, CRC Press, Inc,
F180-181.
CSEDU 2016 - 8th International Conference on Computer Supported Education
420
