Towards Quasi-continuous Heart Rate Variability Estimation
using a Patch Type Electrocardiogram Recorder
Dorthe B. Saadi
1
, Esben Ahrens
2
, Helge B. D. Sorensen
2
, Henning Langberg
3
and Karsten Hoppe
1
1
DELTA Danish Electronics, Lights & Acoustics, Venlighedsvej 4, 2970 Hørsholm, Denmark
2
Department of Electrical Engineering, Technical University of Denmark,
Ørsteds Plads Building 348, 2800 Kgs. Lyngby, Denmark
3
Department of Public Health, University of Copenhagen, Henrik Pontoppidansvej 4, 1014 Copenhagen K, Denmark
Keywords: Heart Rate Variability, Patch ECG Recorder.
Abstract: Changes in different heart rate variability (HRV) measures have been found to possess predictive
information in patients with many different diseases, e.g. myocardial infarction, diabetic neuropathy, and
patients at risk of developing sepsis. At the same time, the emerging of patch type electrocardiogram
recorders facilitates new possibilities for long-term monitoring, real-time data analysis, and wireless
transmission of clinically relevant parameters, e.g. short-term HRV measures. This information might in the
future assist the healthcare professionals in timely notification of changes in the risk stratification profile
obtained from the HRV measures. The purpose of this study is therefore to investigate the possibilities for
quasi-continuous estimation of reliable HRV measures using the ePatch heart monitor. We compared the
physiologically true values of 11 selected HRV measures with the values obtained using automatically
generated RR series from electrocardiograms recorded with the ePatch using four different sampling
frequencies (128 Hz, 256 Hz, 512 Hz, and 1024 Hz). We found no significant differences between neither
the mean nor the median values of the obtained HRV measures for any of the sampling frequencies. This is
very promising for the future application of the ePatch for quasi-continuous monitoring of HRV measures.
1 INTRODUCTION
The application of different heart rate variability
(HRV) measures has become increasingly popular as
a non-invasive clinical estimate of the state of the
autonomic nervous system. One of the major
application areas is risk stratification in cardiac
patients, e.g. patients with myocardial infarction,
congestive heart failure, and left ventricular
dysfunction (Huikuri & Stein, 2013). Other
promising clinical areas that might highly benefit
from risk stratification based on HRV measures
include general health management, patients at risk
of developing sepsis, patients with diabetic
neuropathy, and critically ill intensive care patients
(Buchan et al., 2012; ESC and NASPE, 1996). Many
of these application areas might benefit from
continuous estimation of short-term HRV measures.
Together with reliable continuous estimation of
other vital sign parameters, this might provide a real-
time overview of a potential change in the clinical
condition of the patient.
The emerging of patch type electrocardiogram
(ECG) recorders with embedded processing
capability opens the possibility for this type of
continuous monitoring. One of these patch ECG
recorders is the ePatch designed by DELTA. The
currently CE marked and FDA approved version of
the ePatch stores the recorded ECGs internally for
offline analysis for up to 72 hours. The ePatch
consists of a reusable sensor and a disposable patch.
The patch contains three internal measuring points
that allow the recording of two bipolar ECG
channels without the use of any cables. The ePatch
system is further described in (Saadi et al., 2013)
and (Saadi et al., 2014).
One of the possible future HRV feedback loops
is schematically illustrated in Figure 1. The ePatch
sensor is expected to perform real-time embedded
detection of each QRS complex. The obtained RR
interval curve might then be wirelessly transmitted
to a smart phone or a central monitoring station.
This device could then automatically calculate
preselected clinically relevant HRV measures. This
would allow close to real-time feedback on potential
20
Saadi, D., Ahrens, E., Sorensen, H., Langberg, H. and Hoppe, K..
Towards Quasi-continuous Heart Rate Variability Estimation using a Patch Type Electrocardiogram Recorder.
In Proceedings of the 3rd International Congress on Cardiovascular Technologies (CARDIOTECHNIX 2015), pages 20-29
ISBN: 978-989-758-160-1
Copyright
c
2015 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
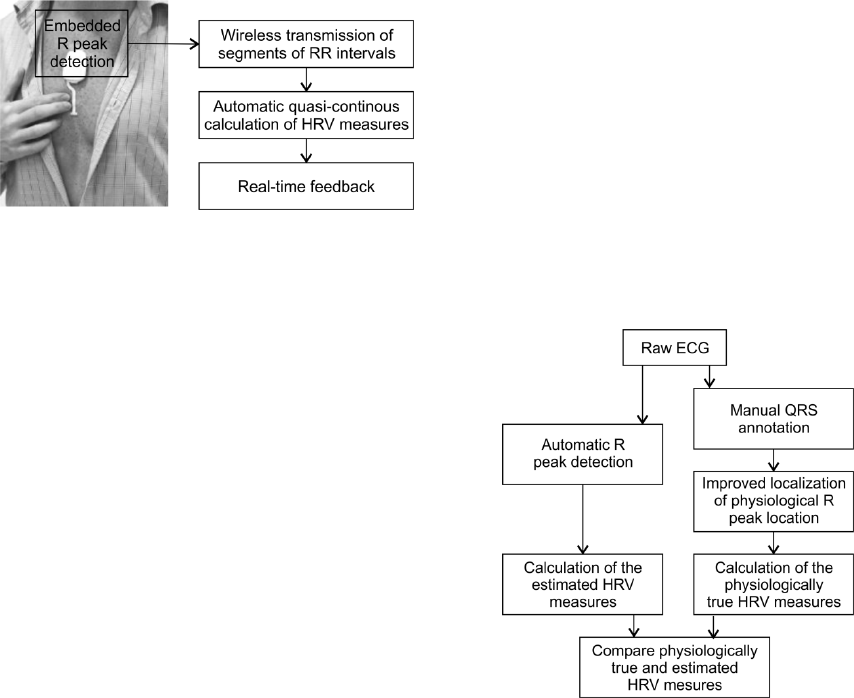
changes in the clinical condition. The information
might be calculated with pre-defined intervals
depending on the specific application. Hence, we
introduce the term quasi-continuous evaluation of
HRV measures. The growing clinical accept of patch
type ECG recorders increases the real-life
applicability of such a system.
Figure 1: Schematic illustration of a possible future quasi-
continuous HRV feedback loop.
One of the important prerequisites for reliable
estimation of the HRV measures is the ability to
obtain a correct RR interval curve. The obtained RR
interval curve might be affected by several factors,
e.g. the sampling frequency, the resolution of the
digitalized signal, artefacts, the automatic R peak
localization procedure, and physiological noise (e.g.
beats that does not originate from the sinoatrial
node). It is very important to obtain an RR interval
curve with as few deviations from the true
physiological variability of the heart as possible. In
this paper, we therefore define the term
“physiologically true R peak position” to describe
the best possible localization of the R peak after
correction for errors in the digitalization (sampling
frequency and signal resolution), errors caused by
inaccurate QRS detection (false or missed
detections), and uncertainty induced by improper
automatic localization of the exact R peak position.
We thus use this expression to describe the R peak
positions that best describe the true physiological
variability of the heart with a minimum of influence
from technical errors. The presence of e.g. ectopic
beats has also been expected to cause errors and
uncertainty in the calculation of the HRV measures
(ESC and NASPE, 1996). On the other hand, several
different automatic or semi-automatic methods for
the editing of the automatically generated RR
interval curve have been proposed recently (Citi et
al., 2012; HASIBA Medical, 2015). It is therefore
not entirely clear whether the manual editing is
strictly required. The purpose of this study is thus to
explore the possibilities for estimating reliable
quasi-continuous HRV measures using the ePatch
ECG monitor.
2 METHODS
An overview of the study design is provided in
Figure 2. The overall purpose was to investigate
whether RR interval series obtained automatically
using the ePatch ECG recorder would be of
sufficient quality to provide reliable estimates of
clinically relevant HRV measures. To investigate
this, we compared HRV measures based on the
automatically generated RR series with an estimate
of the physiologically true HRV measures. The
physiologically true HRV measures were estimated
based on manual annotations of QRS complexes in
5-min ECG segments and a method recently
designed by our group to accurately locate the
physiological R peak position independently of the
applied sampling frequency and bit resolution
(Ahrens et al., 2015).
Figure 2: Schematic overview of the study design. The
input to the analysis is a raw ePatch ECG signal. The
output of the analysis is a comparison between the values
of the physiologically true HRV measures and the HRV
measures obtained using the automatically generated RR
interval series.
2.1 Data Acquisition
The ECG recordings were obtained using the ePatch
recorder. The ePatch stores the recorded ECG
channels locally for later offline analysis. The
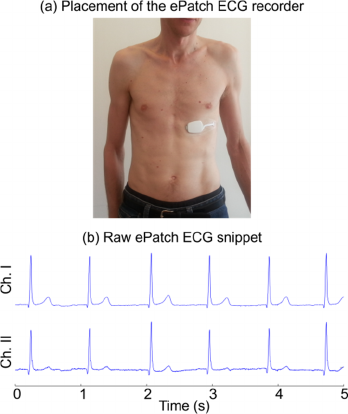
ePatch was placed horizontally on the lower part of
the chest (see Figure 3). The ePatch can record with
four different sampling frequencies (128 Hz, 256
Hz, 512 Hz, and 1024 Hz). With the future
Towards Quasi-continuous Heart Rate Variability Estimation using a Patch Type Electrocardiogram Recorder
21
embedded data processing in mind, it is desired to
apply a low sampling frequency. However, a higher
sampling frequency might induce less R peak jitter,
i.e. less error in the exact R peak localization. We
therefore found it relevant to investigate the
application of all four sampling frequencies. For
each sampling frequency, we obtained six 24-hour
recordings yielding a total of 24 recordings. The
recordings were obtained on healthy, young
volunteers. We had 12 different subjects, and each
subject was therefore monitored with two different
sampling frequencies on two different days. The
subjects were instructed to continue all normal daily
life activities throughout the monitoring period. This
ensures a realistic amount of artefacts and a realistic
impression of the normal changes in the HRV
measures during a full day of normal daily life
activities.
Figure 3: (a) Illustration of the placement of the ePatch on
the chest. (b) Illustration of a two-channel ECG snippet
obtained with the ePatch recorder. It is observed how this
location of the ePatch ensures large R peak amplitudes and
relatively small P- and T-waves.
2.1.1 Manual QRS Annotations
Manually annotated QRS positions are required both
to estimate the performance of the automatic QRS
complex detection algorithm and to obtain the
physiologically true HRV measures. To obtain these,
we automatically extracted and manually annotated
one 5-min ECG segment every third hour of each
recording. The manual annotation procedure was
similar to previous studies conducted by our group
(Saadi et al., 2015). All beats were labelled as
normal.
2.2 Automatic QRS Complex Detection
For the automatic QRS complex detection step, we
decided to apply an algorithm previously designed
by our group (Saadi et al., 2015). We selected this
algorithm based on several considerations: 1) It was
optimized for QRS complex detection in ePatch
ECGs, 2) The algorithm obtained very high clinical
performance on both ePatch ECGs and two large
standard databases, and 3) The algorithm is
computationally efficient enough for real-time
embedded functionality. The algorithm is based on
bandpass filtering, adaptive thresholding, and a
search back mechanism. The algorithm was
originally designed for a sampling frequency of 512
Hz. We therefore made small modifications in the
algorithm to allow automatic QRS complex
detection with all four sampling frequencies. The
modifications are described in the Appendix. The
RR interval series obtained automatically using this
algorithm were applied directly to calculate what we
term the “estimated” HRV measures. This would
correspond to the output from the potential future
feedback loop illustrated in Figure 1. However, in
this study, the QRS complex detection algorithm
was applied offline in MATLAB R2013b.
2.3 Estimation of Physiological R Peak
The digitalization of the physiological R peak
depends both on the sampling frequency and the bit
resolution of the recorded data. It might therefore be
difficult to conduct an accurate estimation of the
physiologically true R peak position based only on
the recorded waveform directly. This might induce
recording jitter in the HRV measures. Recently, our
group has therefore designed a method to estimate
the physiologically true R peak position
independently of the applied sampling frequency
(Ahrens et al., 2015). The input to the algorithm is
an approximate QRS location. In our case, this
location was the manual annotations. The data is
then up-sampled to 8191 Hz. In this high frequency
domain, a template matching is performed to
maximize the alignment of R peaks and hereby
obtain a very accurate assessment of the
physiologically true RR interval series. This RR
interval series is applied to calculate what we term
as the “physiologically true” HRV measures.
CARDIOTECHNIX 2015 - International Congress on Cardiovascular Technologies
22
2.4 Calculation of HRV Measures
A high number of different short-term HRV
measures have been proposed. We decided to
investigate three time domain measures, four
frequency domain measures, and four dynamic
measures: 1) SDNN represents the standard
deviation of the RR intervals, 2) RMSSD is the
square root of the mean squared differences of
successive RR intervals, 3) pNN50 is the percentage
of interval differences of successive RR intervals
that exceeds 50 ms, 4) VLF represents the very low
frequency power component (≤0.04 Hz), 5) LF is the
low frequency power component (0.04 – 0.15 Hz),
6) HF is the high frequency power component (0.15
– 0.4 Hz), 7) LF/HF is the relation between the low
and high frequency components, 8) ApEn is the
approximate entropy (a measure of the regularity of
the RR intervals), 9) SD1 is a geometric measure of
the short-term variations, 10) SD2 is a geometric
measure of long-term variations, and 11) SD1/SD2
represents the relation between the two axis in the
Poincaré plot. The different measures are described
in more details in (ESC and NASPE, 1996).
3 RESULTS
3.1 Performance of QRS Detection
The performance of the automatic QRS complex
detection algorithm was evaluated as the sensitivity
(Se = TP/(TP+FN)) and the positive predictivity (P
+
= TP/(TP+FP)), where TP is the number of true
positive detections, FN is the number of false
negative detections (missed beats), and FP is the
number of false positive detections. The
performance was evaluated using the bxb function
in the WFDB Toolbox (Goldberger et al., 2000).
The performance of the algorithm is provided in
Table 1 for each of the four investigated sampling
frequencies. The algorithm only obtained Se and/or
P
+
of less than 99.0% on seven of the 191 segments
(three obtained with 128 Hz and two obtained with
256 Hz and 512 Hz, respectively). This lower
performance was obtained on segments with high
amounts of artefacts. These artefacts are also
expected to influence the estimation of the
physiologically true HRV measures, and these seven
segments were therefore excluded from the HRV
investigations described in the following sections.
The automatic QRS detection performance on the
184 included 5-min segments is also provided in
Table 1.
3.2 Comparison of HRV Measures
The median values of the physiologically true and
the estimated HRV measures are provided in Table 2
for each sampling frequency. It generally appears
that the automatically generated RR series have a
tendency to slightly overestimate the median values
of most of the HRV measures for all four sampling
frequencies. Therefore, we also investigated the
distribution of the differences between the true HRV
measures and the estimated values. A few examples
of this are provided in Figure 4. It is observed that
most of the differences have slightly negative values
corresponding to a minor overestimation when the
automatically generated RR series is applied.
However Mann-Whitney U tests and anova tests
showed that the differences observed in the median
and mean values, respectively, are not significant for
any of the HRV measures for any of the sampling
frequencies. Both tests were conducted with a
significance level of α = 0.05.
The similarity between the true and the estimated
5-min HRV measures were furthermore investigated
using correlation plots. A few examples are provided
in Figure 5. It is visually observed that there is a
high correlation between the true HRV measures and
the estimated HRV measures for all four sampling
frequencies. This was observed for all of the 11
investigated 5-min HRV measures. The correlation
coefficients between the true and the estimated HRV
measures are provided in Table 3. Statistical tests
showed that all the correlations are significant with a
significance level of α = 0.001.
Table 1: Evaluation of the automatic QRS detection
performances for each sampling frequency with all
segments (top line) and with exclusion of segments with
very poor performance (lower line). N indicates the
number of segments applied.
Fs
N Total
beats
Se
(%)
P
+
(%)
128 Hz
48 17,664 99.58 99.55
45 16,029 99.98 99.93
256 Hz
48 18,283 99.97 99.87
46 17,573 99.99 99.96
512 Hz
48 17,965 99.65 99.64
46 16,651 99.98 99.93
1024 Hz
47 18,338 99.96 99.95
47 - - -
Total
191 72,105 99.79 99.76
184 68,582 99.98 99.94
Towards Quasi-continuous Heart Rate Variability Estimation using a Patch Type Electrocardiogram Recorder
23
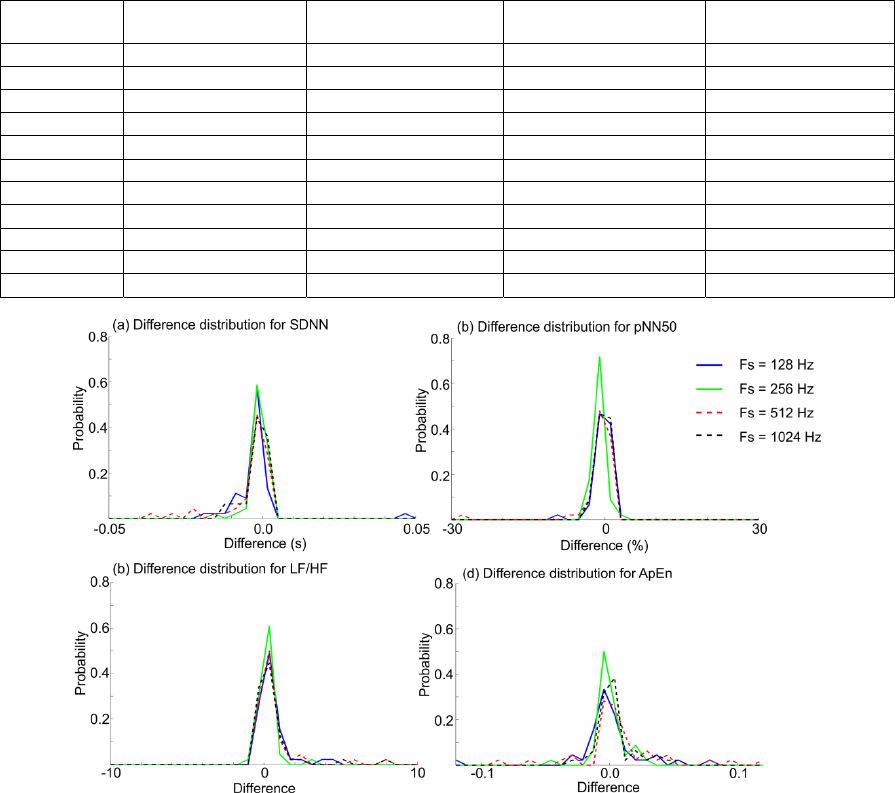
Table 2: Median values of the investigated 5-min HRV measures obtained from the physiologically true RR series (“truth”)
and obtained directly from the automatic QRS complex detection (“estimate”) for each sampling frequency. The median
values are applied because several of the parameters are not normally distributed.
Parameter
Fs = 128 Hz Fs = 256 Hz Fs = 512 Hz Fs = 1024 Hz
Truth Estimate Truth Estimate Truth Estimate Truth Estimate
SDNN (s)
0.0607 0.0681 0.0640 0.0639 0.0606 0.0650 0.0518 0.0529
RMSSD (s)
0.0350 0.0416 0.0316 0.0342 0.0374 0.0439 0.0314 0.0335
pNN50 (%)
13.896 14.402 10.069 11.780 15.205 16.117 10.671 11.024
SD1 (s)
0.0248 0.0295 0.0223 0.0242 0.0265 0.0311 0.0222 0.0237
SD2 (s)
0.0870 0.0888 0.0862 0.0861 0.0758 0.0829 0.0691 0.0704
SD1/SD2
0.2994 0.3270 0.2511 0.2746 0.3611 0.4122 0.2549 0.2985
ApEn
0.5719 0.5700 0.5403 0.5411 0.5889 0.5713 0.5735 0.5767
VLF (s
2
)
3.78·10
5
3.77·10
5
3.53·10
5
3.56·10
5
3.80·10
5
3.80·10
5
3.12·10
5
3.11·10
5
LF (s
2
)
568.14 626.54 484.99 454.93 447.06 477.66 455.94 441.18
HF (s
2
)
248.23 316.00 224.45 230.97 229.96 319.59 159.42 184.05
LF/HF
2.5396 2.1458 2.5691 2.5391 1.9684 1.3634 3.1721 2.9977
Figure 4: Examples of the distribution of the differences between the physiologically true HRV parameter and the estimated
HRV parameter value for each 5-min ECG segment. Negative differences correspond to the estimated value being larger
than the physiologically true value. It is observed that the distribution of the differences is comparable for all four
frequencies. The probability distributions are calculated as histograms with 30 equally distributed bins.
3.3 Applications of the HRV Measures
One of the interesting applications of quasi-
continuous estimation of HRV measures is the
possibility to explore transient changes in the HRV
measures over time. This could be relevant on larger
time scales (e.g. month or years), but also on shorter
time scales (e.g. days). We therefore investigated the
time course of a few selected HRV measures during
the entire duration of our recordings. A few
examples are provided in Figure 6. The daily
variations are especially observed for the first
subject. In order to automatically detect these
transient changes using the estimated HRV
measures, it is necessary to ensure that the before
mentioned tendency to a minor (non-significant)
overestimation of many of the measures does not
interfere with the ability to correctly classify
between simulated groups of high and low
variability, respectively. This ability is also expected
to be important with respect to detection of different
patient populations based on the HRV measures.
CARDIOTECHNIX 2015 - International Congress on Cardiovascular Technologies
24
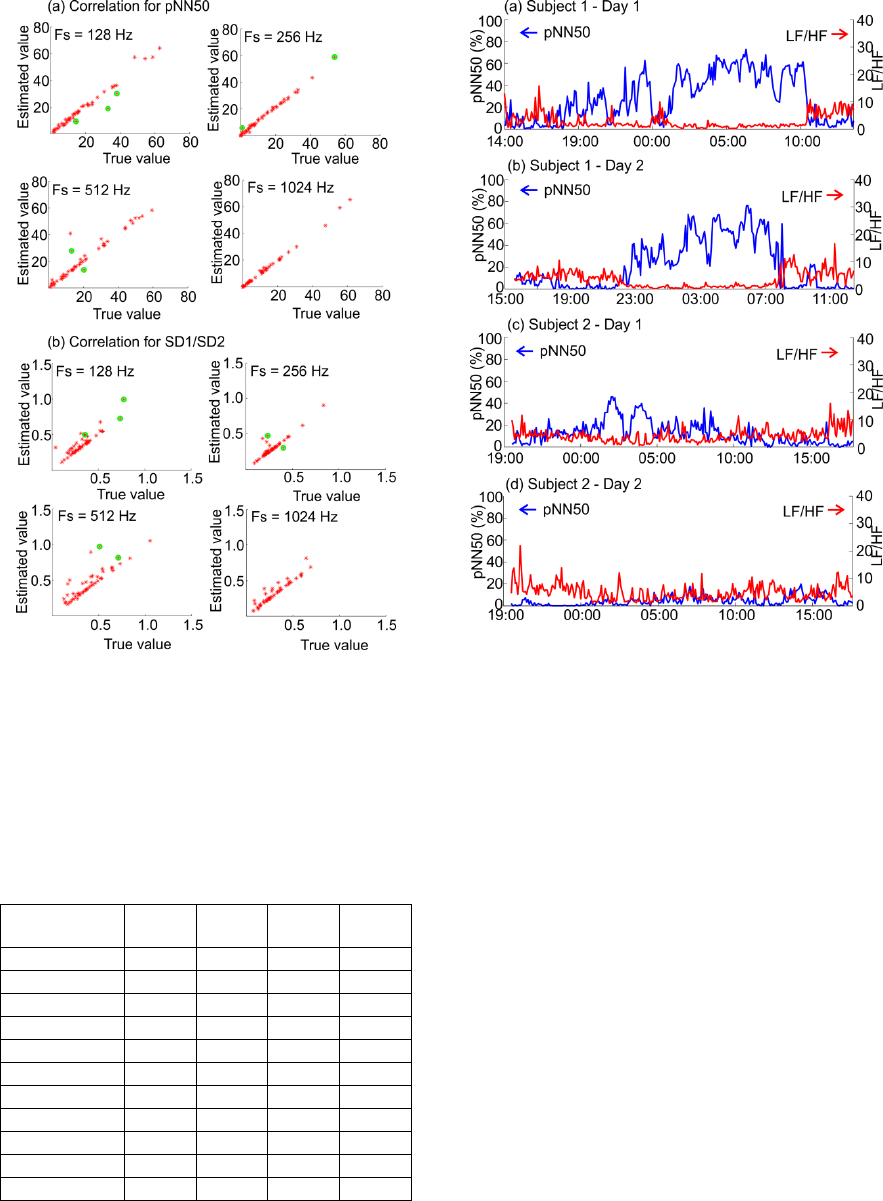
Figure 5: Examples of representative correlations between
the physiologically true HRV measures and the estimated
HRV measures for the four different sampling frequencies.
The green marks indicate segments that were excluded
from the HRV comparisons due to poor QRS detection
performance.
Table 3: Correlation coefficients between the true and the
estimated value of each investigated HRV measure for all
four sampling frequencies. Examples of correlation plots
are provided in Figure 5.
Parameter
128
Hz
256
Hz
512
Hz
1024
Hz
SDNN
0.97 0.98 0.95 0.99
RMSSD
0.93 0.96 0.87 0.95
pNN50
0.99 1.00 0.97 1.00
SD1
0.93 0.96 0.87 0.96
SD2
0.62 1.00 0.80 0.93
SD1/SD2
0.86 0.94 0.88 0.95
ApEn
0.93 0.99 0.96 0.99
VLF
1.00 1.00 1.00 1.00
LF
0.94 0.95 0.89 0.98
HF
0.94 0.99 0.93 0.99
LF/HF
0.89 0.99 0.86 0.97
Figure 6: Illustration of the time course of pNN50 (blue
line) and LF/HF (red line) calculated using the estimated
HRV measures from every 5-min segment throughout the
recording on two different days for two different subjects.
To investigate the ability to classify between
different states of low and high HRV measures, we
divided the included 5-min segments into two
groups. The first group represents the lowest half of
the HRV measures and the second group represents
the highest half for each parameter. The division was
based on the physiologically true HRV measures.
We first confirmed that there was a statistical
significant difference between both the mean and the
median values of the two obtained groups using the
true HRV measures. This was confirmed for all 11
HRV measures for all four frequencies. This
division can thus be applied to simulate two truly
different groups. We then investigated whether the
difference was still significant when the estimated
HRV measures where applied. A few representative
examples of the two obtained distributions are
provided in Figure 7. It is generally observed that
there is a clear difference between the two groups
using the true HRV measures. This difference is
furthermore observed to be correctly reproduced by
the estimated HRV measures. The results were
Towards Quasi-continuous Heart Rate Variability Estimation using a Patch Type Electrocardiogram Recorder
25
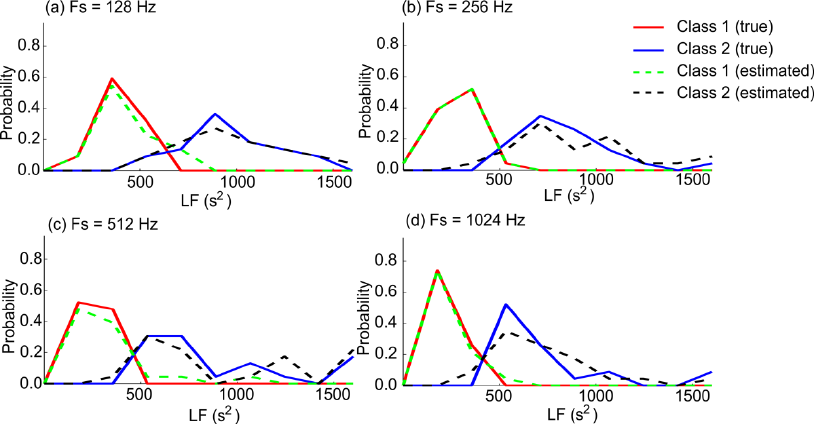
Figure 7: Illustration of division of the LF measure into a high and a low variability group. Each curve illustrates the
distribution of the LF measure for class 1 using the physiologically true values (red lines), class 2 using the physiologically
true values (blue lines), class 1 using the estimated values (green lines), and class 2 using the estimated values (black lines).
The probability distributions are calculated as histograms with 10 equally distributed bins.
similar for all 11 HRV measures for all four
sampling frequencies. Mann-Whitney U tests and
anova tests revealed that the difference between
median and mean values, respectively, were still
significant (α = 0.01) with the estimated values of
the HRV measures.
4 DISCUSSIONS
The performance of the automatic QRS complex
detection algorithm was found to be high for all four
frequencies. This was especially clear when the
seven worst segments were removed. The exclusion
of these segments was considered necessary to
ensure reliable estimation of the HRV measures due
to the lack of manual editing in our setup. However,
this suggests that a reliable HRV estimate can be
obtained in more than 96% of the segments. This is
considered acceptable with the quasi-continuous
application in mind. In future studies, it should be
investigated how these segments could be excluded
automatically. This could for instance include an
automatic pre-qualification of the quality of each
segment. Furthermore, the next generation of the
ePatch is able to record simultaneous accelerometer
data. This could be applied to detect periods of high
activity and thus base the quasi-continuous HRV
estimation on segments with potentially low noise
levels. Furthermore, several studies have recently
investigated the possibility for automatic correction
of errors in the RR interval curve (Citi et al., 2012).
These methods might also be able to decrease the
influence of poor QRS detection performance and
hereby allow inclusion of more of the segments.
However, it should be noted that this study was
conducted on young, healthy, and physically active
volunteers where the amount of abnormal beats is
expected to be low. In cardiac patients or healthy
elderly, it is probably necessary to account for
abnormal beats in the automatically generated RR
interval series before the HRV measures are
estimated. As mentioned, several studies have
recently designed methods to account for these
automatically based on outliers in the RR interval
curve (Citi et al., 2012).
Generally, there was a tendency to overestimate
most of the HRV measures for all the sampling
frequencies. This might indicate that a certain
amount of high frequency noise is induced due to the
finite sampling of the signals. However, looking at
the distributions of the differences in Figure 4, it
appears that the overestimation is quite similar for
all four frequencies. Furthermore, the correlation
was found to be high for all the investigated HRV
measures for all four sampling frequencies and we
found no statistical significant differences for any of
the sampling frequencies. This suggests that all four
frequencies can be applied to obtain a very accurate
assessment of the true physiological variability.
With the embedded implementation in mind, it is
CARDIOTECHNIX 2015 - International Congress on Cardiovascular Technologies
26
therefore appealing to apply the lower sampling
frequencies. However, the usual recommendation
has been to apply a higher sampling frequency,
especially when the HRV measures are expected to
have low values which is often the case in
autonomous dysfunction (Citi et al., 2012; ESC and
NASPE, 1996; García-González et al., 2004;
Tapanainen et al., 1999). Our findings should
therefore be confirmed on a larger population that
includes different clinically relevant patient groups.
It is especially important to investigate whether the
findings can be reproduced in populations with more
complicated ECGs, e.g. patients with ectopic beats,
and patients with reduced HRV measures, e.g.
patients with autonomic dysfunction and critical care
patients. However, it is very promising that our
simulated division into low and high variability
groups are quite similar using the true and the
estimated HRV parameter values.
Figure 6 contains an example of the application
of quasi-continuous HRV measures to investigate
daily variations in the autonomic tone. It is generally
observed that a clear increase in pNN50 is
associated with a clear decrease in LF/HF. This is as
expected: A high value of pNN50 indicates more
high frequency variations and more high frequency
content is associated with a decrease in LF/HF. An
increase in the high frequency components are
believed to be associated with an increase in the
parasympathetic nervous system. For the first
subject, it is clearly observed how the high
frequency components are more pronounced during
the night in both recordings. For the second subject,
this difference is only observed on the first day. It is
furthermore observed that there is a decrease in
pNN50 and hereby a decrease in the high frequency
components, in the middle of the night. That might
indicate that the subject woke up at night. This
illustrates how quasi-continuous estimation of HRV
measures might assist in keeping an eye on the
development of changes in the autonomic tone. This
could for instance prove useful for the monitoring of
critical care patients or patients at risk of developing
sepsis. It might also be helpful in general health
management or for monitoring of improvments
obtained by exercise or stress management
programs. These examples illustrate how new
knowledge about the constantly changing autonomic
tone might be gained by working towards quasi-
continuous monitoring of HRV measures. This paper
is a very early step in the direction of real-time
quasi-continuous monitoring of changes in the
autonomic tone through HRV measures, but the
results are promising and suggest that more research
in this area might prove beneficial.
Generally, there are three requirements for
obtaining reliable estimates of clinically relevant
HRV measures:
1. The patient should be able to comply with
wearing the system for the necessary amount of
time.
2. The system should be able to correctly
reproduce the physiological variability of the
heart in quasi-continuous segments.
3. The system should be able to automatically
select the ECG segments that are suitable for
reliable estimation of the HRV measures.
The first requirement is clearly fulfilled by the
patch type ECG recorders. The second requirement
is bound by the ability to automatically detect and
localize the R peaks with sufficient accuracy. The
focus of this study was to investigate this second
requirement. Overall, we found that when the ECG
is of sufficient quality (defined by the ability to
obtain sufficient automatic QRS complex detection),
it was possible to reproduce the physiological
variability using the ePatch recorder and an
automatic QRS complex detection algorithm. Our
findings thus suggest fulfilment of the second
requirement. However, as mentioned, this was based
on segments from healthy volunteers with expected
low arrhythmia burden and with high QRS detection
performance. This leads to the third requirement that
is related to the ability to automatically select the
segments that are suitable for the quasi-continuous
estimation of the short-term HRV measures. This
might include automatic selection of segments with
sufficient signal quality, automatic selection of
segments without arrhythmias, or automatic
correction of abnormal beats in the RR series before
calculation of the HRV measures. This area should
thus be the subject of further research in the future.
5 CONCLUSIONS
We found a high correlation between the
physiologically true HRV measures and the
measures estimated with an automatically obtained
RR interval series for four different sampling
frequencies (128 Hz, 256 Hz, 512 Hz, and 1024 Hz).
This indicates that the described ePatch system is
able to obtain reliable estimates of clinically relevant
HRV measures. These findings should be further
investigated in larger patient populations with more
complicated ECGs and in patient populations with
expected low variability in the heart rate. However,
Towards Quasi-continuous Heart Rate Variability Estimation using a Patch Type Electrocardiogram Recorder
27
the findings are still promising for the future
application of the ePatch ECG recorder in the
growing area of risk stratification based on HRV
measures.
REFERENCES
Ahrens, E., Sorensen, H. B. D., Langberg, H., Hoppe, K.,
& Saadi, D. B. (2015). Investigation of the Minimum
Conditions for Reliable Estimation of Clinically
Relevant HRV Measures - Introducing a Novel
Approach to the Validation of HRV Measurement
Systems. In CARDIOTECHNIX 2015: Proceddings of
the International Congress of Cardiovascular
Technologies 2015. SciTePress. Accepted.
Buchan, C. A., Bravi, A., & Seely, A. J. E. (2012).
Variability analysis and the diagnosis, management,
and treatment of sepsis. Current Infectious Disease
Reports, 14, 512–521. doi:10.1007/s11908-012-0282-4
Citi, L., Brown, E. N., & Barbieri, R. (2012). A real-time
automated point-process method for the detection and
correction of erroneous and ectopic heartbeats. IEEE
Transactions on Biomedical Engineering, 59, 2828–
2837. doi:10.1109/TBME.2012.2211356
ESC and NASPE (Task Force of the European Society of
Cardiology and the North American Society of Pacing
and Electrophysiology). (1996). Heart rate variability:
Standards of measurement, physiological
interpretation, and clinical use. European Heart
Journal, 17, 354–381.
García-González, M. A., Fernández-Chimeno, M., &
Ramos-Castro, J. (2004). Bias and uncertainty in heart
rate variability spectral indices due to the finite ECG
sampling frequency. Physiological Measurement, 25,
489–504. doi:10.1088/0967-3334/25/2/008
Ghaffari, A., Homaeinezhad, M. R., & Daevaeiha, M. M.
(2011). High resolution ambulatory holter ECG events
detection-delineation via modified multi-lead wavelet-
based features analysis: Detection and quantification
of heart rate turbulence. Expert Systems with
Applications, 38, 5299–5310. doi:10.1016/j.eswa.20
10.10.028
Goldberger, A. L., Amaral, L. A. N., Glass, L., Hausdorff,
J. M., Ivanov, P. C., Mark, R. G., Mietus, J.E., Moody,
G.B., Peng, C.-K. & Stanley, H. E. (2000).
PhysioBank, PhysioToolkit, and PhysioNet :
Components of a new research resource for complex
physiologic signals. Circulation, 101, e215–e220.
doi:10.1161/01.CIR.101.23.e215
Huikuri, H. V., & Stein, P. K. (2013). Heart rate
variability in risk stratification of cardiac patients.
Progress in Cardiovascular Diseases, 56, 153–159.
doi:10.1016/j.pcad.2013.07.003
Li, C., Zheng, C., & Tai, C. (1995). Detection of ECG
characteristic points using wavelet transforms. IEEE
Transactions on Biomedical Engineering, 42.
doi:10.1109/10.362922
Martínez, A., Alcaraz, R., & Rieta, J. J. (2010).
Application of the phasor transform for automatic
delineation of single-lead ECG fiducial points.
Physiological Measurement, 31, 1467–85. doi:10.
1088/0967-3334/31/11/005
HASIBA Medical GmbH. (2015). Cardioscope(TM)
Analytics. Retrieved October 12, 2015, from
https://cardiscope.com
Pan, J., & Tompkins, W. (1985). A real-time QRS
detection algorithm. IEEE Transactions on Biomedical
Engineering, 32, 230–236. doi:10.1109/TBME.1985
.325532
Saadi, D. B., Fauerskov, I., Osmanagic, A., Sheta, H. M.,
Sorensen, H. B. D., Egstrup, K., & Hoppe, K. (2013).
Heart rhythm analysis using ECG recorded with a
novel sternum based patch technology. In
CARDIOTECHNIX 2013: Proceedings of the
International Congress on Cardiovascular
Technologies 2013, SciTePress, 15-21. doi:10.5220/
0004640900150021
Saadi, D. B., Sorensen, H. B. D., Hansen, I. H., Egstrup,
K., Jennum, P. J., & Hoppe, K. (2014). ePatch(R) - A
Clinical Overview. Retrieved from
http://orbit.dtu.dk/fedora/objects/orbit:135692/datastre
ams/file_56776d8b-c232-4bcb-af6c-
20f3a00382a9/content
Saadi, D. B., Tanev, G., Flintrup, M., Osmanagic, A.,
Egstrup, K., Hoppe, K., Jennum, P., Jeppesen, J.L.,
Iversen, H.K. & Sørensen, H. B. D. (2015). Automatic
Real-Time Embedded QRS Complex Detection for a
Novel Patch-Type Electrocardiogram Recorder. IEEE
Journal of Translational Engineering in Helath and
Medicine, 3, 1900112. doi:10.1109/JTEHM.2015.242
1901
Tapanainen, J. M., Seppänen, T., Laukkanen, R.,
Loimaala, A., & Huikuri, H. V. (1999). Significance
of the accuracy of RR interval detection for the
analysis of new dynamic measures of heart rate
variability. Annals of Noninvasive Electrocardiology,
4, 10–18. doi:10.1111/j.1542-474X.1999.tb00359.x
APPENDIX
The automatic QRS complex detection algorithm
was originally designed for a sampling frequency of
512 Hz (Saadi et al., 2015). The performance of this
version of the algorithm on the MIT-BIH
Arrhythmia Database (MITDB) is compared to other
published algorithms in Table 4. Two modifications
were required to adapt the algorithm to the other
three sampling frequencies. The first adaptation was
an adjustment of a threshold that decides the
variability mode of the algorithm. The original
threshold was T
θ
,
original
= 35 samples. This threshold
was updated to T
θ
= 8 samples for fs = 128 Hz, T
θ
=
17 samples for fs = 256 Hz, and T
θ
= 70 samples for
fs = 1024 Hz. The second modification was related
CARDIOTECHNIX 2015 - International Congress on Cardiovascular Technologies
28
to the digital filters. The original filter coefficients
for fs = 512 Hz are provided in (Saadi et al., 2015).
For fs = 1024 Hz, all coefficients were doubled.
Thus the length of all the cascaded filters was
doubled. This keeps the bandpass region for a
doubled sampling frequency. Likewise, every other
filter coefficient was removed to adjust for a
sampling frequency of 256 Hz. This modification
was not possible for fs = 128 Hz. Instead, the two
first bandpass filters were therefore modified
according to (1) and (2).
Table 4: Comparison of performances obtained on the
MITDB by different algorithms published in the literature.
In this study we applied the algorithm designed by (Saadi
et al., 2015). This algorithm was designed and optimized
for detection of QRS complexes in ePatch ECGs.
Algorithm
Se (%) P
+
(%)
(Saadi et al., 2015)
99.90 99.87
(Ghaffari, Homaeinezhad, &
Daevaeiha, 2011)
99.94 99.91
(Martínez, Alcaraz, & Rieta,
2010)
99.71 99.97
(Li, Zheng, & Tai, 1995)
99.89 99.94
(Pan & Tompkins, 1985)
99.75 99.54
3
2
1
2. (1)
2
4
3
2
2
1
2
1
2
23. (2)
For the average filters, half the coefficients were
removed relative to the filters applied for fs = 256
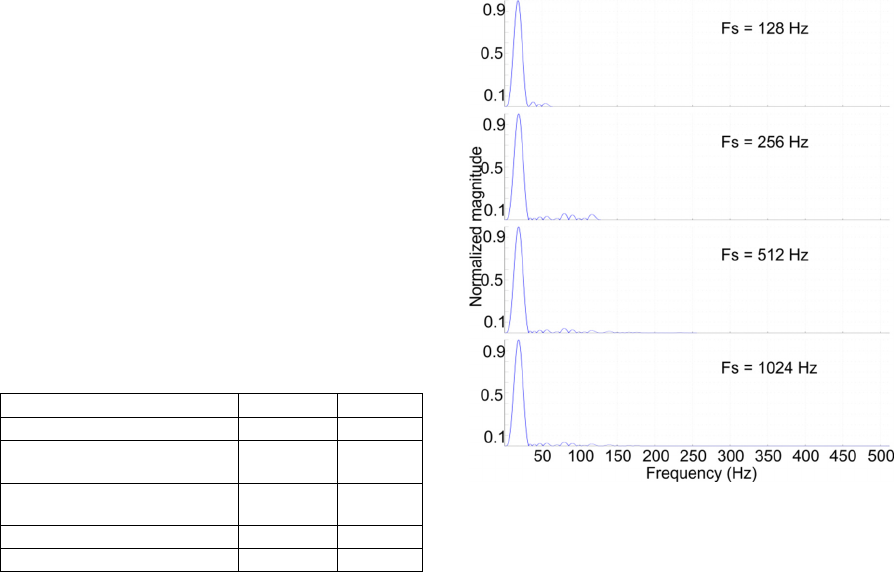
Hz. The bandpass filter characteristics for the four
different sampling frequencies are provided in
Figure 8. It is observed that the filter characteristics
are very similar. It is expected that the performances
obtained by the three modified algorithms (fs = 128
Hz, fs = 256 Hz, and fs = 1024 Hz) will be
comparable to the performance stated in Table 4 for
the original algorithm (fs = 512 Hz).
Figure 8: Amplitude characteristics for the combined
bandpass filters for each sampling frequency.
Towards Quasi-continuous Heart Rate Variability Estimation using a Patch Type Electrocardiogram Recorder
29
