
Wearable Wireless Inertial Sensors for Long-Time Monitoring of
Specific Motor Symptoms in Parkinson’s Disease
P. Lorenzi
1
, R. Rao
1
, A. Suppa
2
,
A. Kita
1
, R. Parisi
1
, G. Romano
1
and F. Irrera
1
1
Department of Information Engineering, Electronics and Communication, Sapienza University of Rome,
Via Eudossiana 18, Rome, Italy
2
Department of Neurology and Psychiatry, Sapienza University of Rome, Viale dell’Università 30, Rome, Italy
Keywords: Biosensors, Wearable Wireless Inertial Sensors, Electronic-Health, Long-Time Home Monitoring,
Parkinson’s Disease.
Abstract: It is proposed an electronic system for the long-time monitoring of specific motor symptoms in patients
affected by Parkinson’s Disease while being at home and making their usual daily activity. The system is
made of a network of non-invasive wireless inertial sensors fixed on the patient body. The muscles activity
is contemporarily analysed through the integration of a circuit for the surface electromyography. Post-
processing algorithms quantify movements in terms of amplitude and power spectrum. Data are
electronically elaborated and wireless transmitted to a receiver in the patient home, to be accessed remotely
by doctors. The challenge is the automatic distinction between specific parkinsonian symptoms including
resting tremor and freezing of gait and patient’s voluntary movements made in daily life. To the aim, the
contemporarily analysis of muscle activity becomes necessary in specific situations, as in the case of
freezing of gait, where accelerometers signals may be misleading. Goal of this research is the
comprehension of all the possible environmental and individual factors which favor worsening of gait
disorders during the patient daily life and the customization of the drug therapy, aiming to preventing
catastrophic events such as falls. Results shown here refer to upper limb tremor and freezing of gait.
1 INTRODUCTION
The analysis of human movement is of major
interest since when, in the last decade, integrated
electronic technologies have finally allowed the
detection, sort and quantification of kinetic
components using the fusion of inertial sensors.
Integrated inertial sensor chips are today
commercially available with a few dollars cost. They
include accelerometers and gyroscopes, an
embedded Micro-Controller Unit (MCU), a non-
volatile memory, a transmission module, in addition
to a battery. Some products devoted to sport training
and rehabilitation and consisting in one sensor with
a dedicated software are commercial. On the
contrary, nothing is available on the market for the
long-time home monitoring of the Parkinson’s
Disease (PD) motor symptoms, although in the last
few years research groups have published papers on
related topics (Patel, 2009; Pantelopoulos, 2010;
Zwartjes, 2010; Patel, 2010; Bächlin, 2010;
Schepers, 2010; Gouwanda, 2011; Becq, 2011;
Taraldsen, 2011; Niazmand, 2011; Sama, 2012;
Caldara, 2014). PD is a chronic neurodegenerative
disorder affecting about 2% of the worldwide
population over 70. Typical PD motor symptoms
include resting tremor, muscle rigidity and
bradykinesia (slowness of movements) (Berardelli,
2001). In PD, motor symptoms manifest when
dopaminergic denervation induces functional
abnormalities in the basal ganglia motor circuits
which in turn drives altered motor inputs in cortical
motor areas. Among PD motor symptoms, tremor is
one the most important and frequently observed in
PD patients. It typically appears in only a single arm
or leg, becoming bilateral later. Frequency of PD
tremor is typically between 4 and 6 hertz.
Tremor crucially worsens when PD patients are not
under dopaminergic therapy (OFF state), whereas it
improves when patients receive their dopaminergic
therapy (ON state). Hence, the long-term monitoring
of tremor amplitude may help the overall clinical
evaluation of PD patients and improve the
therapeutic strategies.
Gait disorders frequently occur in advanced PD
patients and consist of small shuffling steps, reduced
stride length and walking speed during free
ambulation while double support duration and
168
Lorenzi P., Rao R., Suppa A., Kita A., Parisi R., Romano G., Berardelli A. and Irrera F.
Wearable Wireless Inertial Sensors for Long-Time Monitoring of Specific Motor Symptoms in Parkinsonâ
˘
A
´
Zs Disease.
DOI: 10.5220/0005279201680173
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIOSTEC 2015), pages 168-173
ISBN: 978-989-758-071-0
Copyright
c
2015 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

cadence rate are increased. Freezing of gait (FOG) is
typically a transient episode, lasting less than a
minute, in which gait is halted and the patient
complains that feet are glued to the ground. FOG
can be experienced in narrow or tight quarters such
as a doorway or in the presence of an obstacle along
the path and in stressful situations such as when the
telephone or the doorbell rings or when the elevator
door opens. During FOG, PD patients undergo trunk
fluctuations back and forth, move hazardously the
body mass center and load in the forefoot regions,
sometimes resulting in stability loss. At an advanced
stage of the disease, FOG leads to falls in many
instances, in fact, about the 45% of falls of PD
patients occurs forward, due to trunk fluctuations
back and forth. Very recently, some authors have
proposed a IMU based-system which gives an alarm
feedback to assistants or relatives in the case of
patient’s fall (Cabestany, 2013). There are some
evidences that audio stimulations may help the
patient’s to reduce the tendency to undergo FOG.
Auditory stimulations are commonly rhythmic cues,
sometimes embedded in music, set at or slightly
above the patient’s usual cadence. An IMU based-
system has been recently proposed, which gives an
audio feedback to the patient in the case of FOG, to
help the subject to overcome the involuntary block
and prevent the risk of falls (Cabestany, 2013; Sama,
2013; Rodríguez-Martín, 2013). It is evident that the
correct identification of the FOG is crucial, since in
this case any misevaluation of the patient behavior
can be deleterious. For all these reasons, first of all it
is of most relevance to monitor FOG events,
unequivocally distinguishing them from any kind of
voluntary movement, quantifying the daily
frequency, identifying the environmental and the
individual conditions which lead that specific patient
to manifest FOG, finding correlations with the drug
administration and, finally, trying to prevent
catastrophic events such as falls. Optimized drug
therapy can be very effective, especially at an early
stage of the disease. However, drug therapy
optimization is difficult since the response of PD
patients to drugs may vary according to a number of
factors. 24 hours monitoring is the only way to
optimize the therapy and prevent worsening of
symptoms or catastrophic accidents (as falls) due to
incomplete clinical analysis of symptoms during the
day and a consequent not-optimized therapy. On the
other hand, hospitalization is really exceptional
today, due to finance cuts imposed by governments
to national health services. The sensing system
proposed here has the final topic of making possible
the long-time monitoring of specific motor
symptoms of PD while the patient is at home. The
system is composed by a network of several
biosensors disseminated on the patient body which
embed units for the direct non-invasive
measurement of the muscles activity (surface
electromyography, S-EMG). It is being used in the
real-time detection and analysis of PD motor
symptoms. The biosensors are wearable and not
invasive, easy to use and do not need any technical
skill from the patient side. Clinical advantage lies in
the optimization and customization of the drug
therapy for each individual patient. Social benefits
lye in a better quality of life of the patient and the
assisting family.
2 THE SYSTEM
The sensors network system presented in this paper
is designed for both collecting movement signals
and preliminarily analysing them in real-time. This
system is a flexible platform useful for collecting
data via a triaxial accelerometer, a gyroscope and a
magnetometer, with the possibility to incorporate
other information sources in real-time, as the S-
EMG which detects the muscle activity. The Flash
memory stores all inertial data and a Bluetooth
module sends information to other external devices.
The system allows pattern reconstruction of the
kinetic components of movements, discriminates
between voluntary and involuntary movements,
selects only those associated to specific PD
symptoms, reconstructs and, finally, quantifies their
amplitude and frequency. The great challenge of this
work is the automatic association of electronic
signals to specific PD symptoms, filtering all the
signals deriving from voluntary movements. In this
work engineers and neurologists are involved
contemporarily. The engineers develop the
hardware/software system, while the doctors carry
on the clinical research. Patients are clinically
evaluated and movements classified with standard
protocols and compared with voluntary movements
of healthy subjects. This step allows identifying
specific patterns correlated to PD symptoms and is
fundamental for the system calibration. On their
side, engineers optimize the hardware (type of
inertial sensors, protocol of the wireless
communication, entity of the data storage, power
consumption, battery, integration of the EMG, other)
and develop algorithms for data acquisition and
processing. Electrical signals from biosensors are
compared with clinical observations, in order to
achieve the automatic recognition of the disordered
Wearable Wireless Inertial Sensors for Long-Time Monitoring of Specific Motor Symptoms in Parkinsonâ
˘
A
´
Zs Disease
169
movements associated to the disease. The
experiments with patients are performed in the
hospital. A few patients have been studied up to
date, but many others will be studied in the next, to
make reliable the system. In fact, the final goal of
the work is using the system at the patient home, far
from the visual inspection of doctors, and enabling
doctors to access data remotely by a PC. The real
challenge of the research is in the automatic
distinction of voluntary and involuntary movements,
passing through noise filtering, artefacts filtering,
movement reconstruction and the identification of
kinematic components of the PD with quantification
of tremors (amplitude and frequency) and FOG
(duration, trunk fluctuations with associated fall
risk). The complete hard-system, represented in
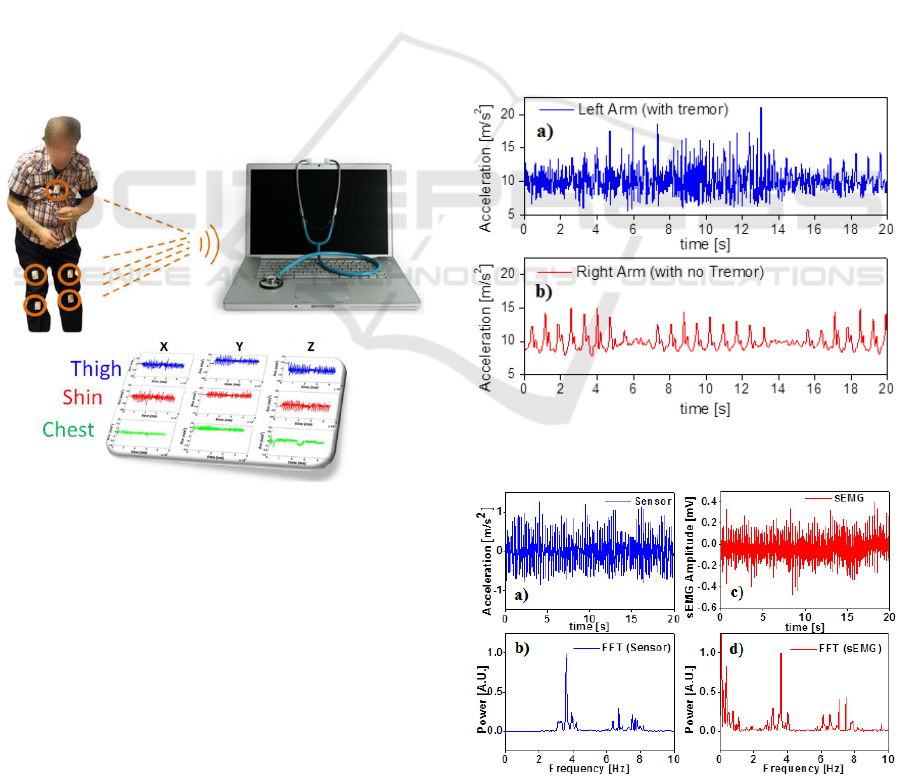
Fig.1, is composed by a network of 5 body sensors.
Two of them are positioned on the thighs, two on the
shins and the last one on the chest. All the sensors
are controlled by a PC trough the LabView software.
Signals coming from the sensors are acquired
through a PC and then they are post-processed and
displayed with MATLAB.
Figure 1: Sketch of the sensing system.
3 EXPERIMENTS
PD patients were recruited at the Movement
Disorders outpatient clinic of the Department of
Neurology and Psychiatry, Sapienza University of
Rome, Italy. All the experiments involving patients
have been performed at the Laboratory of Human
Motor Control at the same Department. Experiments
with patients are still at an early stage, in the sense
that only a few patients have been studied to date,
and only limb tremors and freezing of gate have
been investigated. Nevertheless, although results are
preliminary, the system demonstrated its potentiality
and versatility in recognizing and quantifying
specific disorders associated to the disease.
3.1 Tremor
In this paragraph traces related to tremor are studied.
Patient A had an asymmetric symptomatology, in
fact while the left hand exhibited a tremor, the right
hand did not. A sensor was positioned on the right
forearm while another was position on the left one.
The patient was asked to walk for 5” and then
turn, for three times. Fig.2a and Fig.2b report the
magnitude of resultant accelerations detected while
walking. As one can see, the two traces are very
different. In fact, in the right sensor it is present only
the signal related to arm oscillations during the steps
and the turnings, while in the left sensor the
oscillation during the steps is reduced with respect to
the right one since the left arm was stuck along the
body and only the tremor is present.
Figure 2: Signals from the y-axis accelerometer while
walking: a) Left Arm (tremor), b) Right Arm (no tremor).
Figure 3: Tremor signals from the accelerometer and S-
EMG while sitting a) Tremor; b) FFT; c) sEMG; d) FFT.
BIODEVICES 2015 - International Conference on Biomedical Electronics and Devices
170
Then, the patient was asked to sit with the arms
resting on the leg. In this way, the tremor amplitude
was detected without any contribution from gait.
Results are shown in Fig.3a. The fast Fourier
transform (FFT) of the acceleration trace gave its
power spectrum, highlighting the expected tremor
frequency component around 3.8 Hz (Fig.3b).
The muscle activity during tremor was also
detected positioning the S-EMG electrodes on the
left arm. As one can see in Fig.3c and 3d the EMG
signal and its FFT are of course compatible with the
accelerometer traces (the low frequency component
in the FFT of the S-EMG trace is not meaningful).
3.2 Freezing of Gait (FOG)
Patients have been asked to execute simple
exercises, as the Timed Up and Go test (TUG).
TUG is a simple test used to assess a person's
mobility and requires both static and dynamic
balance. During the test, the person is expected to
wear their regular footwear and use any mobility
aids that they would normally require. Patient A was
asked to execute the TUG. The exercise included the
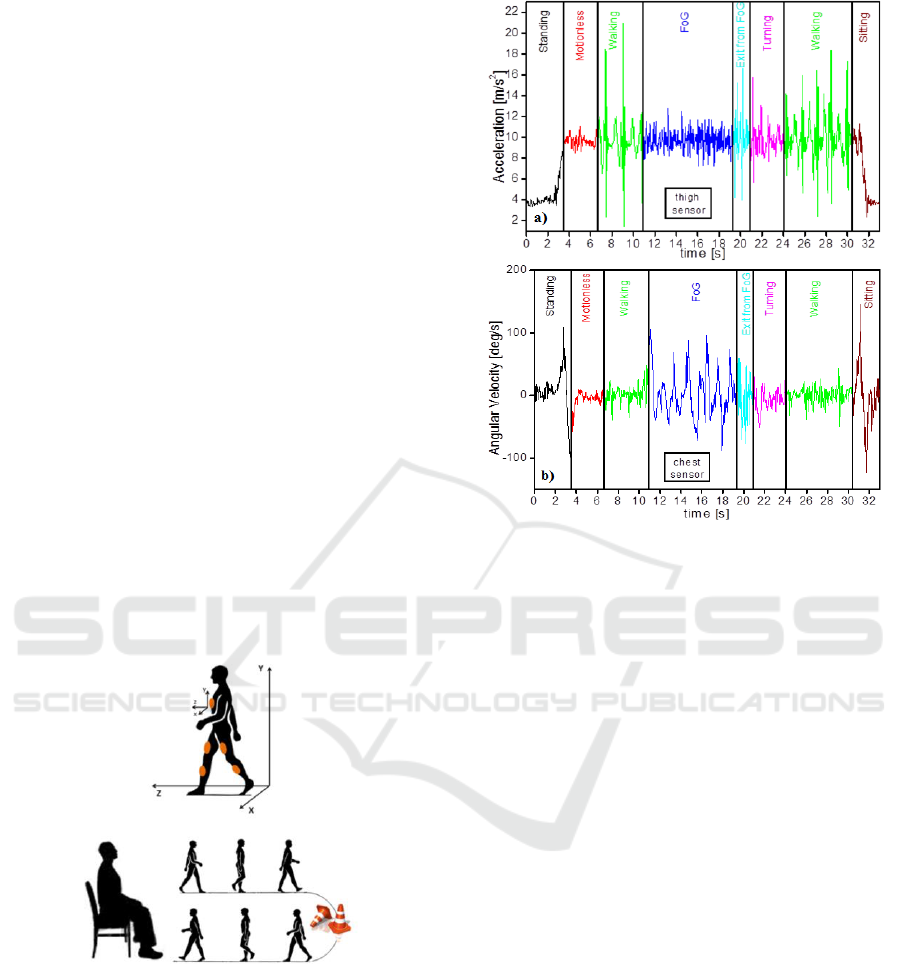
following movements (see Fig.4): 1) standing-up; 2)
motion-less; 3) walking a few meters; 4) turning; 5)
walking; 6) sitting down. An obstacle was
positioned on the floor, along the walking trajectory.
Figure 4: Sketch of the axis orientation and TUG.
Referring to Fig. 4, the patient walking trajectory
is along the z-axis, the y-axis being along gravity
and the x axis perpendicular to the walking
direction, on the same plane.
The main scope of this experiment was the
detection of the FOG and therefore results will be
discussed in the following showing clearly the
occurrence of such an event while walking.
Patient A was asked to execute the TUG. Fig.5a
displays traces relative to the linear acceleration
Figure 5: a) acceleration along the y-axis revealed by the
sensor on the right thigh; b) angular velocity around the x
axis revealed by the sensor on the chest.
along the y-axis revealed by the sensor positioned on
the right thigh. Fig.5b displays the signal from the
gyroscope of the sensor positioned on the chest and
refers to the angular velocity of the trunk around the
x axis. The patient starts his TUG from the sitting
position. In this condition, gravity is not entirely
projected on the y-axis of the thigh sensor, in fact in
Fig.5a the y-axis acceleration is lower than 9 m/s
2
.
Traces outlines that:
1. STANDING-UP: When the patient stands up,
gravity becomes entirely projected on the y-axis of
the thigh sensor. This can be seen looking at the
black line of Fig.5a, which raises to 9 m/s
2
after 3”
approximately from the beginning of the TUG.
While standing-up from the sitting position, also the
gyroscope positioned on the chest clearly detects the
body movement (Fig.5b) and changes its value.
2. MOTIONLESS: the patient is asked to rest
for a few seconds, to assess its postural stability
(which looks quite upright, corresponding to 0 deg/s
from the chest gyroscope and 9 m/s2 from the thigh
accelerometer)
3. WALKING: the postural stability keeps on
while walking, in fact the angular velocity from the
chest sensor is still around zero. The patient makes
just three steps starting with the right leg, as clearly
shown in Fig.6a, where the very first instants of the
green trace indicate that the leg is moved up
Wearable Wireless Inertial Sensors for Long-Time Monitoring of Specific Motor Symptoms in Parkinsonâ
˘
A
´
Zs Disease
171

(acceleration become greater than gravity) and then
moved down for three times. The highest peaks are
inertial impacts when the foot falls on the ground.
4. FOG: At a certain point of his walk, the
patient encounters the obstacle and experiments a
freezing of gait lasting several seconds. In that time
interval the patient makes many attempts to walk,
oscillating the body essentially back and forth and
loading the fore-toes, but he does not make any step
as if feet got glued to the ground. This can be seen in
Fig.5a, where the characteristic peaks related to
steps are absent, but a dense succession of smaller
peaks add to the y-axis acceleration trace, whose
mean value keeps around 9 m/s
2
. At the same time,
the chest sensor detects many changes of the angular
velocity around the x-axis (perpendicular to the
ground, along the walking direction) related to trunk
fluctuations (see Fig.5b). These traces are typical of
a FoG event, which, in this experiment, was favored
by the presence of an obstacle along the walking
trajectory.
It is interesting to compare the traces relative to
“FOG”, to those relative to “motionless”, which is
voluntary. Signals from the accelerometer are
similar, whereas signals from the gyroscope are
quite different. Therefore, just looking at the traces
from the linear accelerometer it is not possible to
distinguish the involuntary FOG from the voluntary
resting position. On the contrary, the measurement
of angular velocity around the x-axis is a clear
indication of trunk fluctuations due to FOG.
We wish to recall that FOG is often the cause of
falls, and its definitive identification is absolutely
necessary. To the aim, traces from the S-EMG are
being analyzed and results will be presented at the
conference.
5. OUT OF FoG: Finally, after about eight
seconds, the patient makes a step and the signal from
the y-accelerometer on the thigh changes its value
and exhibits a shape related to a step. At the same
time, trunk fluctuations tend to stop.
6. TURNING: the patient turns leftward, with a
few small steps. Nothing relevant on the trace.
7. WALKING: the second walking is similar to
the first one.
8. SITTING DOWN: The traces relative to
sitting-down are complementary to those relative to
standing-up. Nothing relevant on the trace.
4 CONCLUSIONS
It was proposed an electronic system for the long-
time monitoring of specific motor symptoms of
patients affected by Parkinson’s Disease while being
at home and making their usual daily activity.
The system is made of a network of non-
invasive wireless inertial sensors fixed on the patient
body. The muscles activity is contemporarily
analysed through the integration of a circuit for the
surface electromyography. Post-processing
algorithms quantify movements in terms of
amplitude and power spectrum.
A few patients of the Movement Disorders
outpatient clinic of the Department of Neurology
and Psychiatry, Sapienza University of Rome were
studied up to date, and asymmetric tremor and
freezing of gait were analysed.
As a result, starting from signals from the
accelerometers signals and the surface-
electromyography tremor was unequivocally
distinguished respect to voluntary movements, and
its amplitude was quantified; the power spectrum
revealed a tremor frequency around 3.8 Hz. On the
contrary, the freezing of gait was distinguished only
thanks to the detection of a gyroscope positioned on
the patient chest, since the accelerometers were not
able to distinguish unequivocally between voluntary
resting in the upright position and the involuntary
gait block.
At the conference, results relative to the
automatic recognition of the freezing of gait will be
presented, obtained on a statistically meaningful
number of patients.
ACKNOWLEDGEMENTS
Authors wish to thank patients of the Movement
Disorders outpatient clinic of the Department of
Neurology and Psychiatry, Sapienza University of
Rome, Italy, who accepted to be involved in the
research.
REFERENCES
Patel, S.; et al.., "Monitoring Motor Fluctuations in
Patients With Parkinson's Disease Using Wearable
Sensors," Information Technology in Biomedicine,
IEEE Transactions on vol.13, no.6, pp.864,873
Nov.2009.
Pantelopoulos, A; Bourbakis, N.G., "A Survey on
Wearable Sensor-Based Systems for Health
Monitoring and Prognosis," Systems, Man, and
Cybernetics, Part C: Applications and Reviews, IEEE
Transactions on vol.40, no.1, pp.1,12, Jan.2010.
Zwartjes, D.G.M et al., "Ambulatory Monitoring of
Activities and Motor Symptoms in Parkinson's
BIODEVICES 2015 - International Conference on Biomedical Electronics and Devices
172

Disease," Biomedical Engineering, IEEE Transactions
on vol.57, no.11, pp.2778,2786,Nov.2010.
Patel, S.; et al., "Home monitoring of patients with
Parkinson's disease via wearable technology and a
web-based application," Engineering in Medicine and
Biology Society (EMBC), 2010 Annual International
Conference of the IEEE , vol., no., pp.4411,4414, Aug.
31 2010.
Bächlin M, et al., “A wearable system to assist walking of
Parkinsons disease patients”, Methods Inf Med. 2010;
49(1):88-95.
Schepers, H.; et al., Ambulatory human motion tracking
by fusion of inertial and magnetic sensing with
adaptive actuation Medical & Biological Engineering
& Computing, Springer-Verlag, 2010, 48, 27-37.
Gouwanda, D. & Senanayake, S. M. N. A. Periodical gait
asymmetry assessment using real-time wireless
gyroscopes gait monitoring system, Journal of Medical
Engineering & Technology, 2011, 35, 432-440.
Becq, G.; et al., P. Classification of epileptic motor
manifestations using inertial and magnetic sensors,
Computers in Biology and Medicine, Computers in
Biology and Medicine, Elsevier, 41,46-55, Jan.2011.
Taraldsen, K.; et al., J. L. Physical activity monitoring by
use of accelerometer-based body-worn sensors in older
adults: A systematic literature review of current
knowledge and applications, Maturitas, Maturitas,
Elsevier, 71, 13-19, 2011.
Niazmand, K.; et al., "Freezing of Gait detection in
Parkinson's disease using accelerometer based smart
clothes," Biomedical Circuits and Systems Conference
(BioCAS), 2011 IEEE, vol., no., pp.201,204, 10-12
Nov. 2011.
Sama, A. et al.; "Dyskinesia and motor state detection in
Parkinson's Disease patients with a single movement
sensor," Engineering in Medicine and Biology Society
(EMBC), pp.1194, 1197, Aug. 28 2012-Sept. 1 2012.
Caldara, M.; et al. “A novel body sensor network for
Parkinson’s disease patients rehabilitation assessment”
2014 11th Int. Conf. on Wearable and Implantable
Body Sensor Network, 81, 86.
Berardelli, A.; et al., “Pathophysiology of bradykinesia in
Parkinson’s disease”. Brain, 2001;124:2131-2146.
Cabestany, J.; et al., “One step towards an automatic aging
people fall detection service," Mixed Design of
Integrated Circuits and Systems (MIXDES), 2013
Proceedings of the 20th International Conference,
vol., no., pp.545,552, 20-22 June 2013.
Cabestany, J.; et al.: “REMPARK: When AI and
technology meet Parkinson Disease assessment,"
Mixed Design of Integrated Circuits and Systems
(MIXDES), 2013 Proceedings of the 20th
International Conference , vol., no., pp.562,567, 20-22
June 2013.
Sama, A.; et al., “A heterogeneous database for movement
knowledge extraction in parkinson’s disease”
European symposium on artificial neural networks,
computational intelligence and machine learning,
2013.
Rodríguez-Martín D, et al., “A wearable inertial
measurement unit for long-term monitoring in the
dependency care area” Sensors (Basel). 2013 Oct 18;
13(10):14079-104.
Wearable Wireless Inertial Sensors for Long-Time Monitoring of Specific Motor Symptoms in Parkinsonâ
˘
A
´
Zs Disease
173
