
Bioinformatics Strategies for Identifying Regions of Epigenetic
Deregulation Associated with Aberrant Transcript Splicing and
RNA-editing
Mia D. Champion
1
, Ryan A. Hlady
2
, Huihuang Yan
3
, Jared Evans
3
, Jeff Nie
3
, Jeong-Heon Lee
4
,
James Bogenberger
5
, Kannabiran Nandakumar
3
, Jaime Davila
3
, Raymond Moore
3
, Asha Nair
3
,
Daniel O’Brien
3
, Yuan-Xiao Zhu
5
, K. Martin Kortum
5
, Tamas Ordog
4,6
, Zhiguo Zhang
7
,
Richard W. Joseph
8
, A. Keith Stewart
5
, Jean-Pierre Kocher
3
, Eric Jonasch
9
, Keith D. Robertson
2
,
Raoul Tibes
5
and Thai H. Ho
5
1
Division of Biomedical Statistics and Informatics, Mayo Clinic, Scottsdale, AZ, U.S.A.
2
Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Rochester, MN, U.S.A.
3
Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN, U.S.A.
4
Translational Program Center for Individualized Medicine, Mayo Clinic, Rochester, MN, U.S.A.
5
Division of Hematology and Oncology, Mayo Clinic, Scottsdale, AZ, U.S.A.
6
Department of Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, U.S.A.
7
Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN, U.S.A.
8
Division of Hematology and Oncology, Mayo Clinic, Jacksonville, FL, U.S.A.
9
Department of Genitourinary Medical Oncology, The University of Texas MD Anderson Cancer Center,
Houston, TX, U.S.A.
Keywords: Epigenetic Modification, Splicing, RNA-editing, Co/Post-Transcriptional Processing, Bioinformatics,
NMD, Alu Elements, Transcriptome, RNA-seq, Methylation, ChIP-seq, Methyl-CpG, DNAme.
Abstract: Epigenetic modifications are associated with the regulation of co/post-transcriptional processing and
differential transcript isoforms are known to be important during cancer progression. It remains unclear how
disruptions of chromatin-based modifications contribute to tumorigenesis and how this knowledge can be
leveraged to develop more potent treatment strategies that target specific isoforms or other products of the
co/post-transcriptional regulation pathway. Rapid developments in all areas of next-generation sequencing
(DNA, RNA-seq, ChIP-seq, Methyl-CpG, etc.) have provided new opportunities to develop novel
integration and data-mining approaches, and also allows for exciting hypothesis driven bioinformatics and
computational studies. Here, we present a program that we developed and summarize the results of applying
our methods to analyze datasets from patient matched tumor or normal (T/N) paired samples, as well as cell
lines that were either sensitive or resistant (S/R) to treatment with an anti-cancer drug, 5-Azacytidine
(http://sourceforge.net/projects/chiprnaseqpro/). We discuss additional options for user-defined approaches
and general guidelines for simultaneously analyzing and annotating epigenetic and RNA-seq datasets in
order to identify and rank significant regions of epigenetic deregulation associated with aberrant splicing
and RNA-editing.
1 INTRODUCTION
Deregulation of epigenetic modifications either
mimics the effects of genetic changes, or provides
additional heritable alterations that contribute to the
development and progression of many cancers
(Feinberg et al., 2006). Epigenetic regulation is not
dependent upon simple binary states of a single type
of modification nor is it a summation of the
activities of regulating methyltransferases. Rather, it
is dependent upon the interplay of both DNA and
histone level modifications that make up complex
“combinatorial codes” (Jin et al., 2012; Cieślik and
Bekiranov, 2014). Epigenetic modifications have a
profound impact on co/post-transcriptional
processes, which also have been identified as having
163
Champion M., Hlady R., Yan H., Evans J., Nie J., Lee J., Bogenberger J., Nandakumar K., Davila J., Moore R., Nair A., O’Brien D., Zhu Y., Kortüm K.,
Ordog T., Zhang Z., Joseph R., Stewart A., Kocher J., Jonasch E., Robertson K., Tibes R. and H. Ho T..
Bioinformatics Strategies for Identifying Regions of Epigenetic Deregulation Associated with Aberrant Transcript Splicing and RNA-editing.
DOI: 10.5220/0005248001630170
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2015), pages 163-170
ISBN: 978-989-758-070-3
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

an important role in cancer progression. For
example, modulation of the levels of the histone
modification H3K36me3 (trimethylated histone H3
lysine 36) either by overexpression, or by silencing
of the SETD2 methyltransferase directly effects
alternative splicing of associated exons (Luco et al.,
2010; Simon et al., 2014). One of these alternatively
spliced mRNAs, FGFR2, commonly undergoes an
isoform switch from a “normal IIIb” FGFR2
transcript isoform to a mesenchymal “IIIc” form in
~90% of kidney renal clear cell carcinomas (ccRCC)
(Zhao et al., 2013). The mechanisms determining
how epigenetic modifications influence differential
mRNA splicing are unknown and may involve
modified histone protein mediated recruitment of
components of the splicing machinery, or on the
DNA level, may involve a regulatory role of mobile
sequence elements (e.g. Alu) that are known to
mediate “exonization” (Makalowski et al., 1994;
Ast, 2004). Alu elements are reportedly enriched for
DNA-methylation as well as active (e.g.
H3K36me3) and repressive associated histone
methylation marks (Huda et al., 2010). It is known
that in some cases, histone modifications involved in
transposable element regulation serve as a “seed
region” from which the marks can spread into
adjacent genes (Kidwell and Lisch, 2000; Feschotte,
2008). Alu elements are also involved in mediating
the post-transcriptional process of RNA-editing and
if oriented in opposition, are a favored substrate for
the ADAR enzymes (Athanasiadis et al., 2004;
Bazak et al., 2014). Computational comparative
studies have revealed that >90% of all A->I
substitutions occur within Alu elements present in
mRNAs (Kim et al., 2004; Levanon et al., 2004;
Athanasiadis et al., 2004), and there are over a
hundred million sites (Bazak et al., 2014). Although
the functional implications of Alu-associated RNA
editing are still largely unknown, it is known that
these variations influence splice site modification
(Rueter et al., 1999), mRNA stability (Wang et al.,
2013), and may affect transport. In addition, RNA-
editing sites have a known role in regulating cell
proliferation during the progression of cancer (Paz et
al., 2007; Choudhury et al., 2012; Chen et al.,
2013). Deeper understanding regarding how aberrant
epigenomic modifications regulate gene expression
and co/post-transcriptional changes during the
development and progression of many cancers
continues to unfold. Here, we present methods
packaged as a Python program used for comparative
analysis of paired patient matched tumor or normal
(T/N) samples, as well as cell lines that were either
sensitive or resistant (S/R) to treatment with the anti-
cancer drug, 5-Azacytidine in order to: 1) identify
regions exhibiting shifts in epigenetic modification
peaks between two paired samples, 2) classify
indicators of co/post-transcriptional mis-regulation
associated with epigenetic deregulation as the
abundance of aberrant splicing events and frequency
of RNA editing variations within identified regions
and, 3) assess the significance of differences for 1)
and 2) between paired samples in order to prioritize
regions for further clinical studies.
2 METHODS AND RESULTS
2.1 Source of Material for
Comparative Studies of Epigenetic
Deregulation and
Co/Post-Transcriptional
Modifications
Comparative epigenetic and transcriptional datasets
can come from a number of sources. The two most
commonly studied epigenetic modifications involve
the binding of proteins (e.g. histones) to DNA and
the methylation of cytosine nucleotides (e.g. CpG
dinucleotide). Differential library preparations are
used to characterize diverse types of histone
methylation patterns that are commonly associated
with repressive or enhanced states of chromatin and
gene expression. Transcriptome datasets are
typically generated by either microarray or RNA-seq
technologies. RNA sequencing is more suitable for
studies focused on assessing the effects of aberrant
epigenetic regulation on transcriptional processing
since it enables assessment of the presence and
abundance of novel transcript isoforms, in addition
to known transcripts (Zhao et al., 2014). Read
counts also provide a means to calculating more
absolute levels of expression and reduce signal-to-
noise ratios that are often problematic when
assessing hybridization experiments (Zhao et al.,
2014). DNA sequencing (either at the genome or
exome level) can be used to do comparative filtering
from RNA variation datasets when identifying
known or novel candidate RNA editing sites. With
regard to parameters influencing studies of
epigenetic regulation of co/post-transcriptional
processes, we discuss below some of the key issues
for dataset generation and processing.
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
164
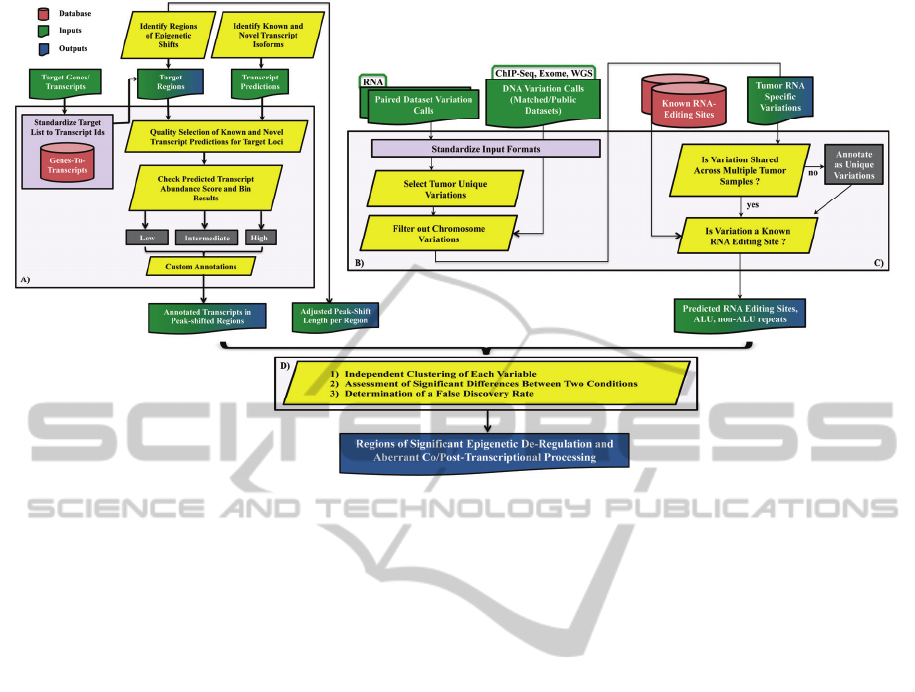
Figure 1: Workflow to expedite identification of diagnostic biomarkers or clinical targets (Champion, 2014).
2.2 Complying with the
“Garbage-In-Garbage-Out”
Concept
Each additional data source used for broad
comparative analysis increases the demand to
comply with the Garbage-In-Garbage-Out (GIGO)
concept. The percentage and distribution of millions
of sequencing reads successfully aligned to a
reference genome greatly impacts predictions of
peak enrichment (ChIP-seq), differential transcript
isoform modeling and abundance (RNA-seq), and
sequence variation identification (DNA and RNA-
seq). Standardizing thresholds for sequencing
quality, number of mismatches allowed per
alignment, and placement of reads mapping to
multiple genomic locations are steps to introducing
consistency that reduces noise when comparing
diverse datasets. Along these lines, community
established “best practices” optimize each
independent workflow, which translates to high
quality comparative datasets when interpreting
convergence of diverse variables (The Encode
Project Consortium, 2011; Landt et al., 2012; The
Broad Institute of MIT and Harvard, 2014).
Different categories of ChIP-seq tag enrichments, or
“peaks”, determine which algorithms or parameter
settings are best for optimization of true signal
prediction. Narrow peaks are characteristic of
sequence specific transcription factor binding or
RNA polymerase II transcription start site specificity
whereas broader peak domains are characteristic of
most histone marks that span a nucleosome sized
region or larger chromatin domain (Pepke et al.,
2014). In our studies (Figure 1A), SICER was used
to identify extended domains of ChIP enrichment by
adjusting the window scan with gaps allowed
parameter to the recommended size of
approximately one nucleosome and linker (~200
bps) (Zang et al., 2009). Number of read pairs from
each peak region in IP and that from the
corresponding region in input was normalized to a
library size of 10 million (FPTM) and the input-
subtracted FPTM values were used for differential
binding analysis. False Discovery Rates (FDRs)
were determined from poisson p-values and
enrichment predictions were further filtered
according to a threshold cutoff (FDR<0.01). For our
genome-wide DNA methylation studies, we used the
Infinium Human Methylation450 BeadChip
(Illumina, 2014) and normalized results using subset
quantile within-array normalization (SWAN)
(Maksimovic et al., 2012). Since there are known
gender specific epigenetic modifications, many
studies typically remove all X/Y associated data
points in order to analyze them separately. However,
we recommend doing this as a final step in the
analysis process since the inclusion of these data
points allow for a more accurate p-value adjustment
for multiple testing. Comparisons of epigenetic
deregulation and co/post-transcriptional processes
are at the gene level, such that the total region of
BioinformaticsStrategiesforIdentifyingRegionsofEpigeneticDeregulationAssociatedwithAberrantTranscriptSplicing
andRNA-editing
165

epigenetic modification affecting identified
transcript isoforms, sequence elements, and
variations is a summation of all significant peaks
within the ORF (Figure 1A).
Differences in alignment methods are also
fundamental to ensuring “best practices” of variant
calling in DNA versus RNA sequencing datasets
(The Broad Institute of MIT and Harvard, 2014).
Correct processing of RNA splice junctions,
avoidance of using soft-clipped bases, and
specialized confidence thresholds minimizes false
positive or negative variation calls. In addition,
RNA-seq library preparation protocols for creating
cDNA from RNA commonly use random hexamers
for the priming step, thus increasing the likelihood
of errors in the terminal 6bp of the read. A
combination of existing GATK parameters (e.g.
FisherStrandFilter, ReadPosRankSumTest (The
Broad Institute of MIT and Harvard, 2014)) provide
best practice filtering approaches instead of
customized methods that likely remove true positive
RNA editing sites (RVboost (Wang et al., 2014)).
Aggressive removal of shared sequence variations
between RNA and comparative DNA datasets is a
first step to identifying known and novel RNA
editing sites (Figure 1B). In addition to variations
identified using DNA sequencing generated from
same individual/cell line collections, DNA SNPs
were also identified from public population
databases (1000 Genomes, HapMap, dbSNP,
BGI200/Danish, ESP6500 European and African
datasets) (dbSNP, 2014; HapMap, 2010; ESP6500,
2014; 1000 Genomes, 2014). Mapping identified
RNA sequencing variations to existing or
customized RNA editing databases and resources
expedites identification of known RNA editing sites
(Champion, 2014; Ramaswami and Li, 2014; Kiran
and Baranov, 2010; Li et al., 2009) (Figure 1C).
Computational prioritization of candidate novel
RNA editing sites includes evaluation of the
proximity of identified RNA sequencing variations
to splice sites or paired Alu elements in opposite
orientation.
RNA-seq alignment methods, such as Tophat
(Trapnell et al., 2010), have been developed to
handle mapping of reads spanning exon-exon splice
junctions (Pepke et al., 2014). Cufflinks uses a
bipartite graphing method to assess a “minimum-
cost-maximum-matching” of bundled fragments
from Tophat read alignments in order to build a
parsimonious set of transcript models (Trapnell et
al., 2010). Cufflinks then also provides an
estimation of expression levels using established
methods (Li et al., 2010; Jiang and Wong, 2009).
We also binned predicted transcript isoforms
according to their scored minor FPKM/major FPKM
ratio in order to identify and compare differences in
transcript isoform abundances (Figure 1A). Unlike
other available algorithms, cufflinks also allows for
the identification of novel as well as known
transcripts, and exhibits superior estimates of
accuracy as measured by the median value of
relative errors in percentage across all genes when
compared to other methods (Nicolae et al., 2011).
Novel transcript predictions are essential for studies
of aberrant co/post-transcriptional processes.
Identifying novel transcripts associated with
epigenetic deregulation is useful for the discovery of
“isoform-switching” events that are associated with
drug resistance or cancer progression, similar to the
mesenchymal “IIIc” FGFR2 isoform abundant in
ccRCC. Correlating the abundance of novel
transcripts identified with regions of epigenetic
deregulation is useful for assessing levels of aberrant
transcription (e.g. “transcriptional-noise”), and
exploring possible regulatory roles of the Nonsense-
Mediated-Decay (NMD) pathway during cancer
progression (Gardner, 2011; Frischmeyer and Dietz,
1999). In addition to evidence that epigenetic
deregulation mediates aberrant splicing, these
processes also affect the rate of transcription (Veloso
et al., 2014; Eswaran et al., 2013); although, the
functional implications of differences in transcript
isoform abundances with regards to tumorigenesis or
drug response are largely unknown. Differences in
“methodological-flow” can also be used to expedite
specific outputs from these types of studies. For
example, transcriptome-profiling studies aimed to
characterize global regulatory shifts in transcript
splicing or abundance would be better done using a
transcriptome-to-peak analysis workflow.
Conversely, identification of diagnostic biomarkers
or potential clinical targets is expedited by starting
with epigenetic deregulated regions (Figure 1A) in
order to identify aberrant target transcript isoforms,
or RNA-editing site variations associated with
phenotype progression.
2.3 Bioinformatics Methods to
Integrate Epigenetic and
Co/Post-Transcriptional Datasets
Historically, methods that integrate epigenetic and
microarray expression datasets were developed to
characterize transcriptional regulons. There are
many available tools and methods available for
comparative analysis via correlative clustering of
significant shifts in epigenetic modification patterns
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
166
with differential gene expression (e.g. Rcade,
TransView (Bioconductor Software Packages,
2014)). However, unique to our approach is the
inclusion of additional variables of co/post-
transcriptional mis-regulation associated with
changes in epigenetic modifications. Although
important biological relationships between the
variables used to evaluate epigenetic influences on
transcriptional processes exist, we find that pairwise
Spearman correlations (R Development Core Team,
2011) between most of the variables assessed in our
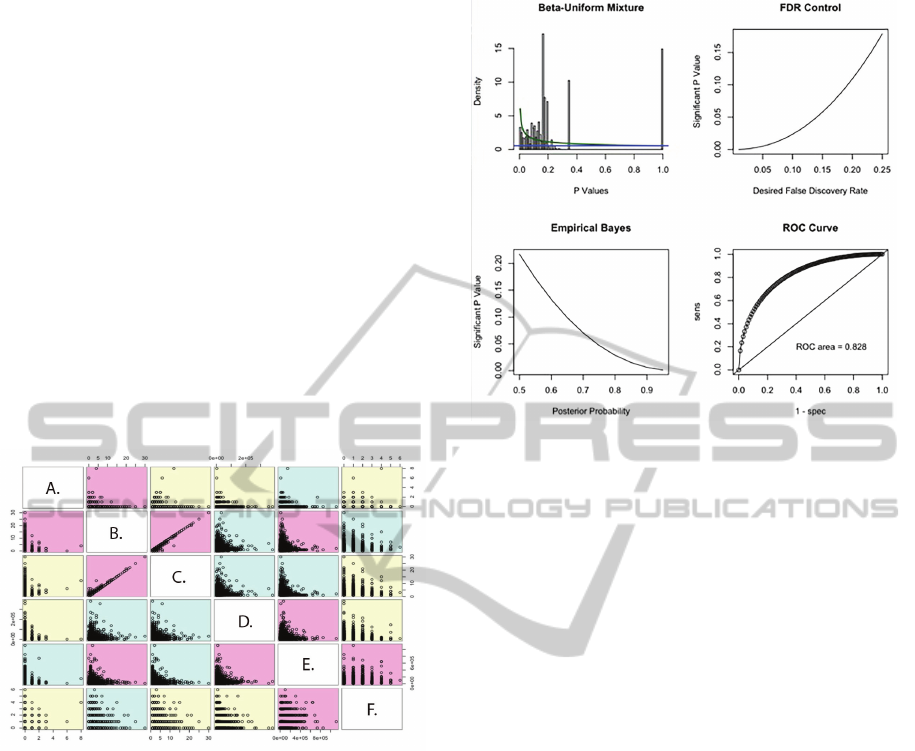
studies are not significant (Figure 2, Frequency of
non-Alu (A.) and Alu (B.) repeats, C. Frequency of
RNA editing sites, D. Adjusted Peak Length Shift,
E. ORF size, F. Frequency of novel transcript
isoforms). An exception is the expected positive
correlation between the distribution of Alu elements
(B.) and RNA editing sites (C.) within a given
region (Figure 2).
Figure 2: RNA-editing sites and Alu-elements are the only
two variables that are significantly correlated in regions of
epigenetic modification shifts.
Therefore, application of any multi-variable co-
clustering approaches would likely be inadequate
and unnecessarily exhaust computational resources.
Rather, we find that first clustering independent
variables followed by a comparative assessment of
significant differences between two conditions (e.g.
T/N, R/S) across a given region using the Student
unpaired t-Test followed by estimation of FDR using
a beta-uniform mixture model (R Development Core
Team, 2011), provides biologically meaningful
results and is useful for ranking the identified
regions of aberrant epigenetic modification and
co/post-transcriptional deregulation (Figure 3).
Finally, functional clustering of candidate clinical
targets identified by our comparative studies into
predicted interaction networks and biological
pathways identified several genes regulating cell
Figure 3: P-values equal to or less than 0.01 are within the
range of desired false discovery rate as estimated by a
beta-uniform mixture model. Significant p-values were
used to select regions for further functional analysis using
network interaction and biological pathway prediction
algorithms.
cycle progression and mRNA processing, which
further supports the validity of our methodologies
and provides an additional level of prioritization for
future diagnostic biomarker or clinical target
development (Table 1).
3 CONCLUSIONS AND
PERSPECTIVES
Advanced sequencing techniques and well-
considered bioinformatics methods provide
unprecedented opportunities for in depth
comparative studies of paired (T/N or R/S) datasets
in order to understand the regulatory roles of
epigenetic modifications on co/post-transcriptional
processes, and how deregulation of these functional
relationships promotes drug resistance and
contributes to the progression of cancer. Our studies
using a developed program to identify regions
exhibiting significant epigenetic modification
changes and aberrant co/post-transcriptional
processing exemplifies one of the workflows we
presented and provides evidence that studies such as
these yield meaningful results of potential high
impact for subsequent diagnostic biomarker or
therapeutic target design endeavors.
BioinformaticsStrategiesforIdentifyingRegionsofEpigeneticDeregulationAssociatedwithAberrantTranscriptSplicing
andRNA-editing
167
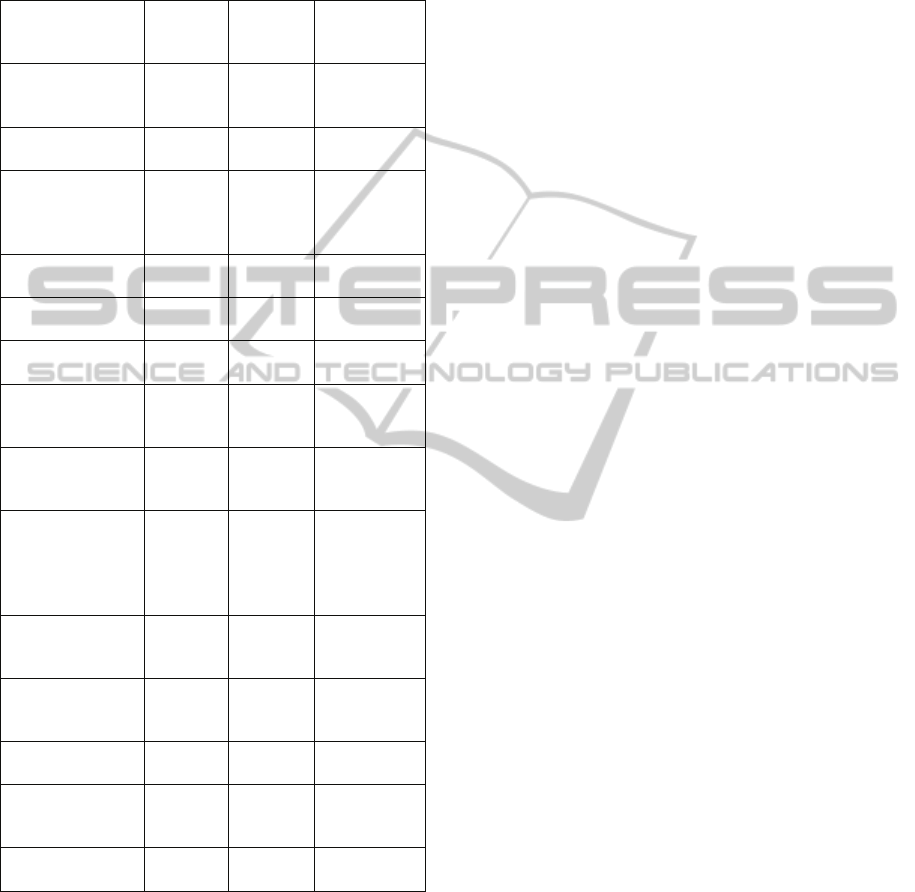
Table 1. Genes exhibiting significant epigenetic
modification shifts associated with aberrant co/post-
transcriptional processing in a 5-Azacytidine resistant
human erythroleukemia cell line, but not in a myeloid
progenitor cell line, cluster into predicted interaction
networks and functional biological pathways.
Biological
Pathway p-value FDR
Frequency
of Genes in
Pathway
Mitotic
Metaphase and
Anaphase 0
<1.00E-
03 10
Mitotic
Prometaphase 0
<5.00E-
04 8
Processing of
Capped Intron-
Containing Pre-
mRNA 0
3.33E-
04 8
RNA transport 0.0001
1.48E-
02 7
PLK1 signaling
events 0.0003
2.06E-
02 4
IL6-mediated
signaling events 0.0003
2.22E-
02 4
Deadenylation-
dependent
mRNA decay 0.0004
2.24E-
02 4
mRNA
surveillance
pathway 0.0004
2.15E-
02 5
Role of
Calcineurin-
dependent
NFAT signaling
in lymphocytes 0.0005
1.97E-
02 4
Regulation of
retinoblastoma
protein 0.001
3.95E-
02 4
IL2 signaling
events mediated
by STAT5 0.001
3.87E-
02 3
Mitotic G2-
G2/M phases 0.0013
4.66E-
02 5
IFN-alpha
signaling
pathway 0.0015
4.74E-
02 2
EPO signaling
pathway 0.0016
4.91E-
02 3
ACKNOWLEDGEMENTS
We would like to thank Ian Davis, W. Kimryn
Rathmell, Kathryn E. Hacker and Jeremy M. Simon
for assistance with genotyping of tissue. We would
like to thank Amylou Dueck for advice regarding
statistical analysis of preliminary studies.
The results published here are in whole or part based
upon data generated by The Cancer Genome Atlas
managed by the NCI and NHGRI. Information about
TCGA can be found at http://cancergenome.nih.gov.
FINANCIAL SUPPORT
T.H.H. is supported by funding from the ASCO
Young Investigator Award from the Kidney Cancer
Association, the Action to Cure Kidney Cancer
Organization, the MD Anderson Hematology-
Oncology Fellowship, a Mayo Clinic CR5 grant,
Mayo Clinic Center for Individualized Medicine
Epigenomics Translational Program and a Kathryn
H. and Roger Penske Career Development Award to
Support Medical Research. This work is supported
in part by the Mayo Clinic Center for Individualized
Medicine Epigenomics Translational Program
REFERENCES
1000Genomes. Accessed November, 2014. Available
from: http://www.1000genomes.org/data#DataAccess.
Ast, G., 2004. How did alternative splicing evolve?, Nat
Rev Genet, vol. 5, no. 10, pp. 773-782.
Athanasiadis, A, Rich, A & Maas, S., 2004. Widespread
A-to-I RNA editing of Alu-containing mRNAs in the
human transcriptome. , PLoS Biol, vol. 2, p. e391.
Bazak, L, Haviv, A, Barak, M, Jacob-Hirsch, J, Deng, P,
Zhang, R, Isaacs, FJ, Rechavi, G, Li, JB, Eisenberg, E
& Levanon, EY., 2014. A-to-I RNA editing occurs at
over a hundred million genomic sites, located in a
majority of human genes, Genome Res., vol. 24, pp.
365-376.
Bioconductor Software Packages. Accessed November,
2014. Available from :
<master.bioconductor.org/packages/release/bioc.
Champion, MD., Accessed November, 2014. ChIP-RNA-
seqPRO: A strategy for identifying regions of
epigenetic deregulation associated with aberrant
transcript splicing and RNA-editing sites. Available
from:
<http://sourceforge.net/projects/chiprnaseqpro/>.
Chen, L, Li, Y, Lin, CH, Chan, TH, Chow, RK, Song, Y,
Liu, M, Yuan, YF, Fu, L, Kong, KL, Qi, L, Li, Y &
Zhang N, TA, Kwong DL, Man K, Lo CM, Lok S,
Tenen DG, Guan XY., 2013. Recoding RNA editing
of AZIN1 predisposes to hepatocellular carcinoma.,
Nature Medicine, vol. 19, no. 2, pp. 209-16.
Choudhury, Y, Tay, FC, Lam, DH, Sandanaraj, E, Tang,
C, Ang, BT & Wang, S., 2012. Attenuated adenosine-
to-inosine editing of microRNA-376a* promotes
invasiveness of glioblastoma cells., J Clin Invest, vol.
122, no. 11, pp. 4059-76.
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
168

Cieślik, M & Bekiranov, S., 2014. Combinatorial
epigenetic patterns as quantitative predictors of
chromatin biology., BMC Genomics, vol. 15, p. 76.
The ENCODE Project Consortium, 2011. A users guide to
the encyclopedia of DNA elements (ENCODE)., PLoS
Biol, vol. 9, no. 4, p. e1001046.
dbSNP. version 137. Accessed November, 2014.
Available from: <http://www.ncbi.nlm.nih.gov/snp/>
ENCODE. DataStandards. Available from: <https://
genome.ucsc.edu/encode/protocols/dataStandards/>.
ESP6500. Accessed November, 2014. Available from:
<evs.gs.washington.edu/EVS/>.
Eswaran, J, Horvath, A, Godbole, S, Reddy, SD, Mudvari,
P, Ohshiro, K, Cyanam, D, Nair, S , Fuqua, SAW,
Polyak, K, Florea, LD, Kumar, R., 2013. RNA
sequencing of cancer reveals novel splicing
alterations, Scientific Reports, vol. 3, p. 1689.
Feinberg, AP, Ohlsson, R & Henikoff, S., 2006. The
epigenetic progenitor origin of human cancer, Nature,
vol. 7, pp. 21-33.
Feschotte, C., 2008. Transposable elements and the
evolution of regulatory networks., Nat Rev Genet, vol.
9, pp. 397-405.
Frischmeyer, PA & Dietz, HC., 1999. Nonsense-mediated
mRNA decay in health and disease., Hum Mol Genet,
vol. 8, no. 10, pp. 1893-900.
Gardner, LB., 2011. Nonsense mediated RNA decay
regulation by cellular stress; implications for
tumorigenesis, Mol Cancer Res, vol. 8, no. 3, pp. 295-
308.
HapMap. Accessed November, 2014. Available from:
<http://hapmap.ncbi.nlm.nih.gov/>
Huda, A, Mariño-Ramírez, L & Jordan, IK., 2010.
Epigenetic histone modifications of human
transposable elements: genome defense versus
exaptation., Mob DNA. , vol. 1, no. 1.
Illumina. Infinium Human Methylation450 Bead Chip.
Accessed November, 2014. Available from:
<http://res.illumina.com/documents/products/datasheet
s/datasheet_humanmethylation450.pdf>.
The Broad Institute of MIT and Harvard. GATK Best
Practices. Available from: <https://
www.broadinstitute.org/gatk/guide/best-practices>
Jiang, H & Wong, WH., 2009. Statistical inferences for
isoform expression in RNA-Seq, Bioinformatics, vol.
25, no. 8, pp. 1026-1032.
Jin, B, Ernst, J, Tiedemann, RL, Xu, H, Sureshchandra, S,
Kellis, M, Dalton, S, Liu, C, Choi, JH & Robertson,
KD., 2012. Linking DNA methyltransferases to
epigenetic marks and nucleosome structure genome-
wide in human tumor cells., Cell Rep, vol. 2, no. 5, pp.
1411-24.
Kidwell, MG & Lisch, DR., 2000. Transposable elements
and host genome evolution, Trends Ecol Evol, vol. 15,
pp. 95-99.
Kim, DD, Kim, TT, Walsh, T, Kobayashi, Y, Matise, TC,
Buyske, S & Gabriel, A., 2004. Widespread RNA
editing of embedded alu elements in the human
transcriptome., Genome Res., vol. 14, no. 9, pp. 1719-
25.
Kiran, A & Baranov, PV., 2010. DARNED: a DAtabase of
RNa EDiting in humans., Bioinformatics, vol. 26, no.
14, pp. 1772-6.
Landt, SG, Marinov, GK, Kundaje, A, Kheradpour, P,
Pauli, F, Batzoglou, S, Bernstein, BE, Bickel, P,
Brown, JB & Cayting P, CY, DeSalvo G, Epstein C,
Fisher-Aylor KI, Euskirchen G, Gerstein M, Gertz J,
Hartemink AJ, Hoffman MM, Iyer VR, Jung YL,
Karmakar S, Kellis M, Kharchenko PV, Li Q, Liu T,
Liu XS, Ma L, Milosavljevic A, Myers RM, Park PJ,
Pazin MJ, Perry MD, Raha D, Reddy TE, Rozowsky J,
Shoresh N, Sidow A, Slattery M, Stamatoyannopoulos
JA, Tolstorukov MY, White KP, Xi S, Farnham PJ,
Lieb JD, Wold BJ, Snyder M., 2012. ChIP-seq
guidelines and practices of the ENCODE and
modENCODE consortia., Genome Res., vol. 22, no. 9,
pp. 1813-31.
Levanon, E, Eisenberg, Y, Yelin, E, Nemzer, R,
Hallengger, M, Shemesh, R, Fligelman, ZY, Shoshan,
A, Pollock, SR & Sztybel, D., 2004. Systematic
identification of abundant A-to-I editing sites in the
human transcriptome, Nat Biotechnol, vol. 22, pp.
1001-1005.
Li, B, Ruotti, V, Stewart, RM, Thomson, JA & Dewey,
CN., 2010. RNA-Seq gene expression estimation with
read mapping uncertainty, Bioinformatics, vol. 26, no.
4, pp. 493-500.
Li, JB, Levanon, EY, Yoon, JK, Aach, J, Xie, B, Leproust,
E, Zhang, K, Gao, Y & Church, GM., 2009. Genome-
wide identification of human RNA editing sites by
parallel DNA capturing and sequencing., Science, vol.
324, no. 5931, pp. 1210-3.
Luco, RF, Pan, Q, Tominaga, K, Blencowe, BJ, Pereira-
Smith, OM & Misteli, T., 2010. Regulation of
alternative splicing by histone modifications., Science,
vol. 327, no. 5968, pp. 996-1000.
Makalowski, W, Mitchell, GA & Labuda, D., 1994. Alu
sequences in the coding regions of mRNA: a source of
protein
variability. , Trends Genet, vol. 10, pp. 188-193.
Maksimovic, J, Gordon, L & Oshlack, A., 2012. SWAN:
Subset-quantile within array normalization for
illumina infinium HumanMethylation450 BeadChips.,
Genome Biol, vol. 13, no. 6, p. R44.
Nicolae, M, Mangul, S, Măndoiu, II & Zelikovsky, A.,
2011. Estimation of alternative splicing isoform
frequencies from RNA-Seq data., Algorithms Mol
Biol, vol. 6, no. 1, p. 9.
Paz, N, Levanon, EY, Amariglio, N, Heimberger, AB,
Ram, Z, Constantini, S, Barbash, ZS, Adamsky, K,
Safran, M, Hirschberg, A, Krupsky, M, Ben-Dov, I,
Cazacu, S, Mikkelsen, T, Brodie, C, Eisenberg, E &
Rechavi, G., 2007. Altered adenosine-to-inosine RNA
editing in human cancer., Genome Res., vol. 17, no.
11, pp. 1586-95.
Pepke, S, Wold, BJ & Mortazavi, A., 2014. Computation
for ChIP-seq and RNA-seq studies, Nat Methods, vol.
6, no. 11, pp. S22-S32.
Ramaswami, G & Li, JB., 2014. RADAR: a rigorously
annotated database of A-to-I RNA editing, Nucleic
BioinformaticsStrategiesforIdentifyingRegionsofEpigeneticDeregulationAssociatedwithAberrantTranscriptSplicing
andRNA-editing
169

Acids Res. , vol. 42, no. (Database Issue), pp. D109-
13.
Rueter, SM, Dawson, TR & Emeson, RB., 1999.
Regulation of alternative splicing by RNA editing,
Nature, vol. 399, pp. 75-80.
Simon, JM, Hacker, KE, Singh, D, Brannon, AR, Parker,
JS, Weiser, M, Ho, TH, Kuan, PF, Jonasch, E, Furey,
TS, Prins, JF, J.D., L, Rathmell, WK & Davis, IJ.,
2014. Variation in chromatin accessibility in human
kidney cancer links H3K36 methyltransferase loss
with widespread RNA processing defects., Genome
Res., vol. 24, no. 2, pp. 241-50.
R Development Core Team. R: A language and
environment for statistical computing. Available from:
<http://www.r-project.org>
Trapnell, C, Williams, BA, Pertea, G, Mortazavi, A,
Kwan, G, van Baren, MJ, Salzberg, SL, Wold, BJ &
Pachter, L., 2010. Transcript assembly and
quantification by RNA-Seq reveals unannotated
transcripts and isoform switching during cell
differentiation., Nat Biotechnol, vol. 28, no. 5, pp.
511-5.
Veloso, A, Kirkconnell, KS, Magnuson, B, Biewen, B,
Paulsen, MT, Wilson, TE & Ljungman, M., 2014.
Rate of elongation by RNA polymerase II is associated
with specific gene features and epigenetic
modifications, Genome Res., vol. 24, no. 6, pp. 896-
905.
Wang, C, Davila, JI, Baheti, S, Bhagwate, AV, Wang, X,
Kocher, JP, Slager, SL, Feldman, AL, Novak, AJ,
Cerhan, JR, Thompson, EA & Asmann, YW., 2014.
RVboost: RNA-Seq variants prioritization using a
boosting method., Bioinformatics.
Wang, IX, So, E, Devlin, JL, Zhao, Y, Wu, M & Cheung,
VG., 2013. ADAR regulates RNA editing, transcript
stability, and gene expression., Cell Rep, vol. 5, no. 3,
pp. 849-60.
Zang, C, Schones, DE, Zeng, C, Cui, K, Zhao, K & Peng,
W., 2009. A clustering approach for identification of
enriched domains from histone modification ChIP-Seq
data, Bioinformatics, vol. 25, pp. 1952-1958.
Zhao, Q, Caballero, OL, Davis, ID, Jonasch, E, Tamboli,
P, Yung, WK, Weinstein, JN & Yao, J., 2013. Tumor-
specific isoform switch of the fibroblast growth factor
receptor 2 underlies the mesenchymal and malignant
phenotypes of clear cell renal cell carcinomas., Clin
Cancer Res., vol. 19, no. 9, pp. 2460-72.
Zhao, S, Fung-Leung, W-P, Bittner, A, Ngo, K & Liu, X.,
2014. Comparison of RNA-Seq and Microarray in
Transcriptome Profiling of Activated T Cells, PLoS
One, vol. 9, no. 1, p. e78644.
BIOINFORMATICS2015-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
170
