
VIRUMILK
Biosensor for CMV Detection in Breast Milk from Lactating Women of Preterm
Infants Less than 33 Weeks
S. Py
1
, B. Wacogne
1,2
, L. Pazart
1
,
A. Coaquette
3
, W. Boireau
2
,
G. Herbein
3,4
and G. Thiriez
5
1
INSERM-CIC 1431, Besançon University Hospital, Besançon, France
2
Institute FEMTO-ST, University of Franche-Comté, Besançon, France
3
Laboratory of Virology, Besançon University Hospital, Besançon, France
4
UPRES EA4266, SFR FED 4234, Pathogens and Inflammation Laboratory, Department of Virology,
University of Franche-Comté, Besançon, France
5
Department of Neonatal Medicine, Besançon University Hospital, Besançon, France
Keywords: Cytomegalovirus, Screening, Biochips, Preterm Infants, Breastfeeding.
Abstract: Cytomegalovirus (CMV) is the leading cause of neonatal viral infection and can have a significant impact
on the neurosensory development of newborns and especially preterm infants. While congenital CMV
infection affects about 2-5% of very preterm infants, the risk of postnatal infection, particularly through
breast milk, is much higher in this population (20%). However, infection could be considerably reduced by
an early and fast screening of breast milk. Indeed, a treatment (freezing or pasteurization) of contaminated
breast milk only could eliminate the virus. The idea of this position paper is that breast milk screening
would help defining an appropriate and personalized feeding strategy. We explain how to develop a CMV
biosensor to detect the virus in milk. It employs specific CMV antibodies grafted on a biochip surface to
capture viral material and additional detection antibodies in a “sandwich assay” type system. Detection is
based on optical absorption. It will be tested with a device developed previously. However, preliminary
results obtained in ELISA technique with breast milk and homemade antibodies are presented in this
position paper. The ulterior motive of this work is the fabrication of an autonomous and automated device
that will be experimented in subsequent diagnosis strategy trial.
1 CONTEXT
Cytomegalovirus (CMV), member of the sub-family
of β-herpesvirus, is only present in humans and 40 to
90% of the world population is infected. This virus,
rarely dangerous for immune-competent person, is a
real threat for immune-depressed people, as for
example, organ transplanted or pregnant women.
Following a primo-infection, CMV diffuses in the
whole body and alternates latency and re-activation
periods. CMV is the most frequent etiologic agent of
congenital and postnatal infection of newborns and
can have a significant impact on the neurosensory
development of newborns and especially preterm
infants (Hayashi et al. 2011). Postnatal transmission
of CMV can occur during blood transfusions, while
absorbing infected biological liquids like mother
cervical secretions during delivery or during
breastfeeding. CMV excretion in breast milk is the
main source of postnatal infection.
1.1 Postnatal CMV Infection via Breast
Milk
Breastfeeding is now clearly recognized as being
superior to artificial feeding for the future of
newborns and, more particularly of preterm infants.
Indeed, preterm newborns are more vulnerable to
digestive and neurological problems. Breast milk is
better accepted and limits the risk of feared
complications like necrotising enterocolitis. Its
incomparable time-varying composition offers the
best chances of cognitive evolution on the long term.
However, breastfeeding plays a major role in the
epidemiology of transmission and postnatal CMV
infection. It is now well established that CMV is
excreted in milk from seropositive lactating mothers,
the majority of whom are asymptomatic, especially
due to a reactivation of the virus. Excretion can start
since the first post-partum week with a low viral
115
Py S., Wacogne B., Pazart L., Coaquette A., Boireau W., Herbein G. and Thiriez G..
VIRUMILK - Biosensor for CMV Detection in Breast Milk from Lactating Women of Preterm Infants Less than 33 Weeks .
DOI: 10.5220/0005251801150120
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2015), pages 115-120
ISBN: 978-989-758-071-0
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)
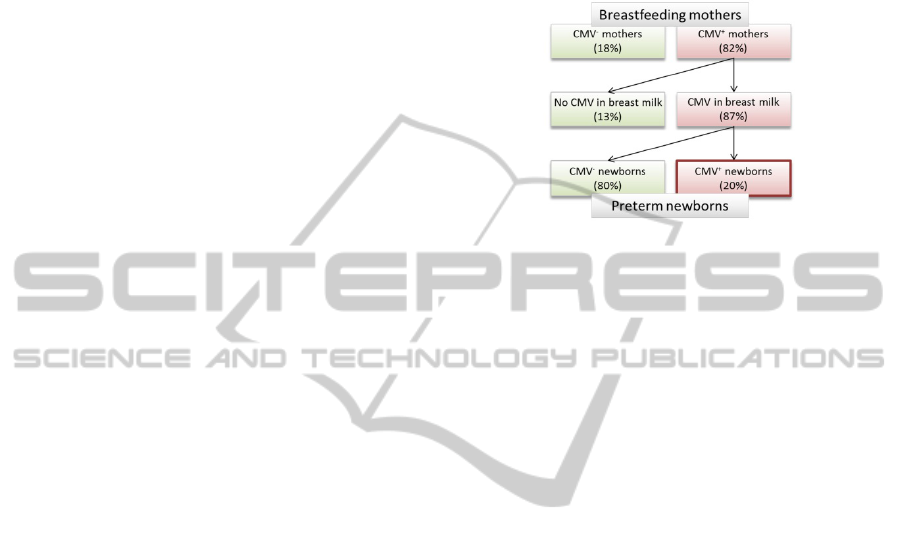
charge and reaches a maximum value 4 to 8 weeks
after birth and declines steadily thereafter. Mother-
to-child transmission generally occurs during the
period where the virus level (DNA or viral particles)
in milk is about its maximum (Hamprecht et al.
2003; Hamprecht et al. 2008).
A review paper (Kurath et al. 2010) related to
CMV transmission by breastfeeding in preterm
infants shows that 87% (median value) of mothers
whose CMV serology is positive for IgG (CMV
+
mothers) excrete CMV in their milk. Among the
children, about 20% (median value) are CMV
positive by PCR or ELISA technique for IgM and
IgG (CMV
+
newborns) and the contamination risk
increases with lactating duration (figure 1). Usually,
for term babies, symptomatic infection does not
exist because mothers start to transmit their
antibodies during the 29th pregnancy week.
Conversely, for preterm infants, the weak
transmission of mother antibodies and the non-
mature immune system increases the risk of
symptomatic CMV infection. A small birth weight
(<1.5 kg) and an early postnatal transmission
constitute risk factors of symptomatic infection
(Lombardi et al. 2012). Recent studies showed that
postnatal CMV infection in preterm infants can lead
to serious clinical consequences like respiratory
distress, neutropenia, thrombocytopenia,
hepatomegaly and septic syndromes, and can lead to
death in rare cases (Lanzieri et al. 2013; Hamele et
al. 2010; Hamprecht et al. 2008). According to
Kurath et al., 3.7% (median value) of the positive
children develop CMV related symptomatic clinical
complication with, for 0.7% (median value) of them,
appearance of a severe septic syndrome (Kurath et
al. 2010). Although the long-term follow-up of the
neurosensory development of congenitally infected
preterm infants is well documented, very few studies
concern postnatal infected preterm infants and
results obtained are generally controversial, in
particular because of the reduce number of infants in
the cohorts
(Kurath et al. 2010; Bevot et al. 2012;
Goelz et al. 2013).
Up to now, there is no consensus between
learned societies of pediatrics concerning the
attitude to be adopted and actions to be started to
prevent CMV infection via breast milk in preterm
infants less than 33 weeks.
Today, almost no national recommendations on
the manipulation of the breast milk of CMV positive
mothers are proposed. However, methods exist to
treat breast milk and consist in heating or freezing
the milk (Forsgren 2004; Hamprecht et al. 2004).
Systematic pasteurization of human milk is not done
because it alters the immune components of milk
which are particularly precious for very preterm
infants (Chang et al. 2013). Freezing at -20 °C does
not completely destroy the virus, but better preserves
biological properties of human milk (Buxmann et al.
2009).
Figure 1: Simplified epidemiology of CMV infection in
breastfed preterm newborns (Kurath, 2010).
For these reasons, neonatologists are squeezed in
their clinical practice between the potential risk to
transmit infection when breast milk is picked up to
be given to the infant, and the risk to favor digestive
complications or not to give the better chances of
neurologic development if the breast milk is not
used. In order to address this issue, the ideal solution
would be to differentiate “at risk” and “non at risk”
situations. In fact, treating the milk of only the “at
risk” population of CMV contamination via the
breast milk would be extremely more satisfactory
than a systematic attitude.
1.2 CMV Screening Tools in Breast Milk
Most of the time in France, the, presence of CMV in
lactating mother’s milk is not screened and milk
does not normally undergo specific pre-treatments in
a breast milk bank. However, an early detection of
CMV in breast milk is feasible. Studies concerning
CMV transmission via breast milk are based on
detecting the viral DNA and/or the infectious virus.
Qualitative or quantitative techniques used to detect
viral DNA are PCR, RT-PCR or nested PCR
(Hamprecht et al. 2008). These techniques are
expensive, time consuming and require the milk to
be previously prepared in 2 or 3 fractions (lipidic,
whey and cellular fractions) using various
centrifugations. Conventional cell culture gives a
result only several days after sampling and often
fails because of the native mother milk cytotoxicity.
This greatly reduces the detection sensitivity of the
infectious virus and further requires fractionation of
mother milk. Therefore, PCR and cell culture are not
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
116

adapted to rapid and early CMV detection in breast
milk.
In this position paper, we show early results
concerning CMV detection in breast milk and we
propose the development of a CMV screening
biosensor. The idea presented in this position paper
is to prevent a postnatal CMV contamination for a
majority of preterm newborns by using an adapted
milk treatment. To do this, it is necessary to detect
CMV in this liquid with a simple, fast and low-cost
system.
VIRUMILK project clearly consists of bridging a
clinical problem to an innovative technological
solution that opens up perspectives of medical
advances. It relies on previous biotechnological
research studies realized during the MEDICALIP
project (Mangeat et al. 2011) whose main scientific
achievements are:
• Homemade production of anti-CMV antibodies,
called PAbH (polyclonal human antibodies) able to
trap viral material at the surface of a biosensor.
• Grafting of these antibodies on the gold surface
of the biosensor while keeping their CMV capture
properties. Indeed, the use of commercially available
antibodies was disappointing because they lost
(partially or completely) their capture properties
when grafted onto the biochip. Grafting methods as
well as chemical environment can, in some cases,
reduce their capture efficiency. Success in producing
homemade and efficient antibodies in the virology
unit of Besançon University Hospital allowed
continuing the project.
• Set-up of methods and opto-fluidic devices for
reactive and sample flow control as well as the
optical detection of the test results.
2 THE BIOSENSOR
2.1 General Concept
The technique we proposed to detect CMV is based
on antigen/antibody recognition. The biosensor
consists of a gold coated biochip grafted with human
polyclonal anti-CMV antibodies. CMV potentially
present in the breast milk sample is captured by
these antibodies. CMV detection uses a specific
secondary antibody coupled to horseradish
peroxidase (HRP) enzyme which subsequently
recognizes the captured virus. After addition of a
substrate of this enzyme, a colorimetric reaction
occurs and allows transforming the substrate to a
blue product. When reaction is stopped, the blue
product turns into yellow. Then, the optical reading
relies on an absorbance measurement around 450
nm. A schematic representation of the biosensor is
given in figure 2.
Figure 2: The CMV biosensor.
2.2 Immunosensor Engineering
Design and production of homemade chips
compatible with Surface Plasmon Resonance
imaging (SPRi) have been performed as previously
described with the help of the MIMENTO
technological platform, Besançon, France (Remy-
Martin et al. 2012). Gold coated biochips are made
using magnetron sputtering. Gold present the
advantage of enhancing the sensitivity, reducing the
detection time and improving the specificity of the
interaction under study.
A strategy of functionalization and grafting of
homemade polyclonal anti-CMV antibodies was
optimized to guarantee an optimum anti-CMV
antibodies surface density and also to reduce non-
specific interactions onto the biochip surface.
This
strategy is based on thiol chemistry and is well
managed by the CLIPP platform (Clinical and
Innovation Proteomic Platform), Besançon, France
(Bruchard et al. 2013).
Chips are incubated in a
solution of 11-mercapto-1-undecanol/16-
mercaptohexadecanoic acid (97/3 by mole) (Sigma-
Aldrich) overnight at room temperature (RT).
Surfaces were rinsed by ethanol and ultra-pure
water. Then, 200 µl of 1-ethyl-3-(3-
dimethylaminopropyl) carbodiimide (EDC) at 200
mM/N-hydroxysulfosuccinimide (sulfo-NHS) at 50
mM (Amine Coupling Kit from Biacore AB,
Uppsala, Sweden) are added on each surface and
incubated during 30 min at RT. This step is
necessary to activate C11/C16 layer. Surfaces were
rinsed by ultra-pure water and different batches of
PAbH directed against viral proteins from various
strains (AD169 and 3 clinical strains) were spotted
(0.27 µl/spot) on the chips during 30 min at RT
under sonication in a humid chamber. To ensure
optimal grafting of the PAbH, antibodies are diluted
in an acetate buffer at 100 µg/mL, pH 5. Then
surfaces were rinsed by ultra-pure water and a
VIRUMILK-BiosensorforCMVDetectioninBreastMilkfromLactatingWomenofPretermInfantsLessthan33Weeks
117
blocking agent (Rat Serum Albumine 40 µg/mL, pH
5.2) was used to passivate the surface by incubation
at RT for 30 min. Surfaces are finally rinsed with
water and C11/C16 layer is deactivated using 200 µl
Ethanolamine-HCl (1 M pH 8.5) during 30 min à
RT. After a last rinsing by water, biochips were
ready for use in SPRi experiments.
2.3 CMV Capture and Detection
Control of the grafting of homemade antibodies onto
the chip surface and CMV capture were performed
in a SPRi-PlexII imager (Horiba Scientific, France)
equipped with an 810 nm wavelength LED and a
CCD camera. Experiments were carried out at 25°C,
in phosphate buffered saline (PBS) 1X. The flow
rate in the chamber was 20 µl/min. CMV was
commercial antigen from AD169 strain (ETI-
CYTOK-M reverse plus, Diasorin). After injection
(volume of 200 µl) the biochip surface was rinsed
for 1 min with detergent (n-Octyl-beta-D-
glucopyranoside, 40 mM)
to remove unbound
ligands.
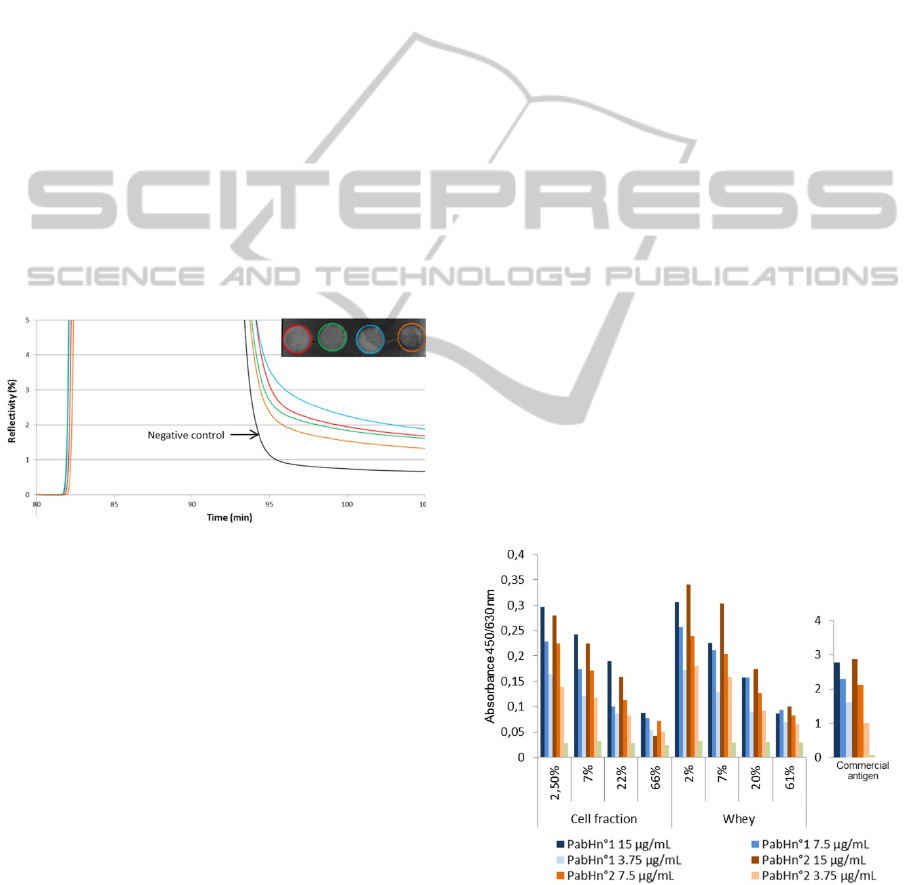
Figure 3: Sensorgram and contrast plasmon image of spots
obtained with the SPRi-PlexII after injection of
commercial CMV on grafted PAbH (4 spots and curves
correspond to different batches of PAbH).
As seen in figure 3, a signal is observed on the
four spots grafted with different batches of PAbH
whereas no significant signal is observed on the non-
grafted spots. The interaction is therefore specific
and shows the absence of undesired non-specific
binding and/or adsorption on the surface. These
results are consistent with those obtained by ELISA
(Enzyme-Like Immunosorbent Assays) experiments
using the same batches of PAbH and the same viral
material (data not shown).
In order to test whether or not similar results can
be obtained with CMV positive breast milk samples,
a direct sandwich ELISA experiment was
performed. One CMV positive breast milk sample
(volume of 5 mL) was centrifuged at 400 g during
10 min at RT in order to separate milk into 3
fractions: the cell fraction, whey and the lipidic
portion which was discarded. The CMV positivity
was assessed by PCR analysis and cell culture. In
the meantime, remaining volumes (slightly less than
1 ml) of cell and whey fractions were stored at -
80°C. When positivity is confirmed, an ELISA
experiment was conducted as follows. Fetal Calf
Serum (FCS) at a concentration of 1 µg/µL or two
batches of PAbH (produce from two different
clinical strains) at a concentration of 3.75 µg/mL,
7.5 µg/mL or 15 µg/mL in 100 µL of
carbonate/bicarbonate buffer were coated overnight
at 4°C in 96 wells microplates. The day after, a
rinsing of wells with PBS 1X followed by a
saturation step of the surface with Bovine Serum
Albumin (BSA) 3% (200 µl/well) during 1 h at RT
was performed. A mixture containing commercial
antigen or the cell fraction (diluted at 2.5%, 7%,
22%, 50% or 66% in PBS 1X) or whey (diluted at
2%, 7%, 20%, 50% or 61% in PBS 1X) and an anti-
CMV antibody conjugated to HRP diluted at 1/70
(ETI-CYTOK-M reverse plus, Diasorin) was added
(100 µL/well) and incubated during 1 h at RT. Five
washing (200 µL/well) were realised with a wash
solution composed of PBS-Tween (ETI-CYTOK-M
reverse plus, Diasorin) and the HRP substrate
(hydrogen peroxide and tetramethylbenzidine) was
incubated (100 µL/well) during 30 min at obscurity
and at RT. The reaction was stopped with 0.2 N
sulphuric acid. Absorbance of the yellow solution
obtained was immediately measured around 450/630
nm. Results are presented in the histograms in figure
4.
Figure 4: Absorbance values obtained by ELISA
experiment using two batches of PAbH and different
CMV sources (commercial antigen, breast milk cell
fraction and milk whey).
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
118

A positive control is represented by the incubation
with commercial antigen in which high absorbance
values are obtained. Decay is observed with the
increasing dilution of capture antibodies.
Concerning the cell fraction and whey, values are
lower overall and decay is observed with the
increasing dilution of capture antibodies but also
with the increasing dilution of milk fraction. As
expected, incubation of CMV with FCS shows a
very low signal.
SPRi and ELISA results show the capacity to
capture CMV with homemade antibodies on
different surfaces. Moreover, viral particles
contained in a breast milk sample can also be
captured and detected in ELISA experiment.
3 A POSSIBLE DEVICE
In the frame of another research program called
SmarTTransfuser (Charrière et al. 2012), a
laboratory prototype had been fabricated (figure 5).
It consists of a fluidic system containing a biochip
inserted into a cartridge. Syringes contain reagents
which are driven on biochips surfaces by fluidics
arrangements. The system allows controlling the
fluid flows and interaction durations.
Immunocapture and immunodetection of CMV are
performed on a biochip. In parallel, a second biochip
is used as negative control.
Preliminary results were obtained with an
experimental protocol approaching as much as
possible the conditions used in ELISA experiments
(concentrations and incubation times of reagents are
the same). Homemade functionalised biochips were
designed and produced as described above and
PAbH were grafted following the same protocol
used for SPRi experiments. BSA was first
introduced manually to the biochip followed by the
mixture of commercial antigen and detection
antibody conjugated to HRP at a flow rate of 100
µL/min during 2 min. A washing was realised by
500 µL of wash solution at a flow rate of 250
µL/min. Finally, substrate was added at a flow rate
of 100 µL/min and incubated at obscurity. The
excess of solution contained at the outlet of the
cartridge was discarded and 100 µL of blue product
was recovered in a tube and directly turned in yellow
by addition of the stop solution. Negative control
consists of a biochip incubated with all the reagents
except the mixture antigen/antibody which is replace
by only antibody diluted at 1/70 in PBS 1X. Optical
reading is performed with a spectrophotometer by
measurement about 450/630 nm.
Figure 5: General concept of the CMV detection in an
already available device (SmartTransfuser project). A
fluidic system ensures the flow of different reagents (BSA,
antigen, antibody detection coupled to HRP, wash solution
and HRP substrate) on the biochip of interest.
Absorbance values obtained in first experiments
were lower in comparison with ELISA results but a
difference in positive and negative biochips was
indeed present (factor of 5). Additional experiments
are necessary to really prove the possibility to detect
CMV in such a laboratory model and particularly
with breast milk samples.
However, this preliminary
result shows that laboratory SPR and SPRi technique
can be transposed to a more bulk device and that
fluid flow condition in this fluidic cartridge allows
specific detection of CMV.
When finalized, the biosensor validated with
breast milk will be integrated into a built-in system
which will be of simple use (presence or not of the
virus indicated with red/green LEDs). It will allow
controlling fluid flows by means of an automated
management of micro-fluidic and also timing of
different biochemical interactions in a similar way to
what we already presented before (Mangeat et al.
2011). It will include the optical reading system of
the test result
which is based on an absorbance
measurement. Finally, it will include a
human/machine
interface to use the device by a non-
expert user.
The finalized biodevice will then allow
rapid and simplified CMV detection in breast milk
of lactating mothers of preterm infants. Contact with
a company was established to develop the device,
but for reasons of intellectual property it is still too
early to assess the cost of producing the proposed
biosensor in a larger scale.
4 CONCLUSIONS
Although the risk of CMV congenital infection is
VIRUMILK-BiosensorforCMVDetectioninBreastMilkfromLactatingWomenofPretermInfantsLessthan33Weeks
119

relatively low (prevalence of about 1%), the risk of
postnatal contamination, in particular via breast
milk, can be dramatic for preterm infants. Currently,
the question is: should we favour a better
development and take the risk of using contaminated
breast milk, or should we use treated milk, even
when the CMV infection is low enough to be
considered safe?
To address this problem, and in the current
context of breastfeeding promotion, we propose to
develop a CMV biosensor based on sandwich
ELISA principle. First SPRi, ELISA and assays in a
laboratory model lead us to assume that a biochip
CMV capture and detection is possible. However,
more tests, especially with positive and negative
breast milk samples, are required to validate our
biosensor.
This position paper presents studies that have
just started, but we think it is possible to set-up an
easy to use and rapid "point-of-care" device to detect
CMV in milk of lactating mothers of preterm
infants. Therefore, a third answer can be proposed to
the above mentioned question. The idea is to screen
CMV on a routine basis and to define a personalized
feeding strategy for “at risk” population only.
Without such a rapid CMV test, this third solution
may never exist.
ACKNOWLEDGEMENTS
The authors would like to thank the French Agence
Nationale de la Recherche, INSERM and the DGOS.
This work is developed in the frame of the Biom'@x
transversal axis at FEMTO-ST.
REFERENCES
Bevot, A., et al., 2012. Long-Term Outcome in Preterm
Children with Human Cytomegalovirus Infection
Transmitted via Breast Milk. Acta Paediatrica, 101 (4),
167-172.
Bruchard, M., et al., 2013. Chemotherapy-Triggered
Cathepsin B Release in Myeloid-Derived Suppressor
Cells Activates the Nlrp3 Inflammasome and Promotes
Tumor Growth. Nature Medicine, 19 (1), 57-64.
Buxmann, H., et al., 2009. Incidence and Clinical
Outcome of Cytomegalovirus Transmission via Breast
Milk in Preterm Infants </=31 Weeks. Acta
Paediatrica, 98 (2), 270-276.
Chang, J.C., et al., 2013. Influence of Prolonged Storage
Process, Pasteurization, and Heat Treatment on
Biologically-Active Human Milk Proteins. Pediatrics
and Neonatology, 54 (6), 360-366.
Charrière, K., et al., 2012. SmarTTransfuser - A Biochip
System for the Final ABO Compatibility Test, in:
SciTePress - Science and Technology Publications,
Vilamoura, Portugal, 257–262.
Forsgren, M., 2004. Cytomegalovirus in Breast Milk:
Reassessment of Pasteurization and Freeze-Thawing.
Pediatric Research, 56 (4), 526-528.
Goelz, R., et al., 2013. Long-Term Cognitive and
Neurological Outcome of Preterm Infants with
Postnatally Acquired CMV Infection through Breast
Milk. Archives of Disease in Childhood. Fetal and
Neonatal Edition, 98 (5), 430-433.
Hamele, M., et al., 2010. Severe Morbidity and Mortality
with Breast Milk Associated Cytomegalovirus
Infection. The Pediatric Infectious Disease Journal, 29
(1), 84-86.
Hamprecht, K., et al., 2008. Cytomegalovirus transmission
to preterm infants during lactation. Journal of Clinical
Virology, 41 (3), 198-205.
Hamprecht, K., et al., 2004. Cytomegalovirus (CMV)
Inactivation in Breast Milk: Reassessment of
Pasteurization and Freeze-Thawing. Pediatric
Research, 56 (4), 529-535.
Hamprecht, K., et al., 2003. Rapid detection and
quantification of cell free cytomegalovirus by a high-
speed centrifugation-based microculture assay:
comparison to longitudinally analyzed viral DNA load
and pp67 late transcript during lactation. Journal of
Clinical Virology, 28 (3), 303-316.
Hayashi, S., et al., 2011. Transmission of cytomegalovirus
via breast milk in extremely premature infants. J
Perinatol, 31 (6), 440-445.
Kurath, S., et., 2010. Transmission of Cytomegalovirus
via Breast Milk to the Prematurely Born Infant: A
Systematic Review. Clinical Microbiology and
Infection, 16 (8), 1172-1178.
Lanzieri, T., et al., 2013. Breast Milk-Acquired
Cytomegalovirus Infection and Disease in VLBW and
Premature Infants. Pediatrics, 131 (6), 1937-1945.
Lombardi, G., et al., 2012. Breast Milk-Acquired
Cytomegalovirus Infection in Very Low Birth Weight
Infants. The Journal of Maternal-Fetal & Neonatal
Medicine, 25 Suppl 3, 57 62.
Mangeat, T., et al., 2011, Detection of the
cytomegalovirus: a mobile device and a disposable
cartridge for detection at the patient's bed. Conférence,
Biodevices 2011, 26-29 January, Rome, Italy, In
Proceedings of the International Conference on
Biomedical Electronics and Devices, 103-108.
Remy-Martin, F., et al., 2012. Surface Plasmon Resonance
Imaging in Arrays Coupled with Mass Spectrometry
(SUPRA–MS): Proof of Concept of on-Chip
Characterization of a Potential Breast Cancer Marker in
Human Plasma. Analytical and Bioanalytical
Chemistry, 404 (2): 423-432.
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
120
