
Towards Voluntary Pupil Control
Training Affective Strategies?
Jan Ehlers, Nikola Bubalo, Markus Loose and Anke Huckauf
General Psychology, Ulm University , 89069 Ulm, Germany
Keywords: Affective Human-Computer Interface, Pupil Size, Biofeedback, Emotions, Voluntary Control.
Abstract: During the past years, increasing attention is being paid to operationalize pupil dynamics for affective
classification (Jacobs, 1996). Thereby it is generally assumed that pupil size displays a genuine impression of
user’s cognitive state but defies any voluntary control (Loewenfeld, 1993). Based on Ekman (2008) we
applied graphical feedback on pupil diameter changes to utilize mechanisms of operant conditioning to
gradually enable voluntary control over pupil size. Participants underwent a training program to exert control
by utilizing affective associations to expand pupil size and relaxation strategies to reduce it. As a result, more
than half of the participants demonstrated to be able to increase pupil sizes relative to baseline recordings.
Training effects did not show up. Furthermore, controlling influence diminishes after about ten seconds.
Intentional increase of sympathetic activity seems to be subject to habituation processes that allow central
inhibition of parasympathetic pathways only over a short period. Beside strategy-based factors, physiological
mechanisms like baseline pupil activity may determine inter-individual differences in exerting voluntary
control. In summary it can be noted that pupil-based communication in HCI extends affective monitoring and
may constitute an active input channel to reliably interfere by means of simple cognitive strategies.
1 INTRODUCTION
In the history of psychological research, pupil size
usually served as a metric of affect or mental effort
(Hess 1972; Janisse, 1974). However, due to the
complexity of the autonomic system, affect is hard to
measure and can’t be predicted with perfect
reliability. This is aggravated by the fact that pupil
size also appears to be influenced by several other
factors like motor responses (Simpson, 1968) or
sensory stimulation (Loewenfeld, 1966). Hyönä
(1995) suggests that the associated dynamics are
related to both, cognitive and affective information. It
is therefore critical to ascribe changes in pupil
diameter exclusively to emotional processing. Still,
controlled studies of the recent past refer to pupil size
as an adequate information channel that provides
insight into the affective state of the user (Partala,
2003).
During the last years, increasing attention is being
paid to operationalize pupil dynamics for affective
classification, even in the traditionally rational
concept of man-machine interaction (Jacobs, 1996).
One main objective behind this inclusion is to reduce
the high amount of accidents and errors in civilian
workplaces that are causally attributed to
impairments resulting from increased stress, sleep
deprivation, cognitive overload or a combination of
these factors (Yu, 2007). Also, the automotive
industry is interested to expand the already good
laboratory registration (Palinko, 2010) on real
environmental conditions. Thereby it is generally
assumed that size and responsiveness of the pupil
display a direct and genuine impression of the user’s
affective and cognitive state but defy any voluntary
control (Hess, 1972; Loewenfeld, 1993).
Classical biofeedback paradigms externalize
covert physiological responses (e.g. heart rate, skin
conductivity or various brain-wave patterns) by
providing visual or auditory online-feedback to allow
strategy-based interference for the purpose of health
preservation or to communicate in affective human-
computer frameworks (Meichenbaum, 1976; Bersak,
2001). Until recently, these parameters were
considered to be an involuntary response; however,
the generation of perceptual awareness led to
successful operant conditioning within all these
physiological functions. Like the aforementioned,
pupil dynamics are regulated by the autonomic
nervous system. Still, the vast majority of studies
5
Ehlers J., Bubalo N., Loose M. and Huckauf A..
Towards Voluntary Pupil Control - Training Affective Strategies?.
DOI: 10.5220/0005240000050012
In Proceedings of the 2nd International Conference on Physiological Computing Systems (PhyCS-2015), pages 5-12
ISBN: 978-989-758-085-7
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

refer to pupil responses as a passive information
channel that defies voluntary control.
With the present study, the view that pupils
function merely as an uncontrollable expression is put
into question. Initial results of Ekman (2008)
suggested that pupil dilation can be suspect to
training. Based on these findings, we applied
graphical real-time feedback to externalize the covert
muscle response and evaluated strategy-based
cognitive attempts to achieve voluntary control over
pupil dynamics. Preliminary data showed that several
participants were able to control their pupils (Ehlers,
2014).
A key question is whether systematic training
gradually increases the ability to control pupil
dilations. Such an adjustment to imagined scenarios
would challenge the concept of pupillary responses as
passive input variables. The capacity of willful
influencing, however, would make the pupil-based
framework susceptible to basic principles of operant
conditioning. Pupillary information would no longer
constitute a true measure during affective monitoring;
on the contrary: progressively exploring the cause-
and-effect relationship between physiological
parameters (arousal, valence, cognitive load) and
dependent changes of a system’s status may trigger
the user’s need for self-efficacy and open a channel
for strategy-based interventions and voluntary
control.
Pupil Dynamics
Size and responsiveness of the human pupil is at any
time determined by the interplay of two antagonistic
muscle groups, governed by the parasympathetic and
sympathetic nervous system. Compared to the
pupillary sphincter, the dilator muscle exerts a much
smaller force; however, this modulation results in a
dynamic equilibrium of pupillary size. An increase in
sympathetic activity is characteristically
accompanied by central inhibitions of
parasympathetic activity and leads to an enlargement
of pupil diameter. In contrast, low autonomic arousal
usually correlates with a reduction in pupil size
(Lowenstein, 1963).
Accordingly, larger pupil expansions during the
presentation of emotionally negative and positive
sounds compared to neutral conditions can be
observed (Partala & Surakka, 2003). In particular,
they report an onset of pupil dilation at about 400 ms
and a gradual increase up to four seconds after
stimulus onset.
Voluntary Control
There is, as far as we know, only few research on the
possibility of voluntary control of pupil reactions.
Ekman (2008) indicate that pupil behavior can be
influenced intentionally by strategies of affective
regulation or cognitive processing. They report pupil
size changes up to 20 % due to various strategies of
self-induced emotions, cognitive tasks, physical
activity and mild forms of pain.
Preliminary studies of our group also show that
pupil dilation can be voluntarily controlled (Ehlers,
2014). However, we assume the true potential of
pupil size-based mechanisms in HCI to be considered
only when certain aspects of iteration and training are
taken into account. That is, we examined the question
of whether and to what extent voluntary pupil
reactions can be trained. Participants had to consult
various strategies known to have modulatory effects
on pupil behavior, including positive/negative
imaginations and individual relaxation strategies.
During training, graphical real-time feedback was
applied to externalize the covert muscle response and
to enable mechanisms of operant conditioning for
gradually achieving voluntary control over pupil size.
2 METHODS
2.1 Stimuli and Apparatus
Pupil diameter of both eyes was recorded using a SMI
iViewX Hi-Speed Eyetracker working at 500 Hz with
binocular tracking. In order to facilitate control of
pupil dynamics, graphical real-time feedback was
presented on a grey screen (1680x1050 pixels at
60Hz) in a distance of 65cm (lum= 48cd/m2; approx.
5.5°visual angle). The feedback scheme disentangles
task-irrelevant variations (divergences beyond one
standard deviation around baseline) from task-
relevant variations (see Fig. 2). Performance during
baseline calibration was displayed as a static black
(mean) against a grey (standard deviation) circle. The
current pupil size (red circle) was reported back in
real-time.
Real-time feedback was provided according to the
single-value approach as specified in Ehlers (2014)
and Georgi (2014, submitted). This calculation model
is based on findings in Bremner (2012) who studied
the correlation between amplitude and peak velocity
of pupil constriction to the light reflex. Results
showed a mean amplitude of 1.92 mm (SD: 0.39) and
an average peak velocity of 5.65 mm/sec (SD: 1.17).
PhyCS2015-2ndInternationalConferenceonPhysiologicalComputingSystems
6
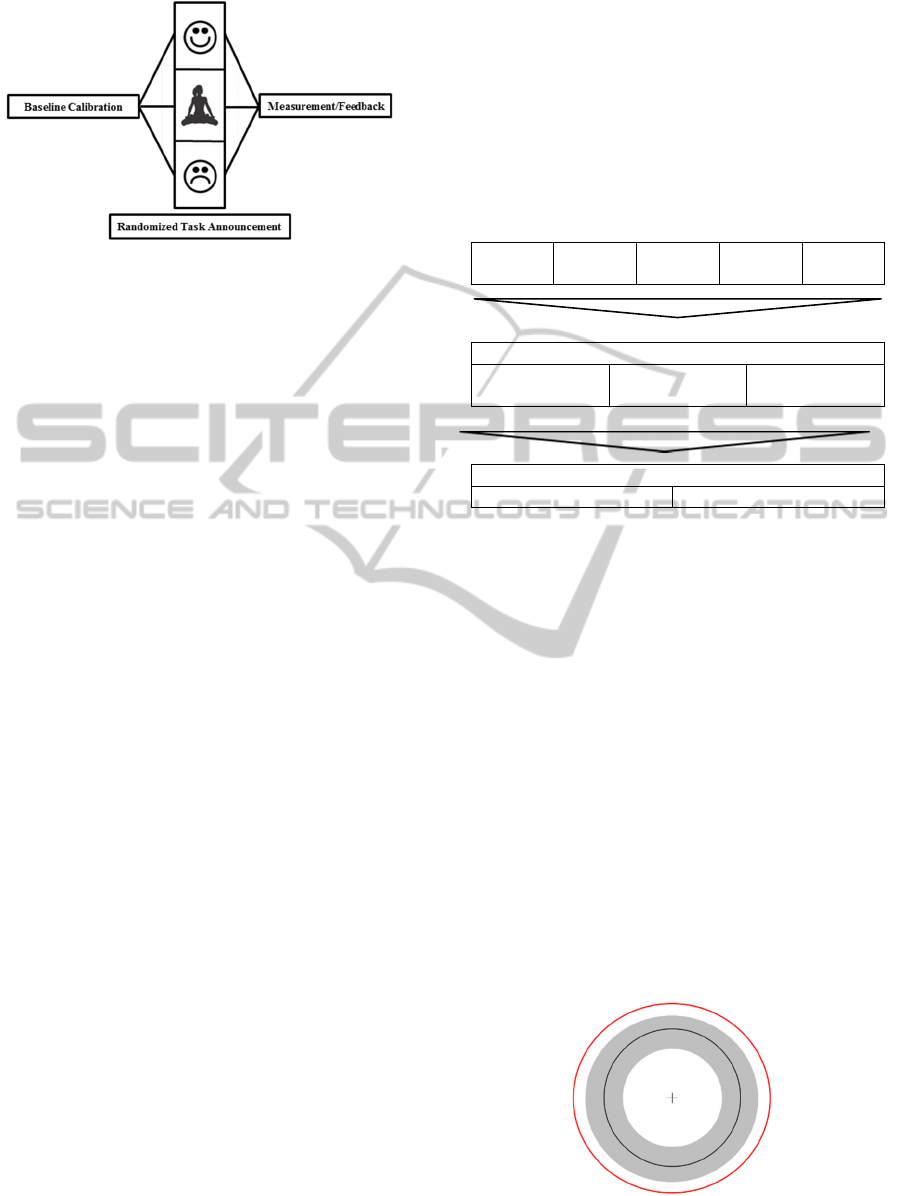
Figure 1: Instruction pictograms for self-induced positive
(upper) and negative (lower) imaginations and the
relaxation task (middle).
For the current implementation the reported peak
velocity is used as a limit for detecting (un-)valid
measurements. Converting the specifications to our
sampling rate of 30 Hz leads to an allowable sample-
to-sample change of 0.1883 mm. If the distance
between two values exceeds this range the latest
measurement is substituted by the last valid value.
Blink artefacts are rejected, and a valid pupil
contraction at the outset is detected. Still, the
dynamics subsequent to the first blink are rejected as
well. Pre-tests showed that constantly received
feedback on the basis of untreated values leads to
shaky expansion movements of the feedback circle
that are difficult to handle. As a consequence, the last
two forwarded values are averaged to smooth the
feedback dynamics and ensure increased usability.
2.2 Training Procedure
Every participant passed an introductory session (test
run) to carefully choose for individual task-specific
strategies and to initially test them with regard to the
anticipated effect. Participants were instructed to
improve their voluntary control by utilizing affective
associations to expand pupil size and by consulting
relaxation strategies to reduce it. In the following
weeks, participants underwent a training program
including four consecutive sessions with no more
than four days in between. Accordingly, the current
within-subjected design with repeated measurements
compensates for the comparable few subjects by
sustainable reducing secondary variances; however,
the experimental set-up is still characterized by a low
external validity.
Each training session consisted of three trials; two
auto-suggestion tasks (negative and positive
thoughts) and the relaxation period. Trials were
carried out in randomized order. As Janisse (1974)
suggested, pupil size is linearly related to the intensity
dimension of the stimuli and behaves curvilinearly on
the valence scale with largest expansions at the
negative and positive ends. Participants were
therefore encouraged to utilize affective
autobiographical memories and to maintain a selected
approach during the envisaged training procedure.
The same applied for the individually designed
relaxation strategies. Baseline recording preceded
every trial (Tab. 1).
Table 1: Testing procedure.
Test run
1
st
Session
2
n
d
Session
3
r
d
Session
4
th
Session
Session (randomized)
Trial 1
(Negative)
Trial 2
(Relaxation)
Trial 3
(Positive)
Trial
Baseline (15 sec) Measurement (30 sec)
Each trial consisted of establishing a baseline and a
subsequent experimental phase. Winn (1993) points
out that the pupil continuously undergoes small
oscillations and that a single „snapshot“ estimate of
its size cannot be accepted as a reliable predictor of
the true mean; it should rather be monitored for a
suitable period. We applied 15 seconds of baseline
recording to enable a reliable estimate of mean pupil
size and standard deviation.
During baseline recording participants were
instructed to avoid any emotionally charged
imaginations. Subsequent measurement time was set
to 30 seconds. Subsequent to baseline recording a
pictogram indicated the respective task (Fig. 1).
Subjects were encouraged to immediately induce
negative/positive imaginations after indicating trial
starts via button-presses. The subjects could therefore
determine the duration of rest periods between single
(randomized) trials to avoid carry-over effects as a
result of previous affective associations.
Figure 2: Feedback scheme (Ehlers, 2014).
TowardsVoluntaryPupilControl-TrainingAffectiveStrategies?
7
2.3 Participants
A total of ten participants (eight female; mean age:
21.8 years; SD: 2.5) were included in the present
study. One participant’s data had to be excluded due
to technical errors during data acquisition. All
participants were right-handed and had normal or
corrected-to-normal vision. Participants reported no
history of head injury and no neurological or
psychiatric disorder. No participant was taking any
kind of medication during the period of testing.
3 RESULTS
3.1 Effects of Training
Due to the sample size of n=9 and deviations in
sphericity, determination of statistic values followed
the non-parametric paired sample Mann-Whitney-U-
Test for comparing means over the 30 seconds
measuring time.
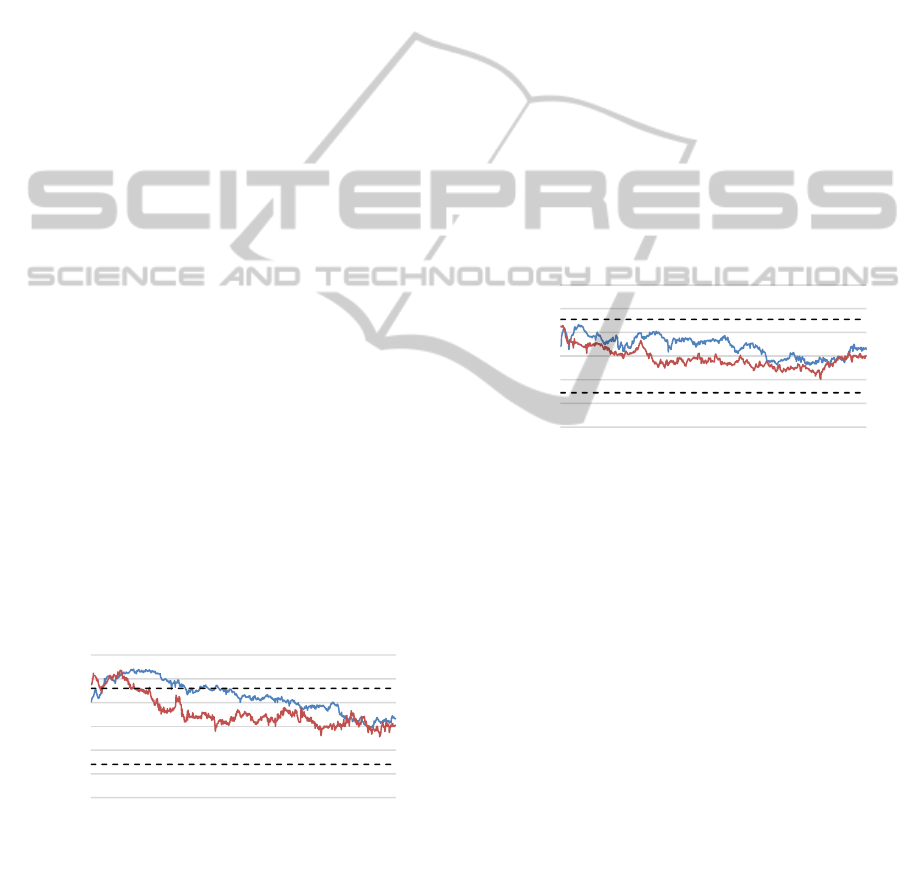
Figure 3 depicts the grand-averaged signal course
of pupil diameter for all subjects within the first and
last session of training. Participants were instructed to
utilize real-time feedback for operant learning and to
enlarge pupil size using affective associations. Since
arousal (and far less valence) seems to constitute the
influencing variable on pupil enlargement (Partala &
Surakka, 2003) and since we did not observe
differences between positive and negative
imagination trials, we forego the separation of results
and present findings as a mean outcome of affective
interventions in general. Dynamics are pictured
relative to individually determined baselines.
Baseline mean is set to zero; the dotted lines illustrate
limits of the averaged standard deviations.
Figure 3: Grand-averaged course of pupil dynamics during
the attempt to voluntary increase pupil size via self-induced
positive/negative emotions. Data points averaged across all
trials of training session one (blue line) and four (orange
line) (n=9). Signals depict variations from baseline mean
(set to zero; averaged standard deviations dotted).
Compared to baselines, self-induced associations led
to a trend of increased pupil diameters already during
the first session of training but solely within the initial
ten seconds (Fig. 3, blue line; Baseline mean: 5.11,
SD: 0.68; Measurement mean for 30 seconds: 5.49;
SD: 1.16) (T(9) = -1.9; p = .06). Subsequently, pupil
sizes decline and remain on the level of spontaneous
fluctuations. A similar tendency can be observed in
session four (orange line); however, deviations from
baseline do not longer emerge statistically significant.
Thus, an overall effect of training could not be
established.
Figure 4 illustrates the grand-averaged signal
courses during the attempt to voluntary decrease pupil
diameter by utilizing individually designed relaxation
strategies. The blue line depicts mean dynamics
during the first session of training, the orange line
traces the averaged course of session four. Though
self-induced relaxation seems to be associated with a
linear decline of pupil size, values do not fall below
spontaneous variations (dotted lines).
Figure 4: Grand-averaged course of pupil dynamics during
the attempt to voluntary reduce pupil size via individual
designed relaxation strategies. Data points averaged across
all trials of training session one (blue line) and four (orange
line) (n=9). Signals depict variations from baseline mean
(set to zero; averaged standard deviations dotted).
3.2 Grouping of Subjects
Ekman (2008) report large variability between
subjects in the general ability of controlled
interference as well as huge variations in magnitude
of the effect. Crider (1971) report individual
differences with regard to the habituation of skin
conductance responses (SCR) and spontaneous
fluctuations in skin resistance. Both variables have
been used to define a trait called “electrodermal
lability” which exhibits high retest-reliability and
reflects inter-individual differences within a variety
of information processing tasks. Subjects with high
rates of spontaneous fluctuations and/or slow SCR
habituation are referred to as electrodermal “labiles”;
in contrast to electrodermal “stabiles” with only few
‐0,6
‐0,4
‐0,2
0
0,2
0,4
0,6
0 4 8 12 16 20 24 28
RelativeChanges(mm)
Time(s)
‐0,6
‐0,4
‐0,2
0
0,2
0,4
0,6
0 4 8 1216202428
Relative Changes (mm)
Time (s)
PhyCS2015-2ndInternationalConferenceonPhysiologicalComputingSystems
8
fluctuations and/or fast SCR habituation. Individual
distinctions within physiological reactions or
habituation to (endogenous) stimuli correspond to our
basic question and we will hereafter try to apply this
concept on pupillary dynamics.
With regard to the assumptions outlined above,
we inspected the current sample in view of inter-
individual differences. It became apparent that five
out of nine participants were consistently (throughout
all trials of the complete training period) able to
utilize auto-suggestive strategies and voluntary
control pupil dynamics with regard to the anticipated
effect (here: expansion of size beyond one standard
deviation from baseline mean). In contrast, the other
four subjects were either incapable of any intentional
influencing in any session, revealed only a one-time
accidental success, or produced contrary patterns.
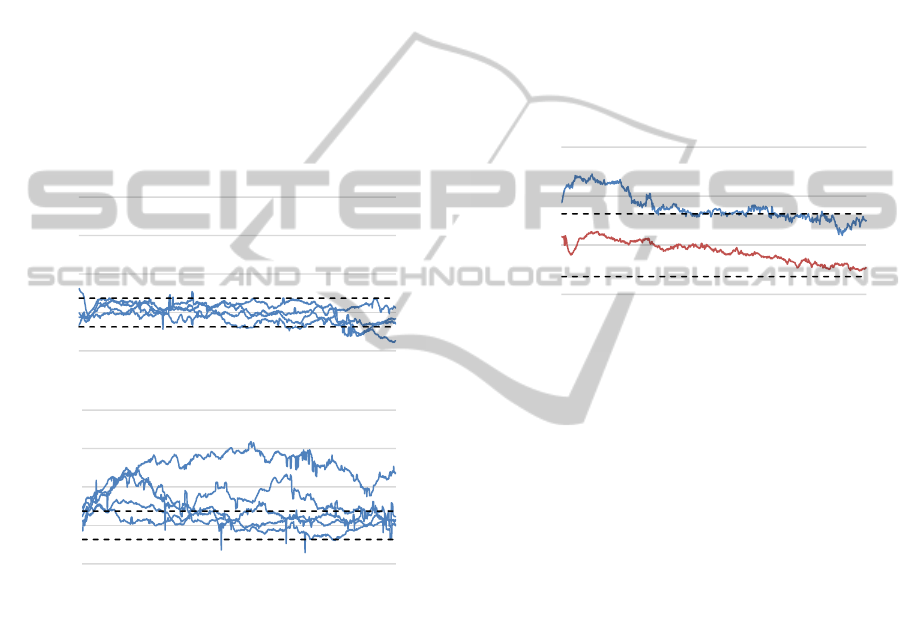
Figure 5: Course of pupil dynamics for “poor performing”
(upper illustration) (n=4) and “performing” subjects (lower
illustration) (n=5) during the attempt to increase pupil size
via self-induced positive/negative emotions. Mean values
averaged across all trials of the four training sessions.
Signals depict variations from baseline mean (set to zero;
averaged standard deviations dotted).
Applying the strict criterion of consistent success
(divergences beyond one standard deviation from
baseline in every trial of every sessions) on group
formation results in the separation depicted in Figure
5. The upper illustration outlines the averaged courses
of individual pupil size variations for poor performers
(n=4) during strategy-based intervention. The lower
figure exhibits mean values for participants
successfully exerting voluntary control over pupil
size. As can be inferred, intervention via emotionally
charged imaginations leads to increased pupil sizes
only for the “performers”. However, even an
exclusive analysis of the successful subjects did not
reveal a clear effect of further improvements during
training sessions.
Figure 6 depicts the grand-averaged course of
pupil dynamics for both groups, whereas data points
are averaged across all four training days. Again, due
to increased pupil diameters during the first ten
seconds we encounter significant higher values for
the group of “performers” (blue) (T(9) = -1.96, p =
.05). In both groups pupil sizes decline during the last
two thirds of recording and fall back on baseline
level.
Figure 6: Grand-averaged course of pupil dynamics for
“poor performing” (orange) and “performing” subjects
(blue) during the attempt to increase pupil size via self-
induced positive/negative emotions. Data points averaged
across all four training sessions. Signals depict variations
from baseline mean (set to zero; averaged standard
deviations dotted).
In contrast to group effects recorded during self-
induced emotional associations which result in
increasing pupil size, we did not discover any
performance differences with regard to the attempt to
voluntary decrease pupil diameter. Both groups failed
to exert intentional influence.
3.2.1 Baselines
According to Crider (1971) individually marked
electrodermal characteristics may serve as a
predictive variable for the performance in a variety of
information processing tasks. In accordance, we
investigated spontaneous pupil behaviour during the
15 seconds of baseline recording to explore for group-
specific differences in the underlying physiological
characteristic.
Figure 7 shows the averaged baselines preceding
the measurement of self-induced affective trials for
all participants. As can be seen, the group of
performing subjects (blue) exhibits higher values in
‐1
0
1
2
3
0 4 8 12 16 20 24 28
RelativeChanges(mm)
‐1
0
1
2
3
0 4 8 12 16 20 24 28
RelativeChanges(mm)
Time(s)
‐0,5
0
0,5
1
0 4 8 1216202428
RelativeChanges(mm)
Time(s)
TowardsVoluntaryPupilControl-TrainingAffectiveStrategies?
9
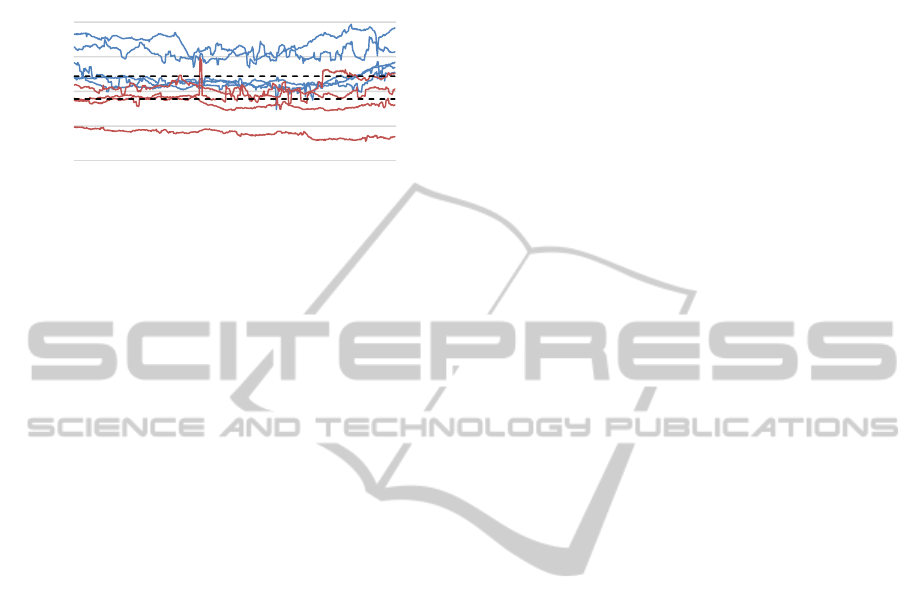
pupil diameter compared to the poor performing
participants (red). However, this trend was not of
statistical significance (T(-1,22); p = .28).
Figure 7: Individual courses of baseline data (averaged over
all sessions) preceding the trials of voluntary affective
interference for “performing” (blue) (n=5) and “poor
performing” (red) (n=4) subjects.
4 CONCLUSIONS
Based on findings of bio-feedback on various
physiological variables (Meichenbaum, 1976;
Bersak, 2001), effects of voluntary control over pupil
reactions were assessed in a training study. Five out
of nine participants demonstrated to wilful increase
their pupil sizes relative to baseline recordings.
Training effects did not show up, at least not over the
current period of four weeks and not even within the
well performing subjects. This latter group slightly
tends to exhibit larger pupil sizes already during
baseline recording.
The present study demonstrates that voluntary
controlling pupil responses is possible; at least for
about half of the participants. Probably, this
behaviour requires intuitive feedback on pupil size
changes providing adequate support for control and
facilitating the opportunity of intentional influencing
in pupil-based frameworks. But still, some
participants were not capable of exerting wilful
control, not even after weeklong training. Beside
strategy- or feedback-based factors it seems obvious
to discuss physiological mechanisms of action
determining the observed inter-individual
differences. However, due to the complexity of the
autonomic nervous system that mediates bodily states
of arousal, group distinctions may arise from a
multitude of different reasons. Though we discovered
only a weak trend at this point, poor performing
subjects featured slightly smaller pupil sizes already
during baseline recording. Further investigations with
larger samples may establish this trend. It would,
however, be reasonable to assume that smaller pupil
sizes are accompanied by a decrease in sympathetic
tone which indicates a lower level of arousal. Such a
reduced activity may limit the ability for spontaneous
self-induced changes between affective states. Again,
the current sample does not permit to test for
psychometric constructs, e.g. to consider whether the
outlined physiological characteristic correlates with
reduced trait anxiety. Norris (2007) reports increased
and prolonged deflections in skin conductance to
affective stimuli for subjects who score high on the
neuroticism scale. Such a factor may act as a
confounding psychological variable that could
prevent participants with lower values from utilizing
fear-laden imaginations to the same extent as anxious
subjects.
Partala & Surakka (2003) find significantly larger
pupil size dilations in response to both, negative and
positive stimulation compared to neutral auditory
stimuli. They report pupil dynamics following a
predetermined course with an increase at about 400
ms after stimulus onset and a plateau phase up to four
seconds followed by a slow decrease. Visual
inspection of the signal characteristics within our
performing subjects reveals an almost identical
course, displaying a slope during the first second and
a continuing dilation until about six seconds after
button-press. These similarities emerge despite the
varying methodological concepts. The stimulus-
driven design should provoke a phase-locked reaction
with high degree of inter-trail stability. In contrast,
the current paradigm consulted a response-locked
approach and is subject to blurred effects due to the
individually generated onset of self-induced
autonomic activation. As a consequence, participants
were encountered to immediately induce
negative/positive imaginations after indicating trial
starts via button-presses. However, though a true
starting point of the endogenous response could not
be determined, it can be stated that voluntary pupil
dilations follow a similar temporal sequence
compared to time-locked reactions. Furthermore,
performing subjects achieved twice the relative pupil
resize due to self-induced activation as reported for
stimulus-related changes (Partala, 2003).
Successful participants were able to expand pupil
diameter repeatedly and reliably beyond the range of
spontaneous fluctuations. Still, even for good
performing subjects, the controlling influence
diminishes after a period of about ten seconds. These
findings are in accordance with initial results
provided by Ekman (2008). Intentional increase of
sympathetic activity via self-induced affective
associations seems to be subject to habituation
processes that allow central inhibition of
3
4
5
6
7
02468101214
PupilSize(mm)
Time(s)
PhyCS2015-2ndInternationalConferenceonPhysiologicalComputingSystems
10

parasympathetic pathways only over a short period of
time. Ekman (2008) reports pupil size expansions
over a corresponding time length, even though as a
result of combined strategies (e.g. physical activity,
self-induced pain, focusing gaze). The current results
indicate a comparable outcome to be already achieved
on the sole basis of auto-suggestive strategies.
Naveteur (2005) shows that physiological
reactions to anxiety-related imaginations are not
universal equal across different subjects. Improved
coping strategies may provoke lower activity in
fearful participants compared to less anxious subjects.
With regard to the current issue, fear seemed
particular useful since it evokes the greatest pupil
expansion of all specific emotions (Al-Omar, 2013).
Cacioppo (2000) consistently states that feelings of
anxiety lead to a stronger increase in physiological
activity compared to various forms of happiness.
However, we did not find considerable differences
between self-induced autonomic activation arising
from positive or negative thoughts. This is in line with
reports from Partala & Surakka (2003) who recorded
no deviant pupil courses to stimulation with negative
and positive auditory stimuli.
Still, the unspecific strategies within the current
study may constitute a methodological deficit. During
training of pupil dilations via self-induced negative
imaginations, subjects consulted individualized
autobiographical associations such as fear, grief or
fury. While fear is closely related to sympathetic
activity, grief may involve a decrease of arousal
(Fredrickson, 2000). Reports from our subjects
indicate that, among others, personal mourning
experiences were consulted. It should therefore be
presumed that countervailing effects were produced
within the range of self-induced negative
associations.
In contrast to the findings mentioned above,
results with regard to voluntary pupil constrictions are
unequivocal. A systematic reduction in size could not
be observed. It is conceivable that the demands of a
relaxation task may increment sympathetic arousal
and produce physiological response patterns that
counter attempt parasympathetic inhibition.
Conversely, the feedback of successful pupil dilation
may cause an additional increase of autonomic
excitation and further reinforce the effect. However,
the inability to voluntary constrict pupil size is in
accordance with findings from Loewenfeld (1966)
who studied the effects of various sensory and
psychological stimuli to pupil dynamics and agreed
that none of them caused pupil constriction except
increased light intensity. Biofeedback paradigms
utilizing heart rate variations report similar findings.
Sakakibara (1994) observed that slowing down heart
rate is more difficult to accomplish than learning to
increase cardiac rhythm. Achieving low states of
arousal in laboratory conditions in which participants
have to continually monitor and process feedback
information appears therefore possible only to a
limited extent. However, Laeng (2013) reports
subjects to voluntary adjust their eye’s pupils to
imaginary light; a corresponding instruction may
therefore constitute a more suitable strategy to apply
voluntary pupil constriction.
Against the backdrop of these outstanding issues
it is mandatory to explore and evaluate further pupil
parameters sensitive to task- or individual-specific
differences. Applying the concept of electrodermal
“labiles” and “stabiles” (Criter, 1971) to pupil
dynamics may serve to further validate the depicted
group-specific distinctions and comprise the
opportunity to conceive a similar typology that
determines individual reactions to affective stimuli.
However, preliminary findings within a second test
series challenge a purely physiologic explanation
approach of the observed differences in performance.
Stricter requirements with regard to the adopted
strategy (e.g. no associations of grief during self-
induced negative imaginations) seem to bring out
clearer results and a considerable effect of training.
In summary, it can be noted that pupil-based
communication in human-computer frameworks
extends affective monitoring by far. If the required
conditions for operant learning are available, the
pupil constitutes an active input channel that allows
several users to reliably interfere by means of simple
cognitive strategies. Further studies will demonstrate
whether pupil-based interference solely contaminates
passive user observation or may even be consulted as
an autonomous input option.
ACKNOWLEDGEMENTS
We like to thank Juliane Georgi and Daniel Staiger
for valuable programming assistance and critical
discussions. This research was supported by the
Collaborative Research Center (sfb transregio 62) by
the Deutsche Forschungsgemeinschaft (DFG).
REFERENCES
Al-Omar, D., Al-Wabil, A. and Fawzi, M. (2013). Using
Pupil Size Variation During Visual Emotional
Stimulation in Measuring Affective States of Non
Communicative Individuals. In Stephanidis, C. (Ed.),
TowardsVoluntaryPupilControl-TrainingAffectiveStrategies?
11

Universal Access in Human-Computer Interaction.
User and Context Diversity (pp. 253-258). Berlin:
Springer.
Bersak, D., McDarby, G., Augenblick, N., McDarby, P.,
McDonnell, D., McDonald, B. and Karkun, R. (2001).
Intelligent Biofeedback Using an Immersive
Competitive Environment. In Abowd, G. D., Brumitt,
B. and Shafer, S. (Eds.), UbiComp 2001-Lecture Note
in Computer Science (Vol. 2201). Heidelberg,
Germany: Springer.
Bremner, F. D. (2012). Pupillometric Evaluation of the
Dynamics of the Pupillary Response to a Brief Light
Stimulus in Healthy Subjects. Investigative
Ophthalmology & Visual Science, 53, 7343-7347.
doi: 10.1167/iovs.12-10881.
Crider, A. and Lunn, R. (1971). Electrodermal Lability as a
Personality Dimension, Journal of Experimental
Research in Personality, 5, 145-150.
Ehlers, J., Georgi, J. and Huckauf, A. (in press). Improving
Voluntary Pupil Size Changes for HCI.
Ekman, I., Poikola, A., Mäkäräinen, M., Takala, T. and
Hämäläinen, P. (2008). Voluntary Pupil Size Change as
Control in Eyes Only Interaction. Proceedings of the
2008
th
Symposium on Eye Tracking Research &
Applications, 115-118.
Fredrickson, B. L., Mancuso, R. A., Branigan, C., and
Tugade, M. M. (2000). The Undoing Effect of Positive
Emotions. Motivation and Emotion, 24(4), 237-258.
Hess, E. H. (1972). Pupillometrics. In Greenfield, N.S. and
Sternbach, R.A. (Eds.), Handbook of Psychophysiology
(pp. 491-531). New York, NY: Holt, Rinehart &
Winston.
Hyönä, J., Tommola, J. and Alaja, A. M. (1995). Pupil
Dilation as a Measure of Processing Load in
Simulataneous Interpretation and Other Language
Tasks. The Quarterly Journal of Experimental
Psychology, 48(A), 598-612.
Jacob, R. J. K. (1996). The Future of Input Devices. ACM
Computing Surveys, 28, 177-179.
Janisse, M. P. (1974). Pupil Size, Affect and Exposure
Frequency. Social Behavior and Personality, 2, 125-
146.
Laeng, B. and Sulutvedt, U. (2013). The Eye Pupil Adjusts
to Imaginary Light, Psychological Science, 27.
doi: 10.1177/0956797613503556.
Larsen, J. T., Berntson, G. G., Poehlmann, K. M., Ito, T. A.
and Cacioppo, J. T. (2000). The Psychophysiology of
Emotion. In Lewis, M. and Haviland-Jones, J. M.
(Eds.), Handbook of Emotions (2
nd
ed.) (pp.173-191).
New York, NY: Guilford Press.
Loewenfeld, I. E. (1966). Comment on Hess’ Findings.
Survey of Opthalmology, 11, 291-294.
Loewenfeld, I. E. (1993). Anatomy and Physiology. In
Loewenfeld, I. E. and Lowenstein, O. (Eds.), The Pupil:
Anatomy, Physiology, and Clinical Applications (pp.
498-510). Iowa City: Iowa State University Press.
Lowenstein, O., Feinberg, R. and Loewenfeld, I. E. (1963).
Pupillary Movements During Acute and Chronic
Fatigue Investigative Ophthalmology & Visual Science,
2(2), 138-157.
Meichenbaum, D. (1976). Cognitive Factors in
Biofeedback Therapy. Biofeedback and Self-
Regulation, 1(2), 201-216.
Naveteur, J., Buisine, S. and Gruzelier, J. H. (2005). The
Influence of Anxiety on Electrodermal Responses to
Distractors. International Journal of Psychophysiology,
56(3), 261-269.
Norris, C. J., Larsen, J. T. and Cacioppo, J. T. (2007).
Neuroticism Is Associated with Larger and More
Prolonged Electrodermal Responses to Emotionally
Evocative Pictures. Psychophysiology, 44(5), 823-826.
Palinko, O., Kun, A.L., Shyrokov, A. and Heeman, P.
(2010). Proceedings of the 2010
th
Symposium on Eye-
Tracking Research & Applications, 141-144.
Partala, T. and Surakka, V. (2003). Pupil Size Variation as
an Indication of Affective Processing. International
Journal of Human-Computer Studies, 59, 185-198.
doi: 10.1016/S1071-5819(03)00017-X.
Peavler, W.S. (1974). Pupil Size, Information Overload,
and Performance Differences. Psychophysiology,
11(5), 559-566.
doi: 10.1111/j.1469-8986.1974.tb01114.x.
Sakakibara, M., Takeuchi, S. and Hayano, J. (1994). Effect
of Relaxation Training on Cardiac Parasympathetic
Tone. Psychophysiology, 31(3), 223-228.
Simpson, H. M. and Paivio, A. (1968). Effects on Pupil Size
of Manual and Verbal Indicators of Cognitive Task
Fulfilment. Attention, Perception, & Psychophysics,
3(3), 185-190.
Winn, B., Whitaker, D., Elliott, D. B. and Phillips, N. J.
(1994). Factors Affecting Light-Adapted Pupil Size in
Normal Human Subjects. Investigative Ophthalmology
& Visual Science, 35(3), 1132-1137.
Yu, M., Kautz, M. A., Thomas, M. L., Johnson, D.,
Hotchkiss, E. R. and Russo, M. B. (2007). Operational
Implications of Varying Ambient Light Levels and
Time-Of-Day Effects on Saccadic Velocity and
Pupillary Light Reflex. Ophthalmic and Physiological
Optics, 27(2), 130-141.
doi: 10.1111/j.1475-1313.2006.00450.x.
PhyCS2015-2ndInternationalConferenceonPhysiologicalComputingSystems
12
