
A Biofeedback System for Continuous Monitoring of Bone Healing
M. Windolf
1
, M. Ernst
1
, R. Schwyn
1
, S. M. Perren
1
, H. Mathis
2
, M. Wilke
1
and R. G. Richards
1
1
AO Research Institute Davos, Davos Platz, Switzerland
2
Institute for Communication Systems, HSR Rapperswil, Rapperswil, Switzerland
Keywords: Bone Healing, Fracture Healing, Biofeedback, Telemetry, Fracture Monitoring, Smart Implants,
Instrumented Plate.
Abstract: A telemetric biofeedback concept for continuous monitoring of bone healing is introduced. The system is
based on an implantable electronic unit with on-board data processing of deformations or displacements of
fracture fixation devices. In contrast to existing solutions, it allows for autonomous long-term data
collection over several months. The system enables observing fracture motion and patient activity under
daily routine conditions. Feasibility of the approach was proven in an animal experiment with an
instrumented plate monitoring axial motion in a transverse osteotomy gap at the sheep tibia. Callus
formation and maturation of the repair tissue was indicated by a decline of the interfragmentary motion
signal over time and by changes in the animal's activity pattern. For improved understanding and
interpretation of such information, extended collection of in-vivo data is the consequent next step.
1 INTRODUCTION
Flexible internal fixation is an essential modality in
today's fracture treatment. It promotes secondary
bone healing by imposing confined mechanical
stimuli at the fracture site, while still permitting
early recovery of limb function. Having reached a
high level of competence, quick, safe and reliable
healing is achieved in the majority of cases. Despite,
healing disturbances under difficult mechanical or
biological conditions such as infections, non-unions
or osteoporosis remain challenging.
Acceleration of bone healing through mechanical
stimulation of the repair tissue has been investigated
over decades (Claes et al., 1995; Goodship and
Kenwright, 1985; Perren,1979). To define the frame
for an appropriate healing environment, numerous
experimental studies investigated active and passive
mechanical stimulation with varying motion
magnitudes (Claes et al., 1995), motion frequencies
(Hente et al., 2001) and directions of motion (Bishop
et al., 2006; Claes et al., 2008; Epari et al., 2007).
However, in clinical practice the actual mechanical
circumstances at the fracture site remain a black box.
Factors such as individual limb loading, patient
activity, fracture patterns or configuration of the
fixation hardware form a complex setting
influencing the healing outcome. An objective
measure to assess fracture healing under in-vivo
conditions is, hence, required. Such information
could be valuable to improve implant designs and
application to better serve the individual
requirements. Moreover, they could have a
considerable impact on patient care, not only to
accelerate healing, but also to steer weight bearing
and early patient mobilization, detect and react on
healing disturbances or define the appropriate time-
point for implant removal.
Clinical methods to determine the state of
healing are based on radiographic evaluation or
clinical examination. Both are highly subjective.
McClelland et al. (2007) showed considerable
intraobserver variability and overall poor prediction
performance of radiographic healing assessment
criteria. Only weak correlations were found between
the radiographically determined diameter of
mineralized callus and fracture stiffness (Eastaugh-
Waring et al., 2009).
Hence, it was suggested that measuring the load
carried by a bridging implant would be an indirect
but valid criterion to assess mechanical stability of
the fractured bone. It is assumed that the load borne
by the fixation device decreases with ongoing
calcification and stiffening of the fracture callus
while interfragmentary motion and strain within the
fracture gap diminish (Cunningham et al., 1987).
243
Windolf M., Ernst M., Schwyn R., M. Perren S., Mathis H., Wilke M. and Richards R..
A Biofeedback System for Continuous Monitoring of Bone Healing.
DOI: 10.5220/0004913002430248
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2014), pages 243-248
ISBN: 978-989-758-013-0
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

This load-sharing relation between implant and bone
serves as basis to track the course of healing by
measuring load transmission through implants.
Current telemetric solutions transmitting such
data from inside the body rely on electric induction
as power source. Some are meant as research tools
to measure internal body loads (Bergmann et al.,
2001; Wilson et al., 2009), some target clinical
application (Seide et al., 2012). An induction coil,
positioned at the injured limb, is required for data
collection and transfer and therefore allows only
short term "snapshot" measurements when long term
data acquisition is actually needed for monitoring
the healing progress. Short term measurements of
implant deformation can be disturbed by several
influencing factors such as current physiological
loading of the bone or artificial conditions in a gait-
lab. Natural patient behavior and individual long-
term activity cannot be captured.
An alternative approach is proposed offering
continuous long-term measurements (approx. 4
months) of biomechanical parameters in-vivo
without external power source. An implantable and
autonomously working electronic unit was
developed for continuous recording of fracture
motion under unimpaired natural locomotion. In a
first instance the device was developed as a research
system to analyze the bone healing progress under
selected defined conditions.
2 DATA ACQUISITION CONCEPT
Targeting a method for autonomous long-term data
acquisition, a novel telemetric data logger unit was
developed. The system comprises a microprocessor
for real-time processing of sensor raw data. The
sensor signal is scanned for peak values employing a
custom-made peak detection algorithm. Assuming a
maximum step frequency of 3 Hz, the sampling rate
is set with a tenfold oversampling at 30 Hz.
Together with a stroke counter, peak values are
continuously cumulated. Results are stored
internally at predefined logging intervals. The
influence of natural variances of functional loading
is thereby averaged out. Additionally, the first
derivative of the raw signal is computed in real-time
and the average deformation rate is calculated,
considered as important parameter characterizing
bone healing. To obtain a histogram of loading
intensities, three peak detectors run in parallel at
different amplitude thresholds to sort load strokes
into distinct bins according to their magnitudes.
Instead of storing and transferring the complete
sensor signal, the data is transformed on-board into
small packages of statistically meaningful
parameters (e.g. average amplitude per stroke within
a defined time interval), using an ultra-low power
microcontroller (MSP430AFE253, Texas
Instruments, Dallas, Texas, USA). This approach
follows the hypothesis that the lion's share of such
raw data lacks meaning and would anyway be
discarded at post-processing.
Figure 1: Electronic unit used for on-board processing of
the sensor signal with wireless interface for data tranfer
via radio frequency identification (RFID).
This lean data management allows the use of an
energy-efficient wireless data transfer technology.
The download of the calculated parameters and
settings adjustment (if required) is realized by means
of Radio Frequency Identification (RFID) with a low
frequency transponder (134.2 kHz). Data download
is independent from the data collection process and
can be done on demand at freely chosen time points.
In the current system version, the patient skin is
approached with an RFID transponder to a
maximum distance of 3 cm to the implanted data
logger. The download process for 1 month of
collected data requires 12 min (at 6 h logging
intervals).
Current consumption of the device is ~60 µA
resulting in a battery lifetime of around 4.5 months
(3 V button cell battery with a capacity of
210 mAh). Size of data logger and battery is 26 mm
diameter x 7.5 mm (Figure 1).
The data collection principle is independent of
the processed signal type. Two versions of the
device with adapted signal conditioning have been
realized for receiving signals of different sensors. 1)
Connecting a conventional strain-gauge rosette,
measuring implant/fixator deformation, and 2)
Attaching a miniature LVDT displacement
transducer (linear variable differential transformer)
for measuring fracture gap motion.
In a first application, the LVDT version was used
together with a research implant system in a sheep
tibia model as described in the following.
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
244
3 PILOT ANIMAL STUDY
Functionality of the developed system under in-vivo
conditions was investigated in an animal model.
Purpose of the experiments was proving the
principle, revealing technical and methodological
issues and using the system to answer specific
research questions. Until now, a total of 10 sheep
were operated and equipped with different versions
of the data logger as part of an iterative development
process. Since settings and scope varied between
experiments, a single case will be described in the
following to illustrate the system function. Statistical
evidence is, hence, not presented.
3.1 Materials and Methods
In-vivo measurements were performed in a sheep
tibia osteotomy model. For stabilization of the
fracture a dynamizable internal fixator system was
used (AO Research Institute Davos). The implant is
axially compressible and comprises a proximal and
distal plate-body connected by two cylindrical rods,
which act as linear guides for implant motion. Two
polymer springs (Polyurethane) allow for passive
dynamization of the fracture through weight bearing
and muscle contraction and provide defined load
sharing between bone and implant. Range of motion
can be freely adjusted from rigid blocking to
macroscopic axial displacement. A miniature
displacement transducer (GHSM-1.0B, Singer
Instruments & Control Ltd., Tirat Carmel, Israel,
2 mm measuring range) was incorporated into one of
the guiding rods to measure axial plate motion
(Figure 2). The electronic unit was connected via a
biocompatible cable and was encapsulated in a
custom-made PEEK housing (Polyetheretherketon)
(Figure 3).
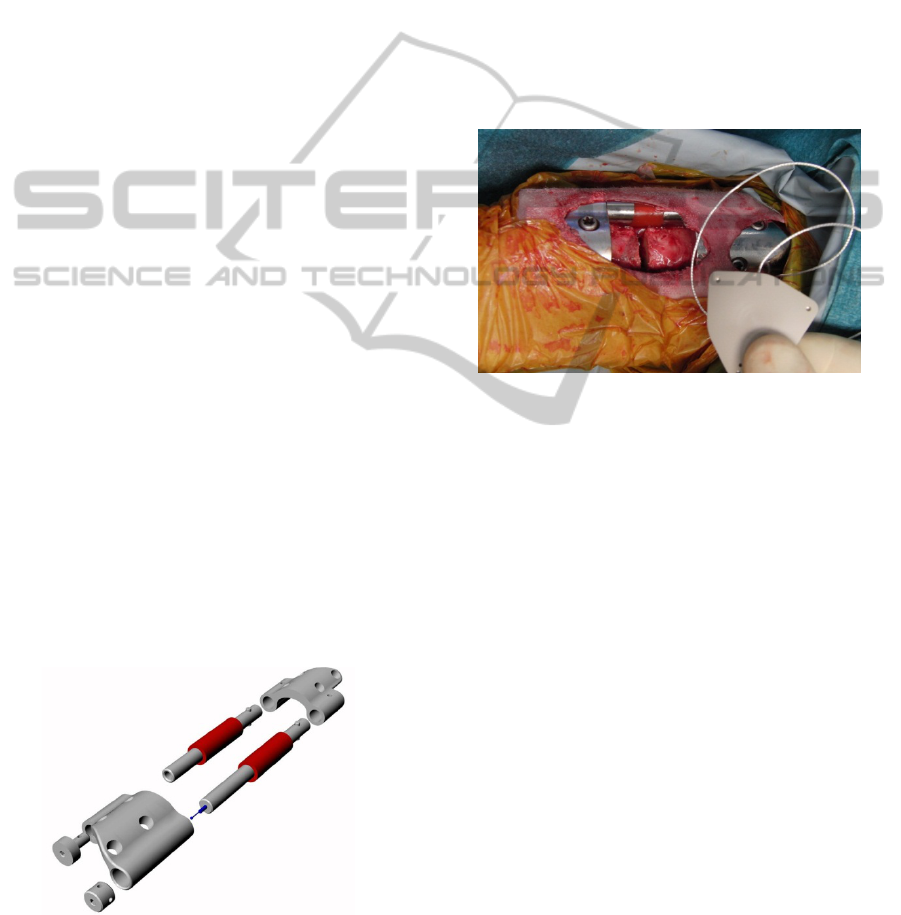
Figure 2: Exploded view of the internal fixator
instrumented with a displacement transducer and polymer
springs for passive dynamization of the fracture gap.
Implant and sensor were calibrated on a material
testing machine. Axial motion was limited to
0.3 mm and the springs were preloaded with 250 N.
Axial stiffness of the implant was ~530 N/mm.
Animal experiments were approved by the local
ethic committee for animal health and were carried
out on Swiss white alpine sheep. Surgery was
performed under general anesthesia. The implant
was placed on the medial aspect of the left tibia with
the sensor wire exiting proximally to the electronic
unit, which was positioned in a subcutaneous pocket
proximal to the implant. Following fixation of the
plate with eight 5.0 mm angular stable locking
screws, a 3 mm transverse osteotomy was created
using an oscillating saw and a guiding jig.
Figure 3: Implantation of the research implant system with
telemetric unit in a sheep tibia model. The electronic is
encapsulated in a PEEK housing and inserted proximally
to the axially compressible plate.
The sheep (body weight 50 kg) was able to bear
full weight immediately after surgery. A loose
harness was installed during the first five days
allowing the animal to rest while protecting the
fixation from excessive loading. Radiographs were
taken biweekly until euthanasia at 18 weeks post-
operation.
Thresholds for the peak detectors were set to
motion amplitudes of 0.2 mm, 0.1 mm and 0.04 mm
(0.02 mm after 6 weeks). The logging interval over
which measurements were averaged, was set to 12
hours, thereby generating two sets of parameters per
day (daytime: 6-18 h, nighttime: 18-6 h). The
recorded data was downloaded to an external
computer once or twice a week. After the animal
was killed at 18 weeks post-operation, both tibiae
were harvested and the implant was removed from
the bone. Torsional stiffness and ultimate torque was
determined for operated and contralateral tibia by
means of torsional mechanical testing to failure.
Mechanical behavior of the implant was reevaluated
after explantation and compared to the initial
mechanical test results.
ABiofeedbackSystemforContinuousMonitoringofBoneHealing
245
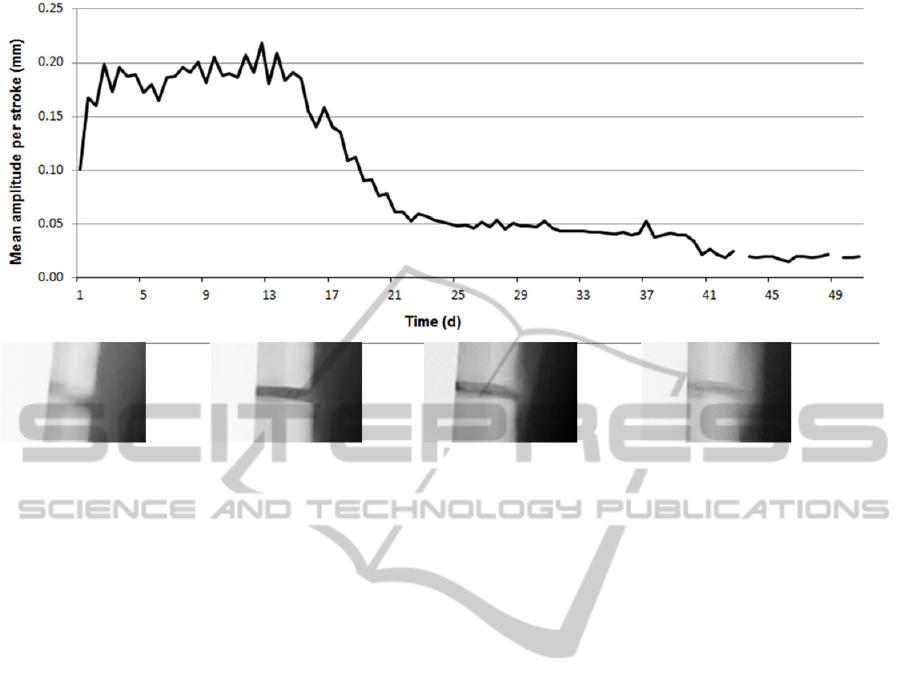
Figure 4: Recorded axial motion per load cycle over time. Displayed values are averaged over an interval of 12 hours.
Below the corresponding radiographs (AP direction) are shown on the same time scale.
3.2 Results
Data logger, sensor and implant kept functioning
throughout the experiment (18 weeks). No
pathological reactions of the animal to the system
were observed.
Mean axial displacement per stroke increased
from initially 0.1 mm after operation to around
0.2 mm within the first three days. It then
temporarily decreased again to 0.15 mm at day 6,
before reaching the highest displacement of
0.22 mm at day 12. From the beginning of the third
week post-surgery, a rapid reduction of the mean
stroke amplitude was found, followed by a slower
decrease until the minimal threshold of 0.02 mm was
reached after eight weeks post-operation. A step in
the displacement curve after day 40 is caused by
changing the lowest detector threshold to 0.02 mm.
(Figure 4).
A maximum of 480 strokes per hour was
recorded at the second day after surgery (Figure 5).
From day 3 the number of strokes/h decreased and
stabilized at a level of 50 - 100 strokes/h for the
following three weeks before the detected activity
faded out. Number of strokes was consistently
higher during daytime than during night (Figure 5).
While initially the strokes divided equally into
the three intensity bins according to displacement
magnitude, the number of strokes in the highest bin
(≥0.2 mm) increased to 50% within the first 10 days.
During the following two weeks, number of strokes
in the lowest bin (amplitude 0.04-0.1 mm) increased
continuously until no more strokes above 0.2 mm
and above 0.1 mm were recorded after 4 and 5
weeks respectively.
First signs of callus formation on radiographs
were found four weeks post-operation; bridging was
observed after six weeks. Mineralization and size of
callus then gradually increased reaching a maximum
at 8 to 10 weeks post operation.
Torsional stiffness of the operated limb was
4.3 Nm/deg (77% of contralateral). Ultimate torque
to failure yielded 58.5 Nm (72% of contralateral).
At the end of the test axial gliding of the plate
remained possible, but implant mechanics were
found altered by the biological environment. Spring
preload was reduced while stiffness of the plate had
slightly increased.
4 DISCUSSION AND OUTLOOK
A telemetric implantable data collection system for
continuous monitoring of bone healing was
introduced and successfully tested in an animal
experiment. To the authors' knowledge, this is the
first time such data could be acquired over a
complete fracture healing cycle. The general
principle of indirect healing assessment by
measuring fixator deformations (Evans et al., 1988)
was confirmed by a decline of the motion signal
while fracture callus forms. A stable response of the
derived signal over days and weeks supports the
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
246
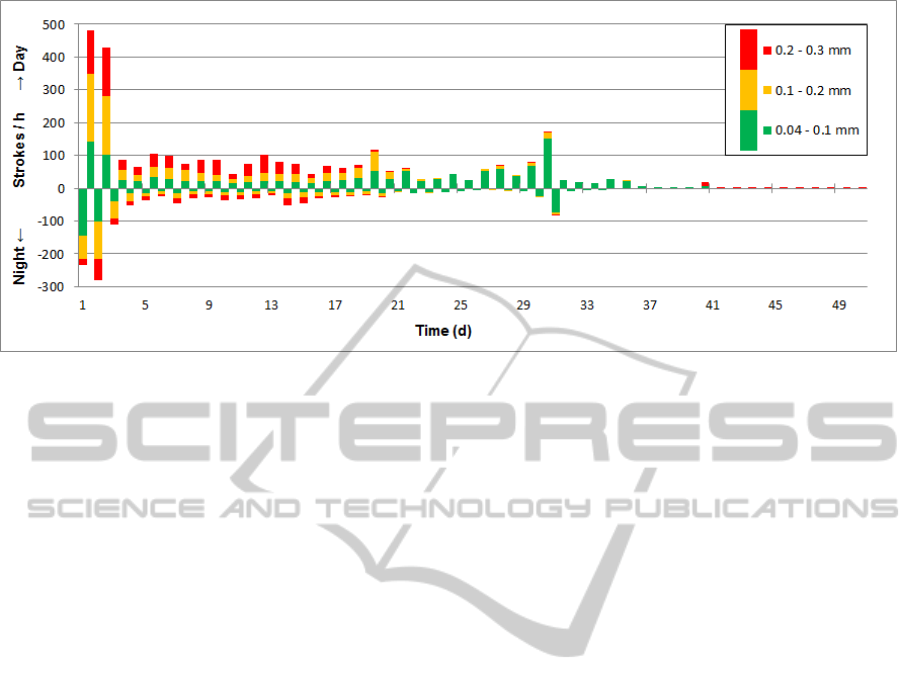
Figure 5: Number of strokes per hour recorded in time intervals of 12 hours. Green bars represent strokes with an amplitude
less than 0.1 mm, yellow bars strokes from 0.1 mm to 0.2 mm and strokes with an amplitude greater than 0.2 mm are
marked in red. Bars for daytime activity point upwards, nighttime downwards.
underlying assumption that variations in functional
loading can be averaged out. Here 12 h averaging
intervals were chosen; other timespans could be
considered. In the present setting, parameters
derived from the biofeedback system indicated
changes in fracture motion earlier than healing
became apparent on radiographs. Whether this is a
valid observation remains to be clarified. It could
also be attributed to the mechanical behavior of the
research implant used. Preloading the springs acts as
a filter shielding the fracture from low intensity
strokes. The plate is, hence, more responsive to
stiffness changes.
In contrast to other solutions (Seide et al., 2012)
the data logger system also enables assessment of
the patient activity. Onset of bone healing is
reflected by an increasingly unbalanced stroke-
intensity distribution. With ongoing fracture
consolidation, occurrence of high-intensity strokes is
fading out in favor of lower stroke intensities.
Overall patient activity in terms of number of
strokes per time-interval is another interesting
parameter which may contribute to a better
understanding of healing processes in the future. A
pronounced activity of the animal directly after
surgery became obvious, but was not necessarily to
be expected. This cannot be explained with
individual animal behavior since a general tendency
was seen in all tested animals. Pain medication
during the first days after surgery may be a reason
for accentuated limb loading. It is unclear how this
initial activity peak influences the healing process
and if the behavior can also be found in humans.
To better understand such information and to
reach an evidence level, the consequent next step is
to build up a database. Distinct healing patterns for
normal and aberrant courses of healing could be
extracted and interpreted (Burny et al., 2012; Claes
et al., 2002). Ideally, data collection should
concentrate on human patients to increase the
significance of results. Therefore, a strategy is
followed where data can be acquired at minimal
patient risk. As a first step, measurement in
combination with external fixation is an interesting
approach (Claes et al., 2002). A non-implantable,
external prototype has been developed for this
purpose. The device can be attached to an external
fixator measuring sidebar deflections without body
contact. A study to collect clinical pilot data is in
preparation.
Technically, the described data logger is still in a
prototype stage to be used for research purposes.
Several minor issues crystalized during animal
testing including robustness of the firmware, timing
accuracy of the internal clock, cable breakage or
sealing of the housing. The next generation data
logger is currently under development targeting
accelerated data transfer, increased communication
range and miniaturization. A size reduction of the
electronics unit is an interesting future option for a
potential internal application in human patients.
However, this may only be considered at a later
stage, when a distinct analysis of acquired pilot data
has been performed regarding potentials and benefits
for clinical fracture treatment.
Another future concept is the idea of active
modulation of fixation stiffness according to
individual healing progressions. The potential of
such an approach is still unknown; further research
is required (Epari et al., 2013). The electronic unit is
ABiofeedbackSystemforContinuousMonitoringofBoneHealing
247

already prepared not only to connect sensors, but
also actuators to perform defined actions for a
closed-loop control strategy.
5 CONCLUSIONS
A telemetric biofeedback concept for continuous
monitoring of bone healing was introduced. The
system allows for autonomous long-term data
collection over several months, carrying a high
potential to significantly improve fracture care.
Feasibility of the approach was proven in an animal
experiment. Collection of further in-vivo data - also
in humans - is the consequent next step.
ACKNOWLEDGEMENTS
The authors would like to thank N. Ramagnano for
his valuable contribution on electronics layout and
development.
This work was performed with the assistance of the
AO Foundation via the AOTRAUMA Network
(Grant No.: AR2012_02).
REFERENCES
Bergmann, G., Deuretzbacher, G., Heller, M., Graichen,
F., Rohlmann, A., Strauss, J., & Duda, G.N. 2001. Hip
contact forces and gait patterns from routine activities.
J.Biomech., 34, (7) 859-871 available from:
PM:11410170.
Bishop, N. E., van, R. M., Tami, I., Corveleijn, R.,
Schneider, E., & Ito, K. 2006. Shear does not
necessarily inhibit bone healing. Clin.Orthop.Relat
Res., 443, 307-314 available from: PM:16462456.
Burny, F., Burny, W., Donkerwolcke, M., & Behrens, M.
2012. Effect of callus development on the deformation
of external fixation frames. Int.Orthop., 36, (12) 2577-
2580 available from: PM:23073925.
Claes, L., Augat, P., Schorlemmer, S., Konrads, C.,
Ignatius, A., & Ehrnthaller, C. 2008. Temporary
distraction and compression of a diaphyseal osteotomy
accelerates bone healing. J.Orthop.Res., 26, (6) 772-
777 available from: PM:18240329.
Claes, L., Grass, R., Schmickal, T., Kisse, B., Eggers, C.,
Gerngross, H., Mutschler, W., Arand, M.,
Wintermeyer, T., & Wentzensen, A. 2002. Monitoring
and healing analysis of 100 tibial shaft fractures.
Langenbecks Arch.Surg., 387, (3-4) 146-152 available
from: PM:12172859.
Claes, L. E., Wilke, H. J., Augat, P., Rubenacker, S., &
Margevicius, K.J. 1995. Effect of dynamization on gap
healing of diaphyseal fractures under external fixation.
Clin.Biomech.(Bristol., Avon.), 10, (5) 227-234
available from: PM:11415558.
Cunningham, J. L., Evans, M., Harris, J. D., & Kenwright,
J. 1987. The measurement of stiffness of fractures
treated with external fixation. Eng Med., 16, (4) 229-
232 available from: PM:3691938.
Eastaugh-Waring, S. J., Joslin, C. C., Hardy, J. R., &
Cunningham, J. L. 2009. Quantification of fracture
healing from radiographs using the maximum callus
index. Clin.Orthop.Relat Res., 467, (8) 1986-1991
available from: PM:19283438.
Epari, D. R., Kassi, J. P., Schell, H., & Duda, G. N. 2007.
Timely fracture-healing requires optimization of axial
fixation stability. J.Bone Joint Surg.Am., 89, (7) 1575-
1585 available from: PM:17606797.
Epari, D. R., Wehner, T., Ignatius, A., Schuetz, M. A., &
Claes, L. E. 2013. A case for optimising fracture
healing through inverse dynamization.
Med.Hypotheses, 81, (2) 225-227 available from:
PM:23688741.
Evans, M., Kenwright, J., & Cunningham, J. L. 1988.
Design and performance of a fracture monitoring
transducer. J.Biomed.Eng, 10, (1) 64-69 available
from: PM:3347037.
Goodship, A. E. & Kenwright, J. 1985. The influence of
induced micromovement upon the healing of
experimental tibial fractures. J.Bone Joint Surg.Br.,
67, (4) 650-655 available from: PM:4030869.
Hente, R., Lechner, J., Fuechtmeier, B., Schlegel, U., &
Perren, S. 2001. Der Einfluss einer zeitlich limitierten
kontrollierten Bewegung auf die Frakturheilung. Hefte
Unfallchirurg (283) 23-24.
McClelland, D., Thomas, P. B., Bancroft, G., &
Moorcraft, C.I. 2007. Fracture healing assessment
comparing stiffness measurements using radiographs.
Clin.Orthop.Relat Res., 457, 214-219 available from:
PM:17159575.
Perren, S. M. 1979. Physical and biological aspects of
fracture healing with special reference to internal
fixation. Clin.Orthop.Relat Res. (138) 175-196
available from: PM:376198.
Seide, K., Aljudaibi, M., Weinrich, N., Kowald, B.,
Jurgens, C., Muller, J., & Faschingbauer, M. 2012.
Telemetric assessment of bone healing with an
instrumented internal fixator: a preliminary study.
J.Bone Joint Surg.Br., 94, (3) 398-404 available from:
PM:22371550.
Wilson, D. J., Morgan, R. L., Hesselden, K. L., Dodd, J.
R., Janna, S. W., & Fagan, M. J. 2009. A single-
channel telemetric intramedullary nail for in vivo
measurement of fracture healing. J.Orthop.Trauma,
23, (10) 702-709 available from: PM:19858978.
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
248
