
How to Cross the Border from R to D?
The Example of Conception of New Medical Devices
Lionel Pazart
INSERM CIC 1431, Besançon University Hospital,
Place Saint Jacques, 25030 Besançon cedex, France
Keywords: Translational Research, Medical Device, Innovation, R&D, Clinical Trials.
Abstract: The border between Research and Development for a new medical devices is often unclear since the process
of development of a new medical device remains non linear, with the need of feedback from trials in clinical
situation to new conception of the product. More importantly, the classification of the different steps of a
project impacts on 1/the identification of right partners for the project, 2/ state aid intensities, generally
lower for activities linked to development than for research related activities 3/impact factor of publication
related to the phase of the project. Sometimes researchers under-estimate these studies because it is thought
that, although essential to set-up new investigation tools, they do not lead to an increase of fundamental
knowledge. However, and especially in the field of medical devices, users have to face specific difficulties
due to the variability of the biological systems under study. Results obtained in translational research often
depend on this variability and new questions or scientific obstacles arise from the confrontation to the real
world. In order to address these new challenges, reverse translational research is required. Fundamental
research is then fed from the results of translational research. In this position paper, we would like to present
a useful model of medical device development through several examples of translational research to
illustrate the adequacy of research to bridging fundamental research results to the closest to the patients.
1 INTRODUCTION
Basic research rarely knows what discovery will
serve and disruptive innovations in health mostly
come from basic research whose authors have not
suspected the consequences (for instance, the
discovery of electron spin in 1922 to the MRI in
70’s). At the opposite, applied research is primarily
directed towards a specific practical objective (for
instance the long story to capture and preserve
images began with the Egyptians some ten thousand
years ago when they noted the ability of light to
transmit images). Between these both enemies’
brothers, experimental development is a systematic
work, using knowledge gained from basic research
and/or practical experience, which is directed to
produce new products or to improve substantially
those already existing. To transform basic research
results into a practical innovation, translational
research needs big efforts to conceive future
application, and requires thinking differently and
changing our mind. Research and Development for a
new medical devices is often unclear since the
process of development of a new medical device
remains non linear, with the need of feedback from
trials in clinical situation to new conception of the
product. Sometimes basic researchers neglect these
further studies because it is thought that, although
essential to set-up innovative technologies, they do
not lead to an increase of scientific knowledge.
However, and especially in the field of medical
devices, users have to face specific difficulties due
to the variability of the biological systems under
study. Variability is easily understood from one
patient to another one. But there is also the
variability of a single patient whose metabolism
evolves naturally by the time and additionally with
the therapeutic actions. Translational research has to
understand these variability and new basic research
questions arise from the confrontation to the real
world. In order to address these new challenges,
reverse translational research is required.
Fundamental research is then fed from the results of
translational research.
In this overview, we would like to present a
useful model of translational research for medical
Pazart L.
How to Cross the Border from R to D? - The Example of Conception of New Medical Devices.
DOI: 10.5220/0006803100010001
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIOSTEC 2015), pages 7-12
ISBN: 978-989-758-071-0
Copyright
c
2015 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

device development through several examples to
illustrate the adequacy of research to bridging
fundamental research results to the closest to the
patients.
2 CLASSIFICATION OF
RESEARCH ACTIVITIES
The Frascati Manual
1
defines R&D as “creative
work undertaken on a systematic basis in order to
increase the stock of knowledge”. The term R&D
covers in fact three activities: basic research, applied
research and experimental development.
Basic research is experimental or theoretical work
undertaken primarily to acquire new knowledge of
the underlying foundation of phenomena and
observable facts, without any particular application
or use in view.
Applied research is also original investigation
directed primarily towards a specific practical aim.
In technical fields, applied research could be
associated with the ‘industrial research’ for
developing new products or processes.
Experimental development is systematic work,
drawing on existing knowledge gained from
research and/or practical experience, which is
directed to producing new materials, products or
devices, to installing new processes, systems and
services, or to improving substantially those already
produced.
For example, the determination of the amino acid
sequence of an antibody molecule would be basic
research. Investigations undertaken to identify the
right antibody for a specific membrane protein of a
virus would be applied research. Experimental
development would then consist of designing a
biochip functionalized with the right antibody for the
disease on the basis of knowledge of its structure
and clinically testing with biological liquid of
interest (blood, urine, etc.) in order to make the right
diagnosis.
Translational Research involves, for the National
Institutes of Health (NIH), “the extensive body of
work required to move a discovery from bench to
bedside” and Wikipedia definition insists on the
capacity of translational research to shorten the time-
frame to reach the market. Accordingly to these
definitions, translational research covers applied
research and experimental development till the
market launch of the product. Some others add in
healthcare fields the feedback loop “from the bed to
the bench”
3 CHALLENGES OF R&D ON
MEDICAL DEVICES
With the great diversity of the medical devices from
crutches to programmable pacemakers it is not
feasible to subject all medical devices to the same
development scheme. Much specificity of MDs vs
drugs should be taken into account
2
, and
methodological adaptation could be performed to try
to exercise discretion to propose a feasible
methodology:
• Clinical investigation is particularly needed to get
CE mark for class IIb and III MDs
3
. First tests in
human need to answer to essential requirements and
to assume the safety of the device by in vitro, on
bench and in vivo (animals) tests. For the other ones
(ex: glove, eyes occlusion plasters, conductive gels,
non-invasive electrodes, image intensifying screens)
predictability of performance could be a useful
manner to answer the question.
• The interest of product may concerns either
therapeutic, diagnostic, or compensation of disability
area and the methodology of clinical assessment
should therefore be adapted classically to the main
objective of the study. For instance, clinical trials of
diagnostic tests are sometimes divided into
exploratory phases, challenge phases and advanced
phases to see how effective and how accurate the
tests are
4
. In all cases, a distinction should be made
between the clinical proof of efficacy and safety in
order to get the market approval and the place in the
diagnostic or therapeutic armamentarium in order to
define the price or the reimbursement of a MD. In
the latest intent, randomized controlled trials are
generally conducted to compare a new intervention
or strategy to the classical one.
• The level of innovation; should the new MD be
considered as an incremental evolution or an
evolution of rupture ? Minor or incremental changes
on an existing medical device are the most frequent
type of innovation activity in companies. Activities
leading to minor, incremental changes or adaptations
should in principle not be counted as R&D activities,

unless they are part of, or result from, a formal R&D
project in the firm.
• The equivalence with a predicate; substantial
equivalence means that the new device is at least as
safe and effective as the predicate
5
. This concept can
be applied to many products including high-risk
products, such as coronary stent or hip prosthesis.
To prove the equivalence, technical bench tests and
preclinical study could be done. Production of
specific clinical data could be limited to a cohort
study in order to retrieve the similar results of
predicate. However, this applies only if the
equivalence criteria are not affected claim, clinical
and technical data and environment.
• The operator/MD interaction; the clinical benefit
may depend not only on MD itself but also the
performance of the medical team (operator
dependent nature, learning curve) and the technical
platform, this organizational dimension is an
element which must be taken into account in the
early investigations of a new MD; trials should
incorporate this learning curve by providing a first
acquisition phase, in the number of subjects required
for example, and / or any interim analyzes. Another
possibility is to use a sequential adaptative Two
stages design (i.e Fleming methods)
• The diversity of use; one or more studies are
needed to develop the implementation of a new MD
and describe different operator (medical staff or the
patient himself), operating times, the technical
facilities and personnel skill to the success of the
procedure.
• The reduced life cycle. The clinical assessment
should be realized in short-term monitoring, on
technical and clinical intermediate parameters.
Nevertheless, a long term monitoring should be
performed till failure occurrence for all patients who
were implanted with an old version of MD
(particularly for implantable devices like cardiac
prosthesis, breast implants, cochlear implants etc.).
• The small size of target population. Of particular
methodological solutions can be proposed:
conducting multicentre clinical trials in Europe
(within ECRIN network for instance), or exhaustive
survey of patients through national or international
register.
• The short track of development; a lot of MDs could
be developed with few technical experimental tests
to get the Proof of Concept without clinical test, like
for instance dental impression materials, tubes used
for pumping the stomach, urinary catheters intended
for transient use etc. For other MDs category, the
absence of an animal model to test preclinical MD
and the futility to test it on healthy volunteers
contribute to go quickly to the patient, for instance
for hip prosthesis or implantable analgesic pump.
4 PRACTICAL SITUATIONS OF
TRANSLATIONAL RESEARCH
4.1 “Optical Biopsy”
Invasive biopsy is still today the reference diagnostic
technique of a lot of skin or mucosa pathologies
(inflammation, tumours). Nevertheless, several
situations of diagnosis should be kept as
conservative as possible. Consequently, non-
invasive imaging methods (ultrasounds, computed
tomography, magnetic resonance imaging) have
been developed for clinical use. Based on the
principle of white-light interferometry and
developed initially in 1991 for in-vivo imaging of
the human eye
6
, OCT was investigated by a large
number of groups worldwide. With regards to
penetration depth and resolution, OCT could be a
perfect trade-off between ultrasound and confocal
microscopy. The use of optically pumped based on
specific swept sources for OCT was first
demonstrated in 2011 but since that time, the
threshold towards the use of low-cost electrically-
pumped devices is still not crossed.
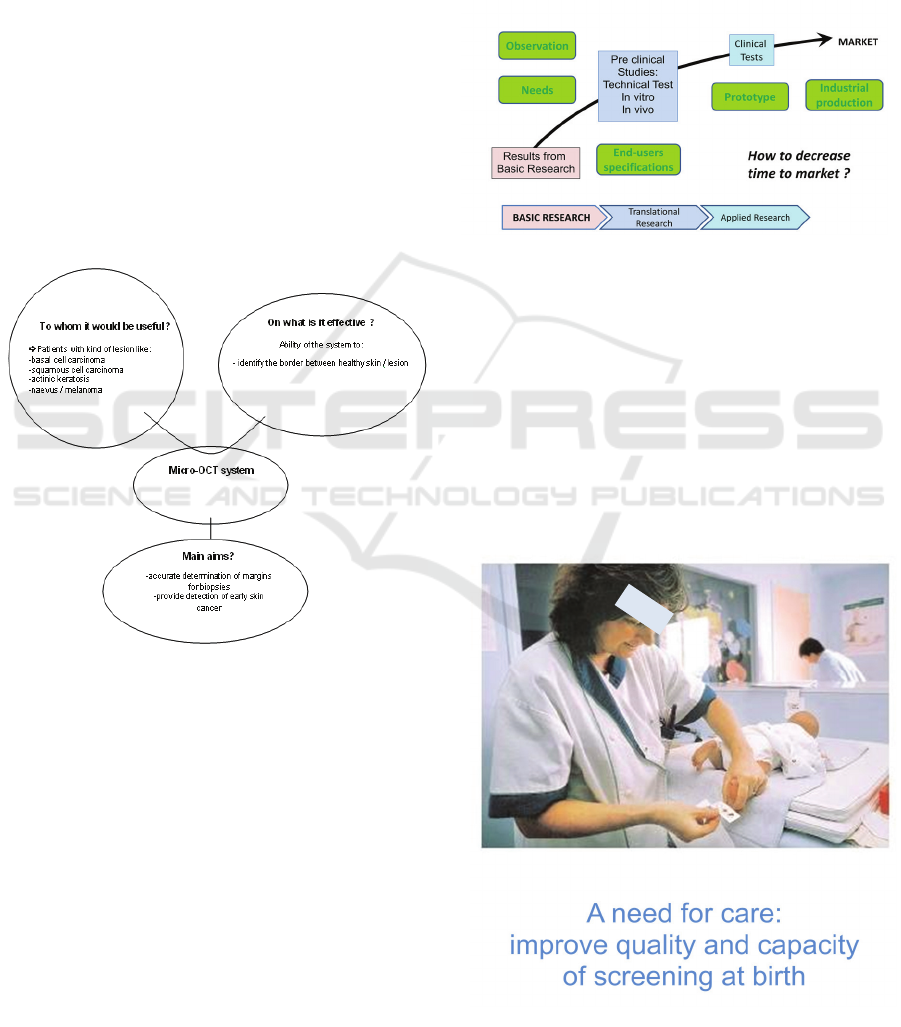
How to translate the basic knowledge to a
practical application in healthcare?
A first way could be to fix the possible
application fields (for instance skin biopsy) and ask
the specialists (here university dermatologists) about
the possible clinical use with the technical
characteristics of the future device concerning the
spatial resolution of the system, the field of view and
imaging magnification. The design parameters will
be selected according to the system specifications
and technological constraints, for instance a
miniature (< 15cm3), low cost OCT imager
providing cross-sectional 3-D tomograms with a
depth around 0.5 mm, axial and transverse
resolutions of 5 µm and imaging field of 5x5 mm2.
Of course, specialists could imagine possible clinical
applications
7
such as superficial baso-cellular
cancer, follow up healing after an injury or surgery,
assessment of new wound dressing or graft,
determination of the degrees of skin burns, the local
efficacy and tolerance of topical treatment etc. But
the usefulness of such an OCT imager remains
questionable, and clinicians are doubtful of pictures
interpretation since they have no experience
feedback about such imaging. The learning curve is
for the moment very slow with those new technics
(new images, new colors, new field of view…),
which is currently a real limiting factor for the
diffusion of those technologies.
A second approach could be clinical use based
specifications. First of all, dermatologists are invited
to express their will. In this way, they claim for a
new device able to provide detection of early skin
cancer by discerning diseased and healthy skin, and
helping the practitioner to accurately determine the
margins for resection, which is usually affordable by
the examination of the overall architecture of
epidermis and identifying the number of atypical
cells per unit of area.
Figure 1.
Thus, the parameters of new OCT microsystem
design have to be determined by examining the
biomedical application requirements as well as the
instrumental characteristics of selected
interferometric architecture including array-type as
well as high-speed camera requirements. The
Medical ISO13485 methodology requires also a
Risk Analysis of the final product. It must be
initiated with every participant and especially the
future users. A Functional Analysis can then
describe what we expect from the MD and split it in
building blocks.
Current works are trying to improve the
accuracy, resolution, penetration depth of these
devices. Manufacturers and researchers should focus
their insights on the easiness of recording,
measuring and analyzing, the daily practice in doctor
office, their reliability, and the prize.
In summary, the best way could be analyzing the
constraints of available techniques, defining the
needs from the end-user (medical) point of view and
adapting research program to conciliate both
requirements. The following scheme tries to
represent this approach:
Figure 2.
4.2 Screening at Birth
Routine screening by capillary blood sample at birth
concerns several diseases in France. The lateral edge
of the feet was chosen as sampling area by scientific
societies, on an anatomical removal of the main
neurovascular bundles and to avoid the risk of
osteomyelitis of the calcaneus, previously found
with bites to the posterior heel. The method is
painful for the newborn and quantitative failure
often leads to sample a second time.
Figure 3.
How to improve the quality and the capacity of
screening at birth, particularly to reduce newborns’
pain?
The first way consists to search available
techniques on the shelves, then to try to adapt them
to the need.
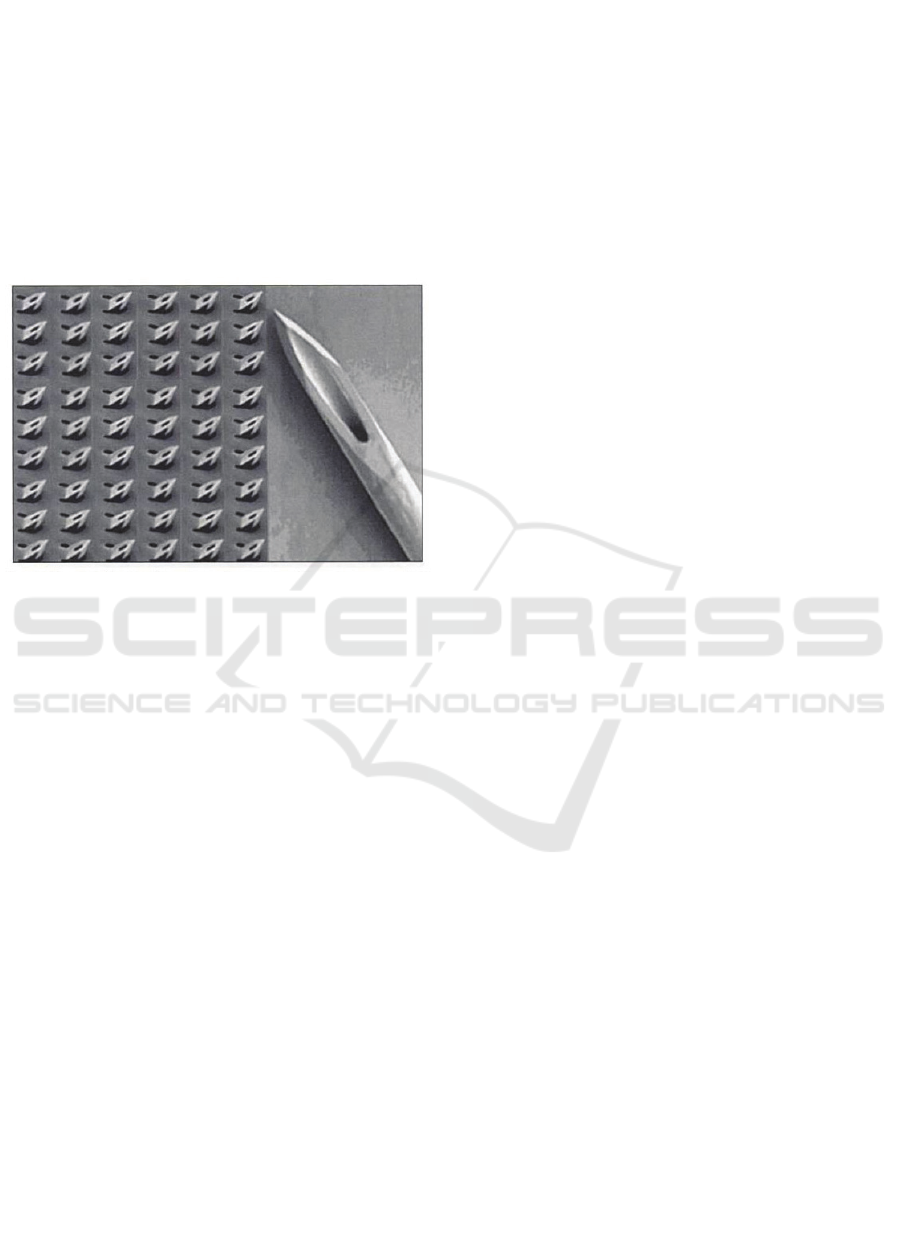
Micro-needles array appears to be a good solution to
replace the lancet (see picture). This matrix would
be applied on the heel as a patch, the multitude of
micro-needle (deemed not painful) replacing the
wide blade of the lancet.
Figure 4.
But a lot of questions emerge to adapt this
technology to the heel of newborns:
¾ How deep to prick ?
¾ Which density of needle should be
compose the network?
¾ Which size for the channel, if any ?
A better understanding of the distribution of
capillary networks could improve the specifications
for a new device based on micro-needles array. To
acquire these data, we conducted a clinical study
using ultrasound (device Dermacup Atys) and
videocapillaroscopy (device Moritex, MS-500C
Micro-Scopeman) on both sides (lateral and medial
edges) of the heel of 62 newborns according to
gestational age at birth. The parameters of the
microcirculation were obtained by ultrasonography
(depth of dermis) and capillaroscopy: capillary
density and distribution, inter-capillary distance and
average diameter of the capillaries. The results show
that on average, the density of the capillary network
is 60 capillaries / mm2, the inter-capillary distance
of 155 microns and a diameter of 22 microns.
Another result shows that the capillary network is
oriented mainly parallel to the lateral edge of the
foot and less on the medial edge.
From our study, the results of capillaroscopy and
skin ultrasound will help determine the right micro-
needles array configuration as follows:
¾ The area provided for the needle plane is 25
mm², and the number of micro-needles
depend on the density of capillaries and of
the inter-capillary distance
¾ The depth of the dermis specified the
maximum depth of the micro-needles.
After some prototypes adaptation, we performed
several tests on animals. The results show that a
network of 8 micro-needles could be acceptable, and
avoid any “fakir effect” of the skin. But these micro-
needles must penetrate about 1 mm in the heel of the
newborn and three applications of the matrix are
needed to achieve a 96% probability of blood
collection. Under these conditions, it is difficult to
talk about withdrawal without pain.
A second way to solve the problem of pain consists
firstly to understand the mechanisms of this
painful process to provide input for improvement in
terms of medical devices and also to explore new
avenues for screening at birth.
A systematic clinical observation of blood collection
steps at the 72 th hour of life was conducted on a
sample of 50 newborns (PREVMAL study). The
purpose of this observation was to get confirmation
of the frequency of pain and when it appears and on
another hand to understand the factors behind its
occurrence on which further action could be taken.
The anatomical data from videocapillaroscopy and
ultrasound were also collected to be correlated with
results of observation of the act of screening in terms
of pain (DAN scale) and quantity of blood obtained.
89% of newborns have expressed a pain
8
. It
appeared that the pain is mainly observed when
pressure is applied on the newborn's heel to collect
the blood on the blotter paper and secondarily at the
heel prick with the lancet. So, the most painful is not
the bite but the pressure of the foot (p = 0.0005). The
pain at the sting of the lateral edge appears less
important than the sting of the medial edge, but this
result should be confirmed by a more powerful study
with more cases. No correlation was found between
pain and deep dermis, or density, or the diameter of
the capillary.
Considering these results, we were committed to
finding new methods of blood collection,
particularly to avoid pressure on the heel and the
proliferation of bites to get the blood in sufficient
quantity on the blotter paper. We have therefore
developed a system provided with a micro-machined
nozzle. This tip is applied to the heel, after bite of

the lancet. The blood viscosity properties and the
geometry of the tip make possible to maintain the
blood captive inside a reservoir. This tip is then
stamped on the blotter paper. Tests have shown that
a volume of less than 800 µl of blood sufficient to
properly soak the spaces provided on the blotter
paper.
From clinical needs to fundamental research, this
approach could be summarized as follow:
Figure 5.
To go on with the development of the new device, it
is planned to conduct clinical trials on parallel
groups for ethical reasons, with prototypes and
classic screening methods.
5 CONCLUSION
To conclude, in this position paper we considered
examples of the conception of MD either based on
technology availability or clinical use requirements.
In both approaches, identification of clinical useful
technical characteristics are critical issues to faster
the development of new medical device. As
mentioned in the introduction of the paper,
translational research activities are sometimes under-
estimated and postponed because they do not lead to
an increase of “scientific” knowledge.
However, translational research covers applied
research and experimental development and it is
essential to set-up new tools especially in the field of
medical devices. It isn’t only a question of semantic
as illustrated few years ago in a similar congress, but
a way of thinking.
If we design a clinical study to describe the
capillary network of newborn, without any objective
other than knowledge, this study should be qualified
of basic research. If the same study is intended to
complete technical specifications for a new
screening device, it becomes applied research. The
translation is there, behind the aims of the study
protocol: what might be the usefulness of study
results? Basic Researchers should be aware of this
new paradigm, even if they haven’t to focus their
attention on application anymore. Louis Pasteur
perfectly summarized this necessary connection:
“There is not basic research on a side and applied
research on the other. There are research and
applications thereof, united to each other as the fruit
of the tree is joined to the branch that has worn.”
ACKNOWLEDGEMENTS
The studies presented in this paper are funded by
public grants, under the European Commission's 7th
Framework Program, or French Health Ministry and
French National Agency for Research.
REFERENCES
OECD, Frascati Manual 2002: Proposed Standard Practice
for Surveys on Research and Experimental
Development, The Measurement of Scientific and
Technological Activities, OECD Paris.
Parquin F, Audry A and Giens XXVII N° 1 group
members. Constraints and specificities of the clinical
evaluation of medical devices. Thérapie 2012 Juillet-
Août; 67 (4): 301–309
Annex IX from 93/42/EEC concerning medical devices as
amended by Directive 2007/47/EC
ISO 14155:2011 Clinical investigation of medical devices
for human subjects - Good clinical practice
Food and Drugs Administration, Premarket Notification
(510k)
Fujimoto J. et al., Biomedical Optical Imaging, Oxford
University Press, 2009.
Sattler E, Kästle R, Welzel J: 2013 Optical coherence
tomography in dermatology, J. Biomed. Opt. 18(6),
061224
Robieux C., Sainthilier J.M., Vidal C. & al. Study of the
correlation between the structure of the capillary
network of the heel of newborns and pain during
screening at birth, J Invest Dermatol 2011, 131:2144
