
Rule-based Classification of Visual Field Defects
Enkelejda Kasneci
1
, Gjergji Kasneci
2
, Ulrich Schiefer
3
and Wolfgang Rosenstiel
1
1
Department of Computer Engineering, University of T
¨
ubingen, T
¨
ubingen, Germany
2
Hasso-Plattner-Institute, Potsdam, Germany
3
Centre for Ophthalmology, Institute for Ophthalmic Research, University of T
¨
ubingen, T
¨
ubingen, Germany
Keywords:
Scotoma, Rule-based, Classification, Visual Field, Defect.
Abstract:
The automated recognition of the visual field defect type from results of visual field testing is crucial for the
adequate diagnosis and treatment of the underlying disease of the visual system. This paper presents a reliable
rule-based classifier that emulates the decision strategies of expert ophthalmologists based on a two-level
approach that combines methods of unsupervised learning.
1 INTRODUCTION
Diseases affecting the optic nerve (e.g., glaucoma),
or the brain (e.g., stroke, trauma, brain injury) may
lead to blind areas or areas of reduced visual percep-
tion in the visual field. Such areas, also known as vi-
sual field defects (or scotoma), are identified through
the measurement of the visual field, i.e., perimetry.
During a perimetric examination, light stimuli of dif-
ferent luminance levels are projected onto a uniform
background at predefined locations in the visual field.
The subject confirms stimulus perception by pressing
a button; no response to a stimulus projection is in-
terpreted as failure to see the stimulus. Visual field
defects are areas at which stimuli are not perceived or
only perceived when the stimuli have high light inten-
sity.
Figure 2(a) depicts a perimetric result showing a
central visual field defect. Locations of perceived
stimuli are marked by black dots. Rectangles rep-
resent stimuli locations with reduced visual percep-
tion. The darker the rectangle, the lower the sensi-
tivity at that location. The detected location, shape
and size of defects in the visual field are hints for the
underlying disease of the visual system. In the clin-
ical routine, the classification of the visual field de-
fect type from perimetric measurements is performed
manually based on the T
¨
ubingen Scotoma Classifica-
tion Scheme (TSCS), which combines expert know-
how and long-term experience. This scheme distin-
guishes between eight classes of visual field defects
as depicted in Figure 1.
The high variability in the manifestation of visual
field defect types makes the automated classification
of visual field defects from perimetric results highly
challenging. Moreover, the measurement data may be
sparse or noisy.
Most prior techniques (Bizios et al., 2007; Boden
et al., 2007; Brigatti et al., 1997; Goldbaum et al.,
1994; Goldbaum et al., 2002; Goldbaum, 2005; Gold-
baum et al., 2009; Henson et al., 1997) have focused
on the automated detection of glaucoma from peri-
metric results, as it is a progressive disease that is
becoming more present due to demographic aging.
Other methods have considered a subset of the vi-
sual field defects in terms of the TSCS (Keating et al.,
1993; Mutlukan and Keating, 1994). The extension
and applicability of these methods to the recogni-
tion of other defect types has not been investigated.
Classification techniques for the detection of all vi-
sual field defect types based on neural networks have
been presented in (Fink, 2004; J
¨
urgens et al., 2001).
These approaches, however, do not report how the
performance of the algorithms varies with decreasing
quality of the data from perimetric results. The main
drawback of methods that are based on neural net-
works is their dependence on the training procedure,
as the overall classification performance can be nega-
tively influenced by missing correlations and noise in
the input data (Bengtsson et al., 2005; Henson et al.,
1997).
In this work we consider all the TSCS types of vi-
sual field defects and provide a highly accurate rule-
based classification technique that integrates expert
knowledge with parameters that were statistically es-
tablished from a large number of real-world visual
34
Kasneci E., Kasneci G., Schiefer U. and Rosenstiel W..
Rule-based Classification of Visual Field Defects.
DOI: 10.5220/0004746200340042
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2014), pages 34-42
ISBN: 978-989-758-010-9
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)
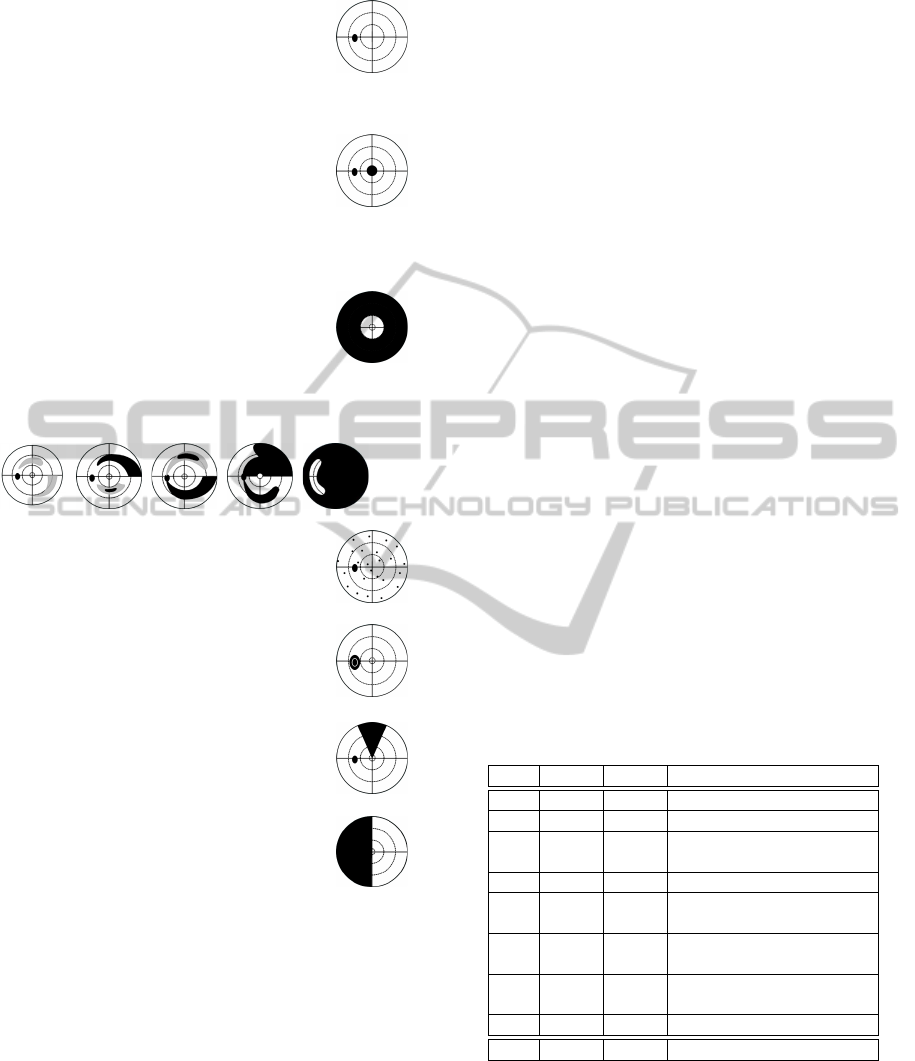
Normal visual field: Less than 7 relative de-
fects may occur anywhere in the visual field,
but not in the edges of the grid. No defect
clusters besides the physiological blind spot
are visible.
Central scotoma: Defect that occurs within
5
◦
eccentricity without respecting the verti-
cal or the horizontal meridian. Includes also
the Paracentral scotoma (normal blind spot
position) and the Centrocecal scotoma (ex-
tending from the blind spot towards the cen-
tral area symmetrically above and below the
midline).
Concentric constriction: Visual field loss
sparing the central visual field.
Glaucoma: Progressive defect that occurs in five stages
(Aulhorn and Karmeyer, 1977), from relative (left) to
massive absolute defect (right).
Diffuse visual field defect: More than 7 rela-
tive defects that are disseminated across the
visual field.
Blind spot enlargement: Defect involving at
least two points that are contiguous with the
blind spot.
Sector- oder wedge-shaped defect: Wedge-
shaped defect respecting neither the vertical
nor the horizontal meridian.
Hemianopic: Defect respecting, at least
locally, the vertical meridian.
Others: Defects that cannot be attributed to any of the
above mentioned classes.
Figure 1: T
¨
ubingen Scotoma Classification Scheme
(TSCS) (Nevalainen et al., 2007)
field examinations. Thus, our method is not neces-
sarily dependent on correlations in the training or in-
put data. In contrast to related methods, our approach
has been evaluated on perimetric results of different
quality levels.
2 INPUT DATA
Perimetric results consisting of 192 concentrically ar-
ranged stimuli locations (e.g., Figure 2(a)) are input
data to our classification method. The result con-
cerning the detection of a stimulus location by the
test subject is represented by a stimulus vector s
i
=
(x
i
, y
i
, dd
i
), i ∈ {0, . . . , 191}. (x
i
, y
i
) represents the
position of the stimulus with x
i
, y
i
∈ {−30,···, 30}.
dd
i
represents the defect depth that is related to the
measured differential luminance sensitivity at loca-
tion (x
i
, y
i
). The range of defect values from 0 dB
to over 30 dB is subdivided into 7 intervals, i.e.,
dd
i
∈ {0, ··· , 6} classes. A perimetric result can be
represented as a matrix of stimuli vectors:
M =
x
0
y
0
dd
0
···
x
191
y
191
dd
191
(1)
The classification method was evaluated on 8,868
anonymized visual field examination results provided
by the University Eye Hospital T
¨
ubingen. The data
was hand-labeled by expert ophthalmologists. In ad-
dition, a classification quality, Q ∈ {2, 3, 4}, repre-
senting the physician’s certainty for the identification
of the visual field defect, was provided. Q = 4 is the
highest quality level, i.e., the defect type could clearly
be identified. The distribution of perimetric results
among the defect classes is presented in Table 1.
Table 1: Distribution of defect types in the evaluation
dataset of perimetric results with quality level (Q4-Q2).
Q4 Q3 Q2 Visual field defect class
690 1,622 123 C
1
: Normal visual field
16 634 430 C
2
: Central scotoma
13 111 51 C
3
: Concentric constric-
tion
125 2,956 999 C
4
: Glaucoma
1 16 2 C
5
: Diffuse visual field
defects
9 268 153 C
6
: Blind spot enlarge-
ment
0 19 8 C
7
: Sector- or wedge-
shaped defect
105 315 202 C
8
: Hemianopic defect
959 5,941 1,968
3 CLASSIFICATION METHOD
Our method for the recognition of the visual field de-
fect type from a perimetric result consists of three
steps:
Rule-basedClassificationofVisualFieldDefects
35

1. In a first step, structures in perimetric exami-
nations, i.e., clusters of stimuli locations with
impaired visual perception, are found. This is
achieved by two methods of unsupervised learn-
ing, namely Hierarchical Agglomerative Clus-
tering (HAC) (Duda et al., 2000) and Self-
Organizing Maps (SOMs) (Kohonen, 1990). In
a two-level approach, SOMs are used to compute
prototype clusters, which are then combined to fi-
nal clusters using HAC. The most decisive benefit
of the SOM-based pre-clustering is noise reduc-
tion (Mangiameli et al., 1996; Henson et al., 1997;
Tafaj et al., 2011a).
2. In the next step, for the original perimetric result
new features are derived from the clusters found
in Step 1, e.g., centroid position, cluster size, aver-
age defect depth of stimuli locations in the cluster,
etc.
3. Finally, first-order logic rules check the class
membership of the enriched perimetric result (i.e.,
with the above features). The rules have been
designed in close collaboration with expert oph-
thalmologists. Uncertain decision parameters
(e.g., decision thresholds) have been optimized by
means of correlation analysis.
Step 1: Clustering for the Feature
Enrichment of Perimetric Results
Pre-clustering through Self-Organizing Maps: A
Self-Organizing Map (SOM) is an unsupervised
learning method introduced by Kohonen (Kohonen,
1990). In a SOM network, the N-dimensional in-
put data is mapped to a lower dimensional arrange-
ment (grid) of neurons, such that the topological or-
der is maintained (Kohonen, 1990). Thus, adjacent
units map to similar data points. The particularity
of a SOM is that it can be used at the same time for
both the reduction of data complexity (by clustering)
and the nonlinear projection onto a lower-dimensional
space (Kaski, 1997). Each neuron, also called unit, is
assigned a reference vector m
i
containing the synaptic
weights. These reference vectors are initialized prior
to the SOM training, usually randomly. During train-
ing, the reference vectors of the map units are iter-
atively adapted to fit the input data best. Given the
input data point x, for each unit, the distance between
its reference vector and x is computed. The Kohonen
unit c with the minimum distance to x (usually the
Euclidean metric is used) is the winner neuron, also
called Best Matching Unit, BMU, Equation 2:
c = c(x) = argmin
i
{
k
x −m
i
k
2
} (2)
Thus, the BMU determines the spatial location
of a topological neighborhood of excited neurons,
thereby providing the basis for cooperation among
neighboring neurons. In each new iteration step t + 1,
the reference vectors are adapted to the input from the
previous iteration step, x(t), according to the gradient
descent rule in Equation 3:
m
i
(t + 1) = m
i
(t) + α(t)h
ci
[x(t) −m
i
(t)] (3)
α(t) represents the learning rate over time (which
typically decreases with learning progress), whereas
h
ci
represents the neighborhood kernel around the
BMU c.
Hierarchical Agglomerative Clustering: In HAC
clusters of points are constructed in a bottom-up man-
ner; starting with each point as a singleton clus-
ter, clusters that are close to each other, according
to a predefined similarity or distance measure, e.g.,
the Euclidean distance, are merged iteratively until a
single, all-encompassing cluster remains (Jain et al.,
1999; Tan et al., 2005). We use HAC with centroid-
linkage on top of the SOM-based pre-clustering of
stimuli locations to derive coherent defect clusters.
Centroid-linkage clustering defines the cluster dis-
tance as the distance between their centroids (Flach,
2012). The goal of every merge step is to find and
merge a pair of clusters c
i
, c
j
that minimize the link-
age function L
centroid
(c
i
, c
j
):
L
centroid
(c
i
, c
j
) = D(µ
i
, µ
j
) (4)
The SOM-based clustering approach has several
advantages over other popular alternatives, such as k-
means or expectation-maximization-based methods.
Such methods require that the number of clusters
is known in advance. In our scenario, we have no
prior knowledge on the number of clusters. In the
SOM approach, the only parameter that is decisive
for the number of clusters is an empirically estab-
lished threshold on the distance of neighboring neu-
rons. That is, neighboring neurons with a distance
greater than the threshold belong to different clus-
ters. While several flat-clustering algorithms can be
adapted to take such a threshold into account, they
often face the problem of unstable results in the pres-
ence of noise. In fact, noise is the most difficult aspect
to deal with in the scenario of clustering stimuli loca-
tions.
Figure 2 illustrates how this two-level approach
was used for the analysis of visual field defects in
perimetric examination results. Figure 2(a) depicts a
perimetric examination result showing a central visual
field defect. A SOM grid (12 ×6 units) was trained
on the normalized perimetric data from Figure 2(a)
HEALTHINF2014-InternationalConferenceonHealthInformatics
36
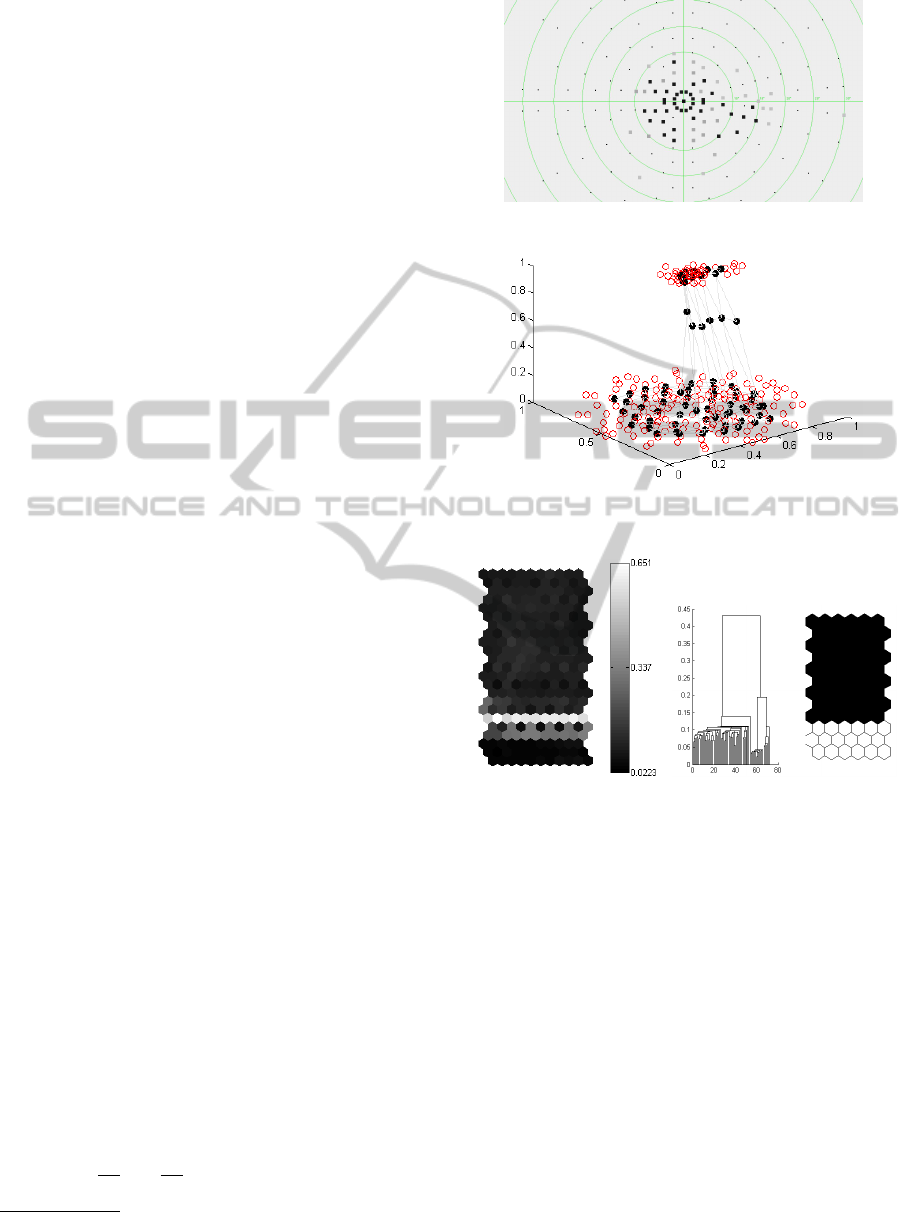
based on the Matlab SOM Toolbox
1
. Figure 2(b)
shows the mapping of the SOM units (represented by
black circles) on the normalized perimetric data. Note
that a 3-dimensional representation has been used for
the sake of better visualization. In this example, the
visual field defect and the intact area of the visual
field form well-separated clusters. Figure 2(c) shows
a so-called U-Matrix for the SOM trained in Fig-
ure 2(b) visualizing the Euclidean distances between
reference vectors of neighboring units (black repre-
sents small Euclidean distance). As is can be seen in
Figure 2(c), a bright set of cells separates two dark
areas that correspond to the clusters in the perimetric
result. Note that separating the clusters of the SOM
from Figure 2(b) according to the corresponding U-
Matrix is straight-forward for the presented example,
which was chosen for the sake of a comprehensible
demonstration of the method. However, note that in
general, the U-Matrix may suggest multiple clusters
with non-obvious boundaries between them. For such
cases further analysis is needed to separate the clus-
ters. Therefore, we apply HAC on top of the SOM
results to separate clusters based on their hierarchical
dependencies derived from the centroid-linkage strat-
egy. This was also done to detect the clusters of units
on the SOM from Figure 2(c). The corresponding
dendrogram and the clustering result are presented in
Figure 2(d).
Step 2: Feature Enrichment
The features of the clusters derived in Step 1 (e.g.,
cluster centroid, cluster size, average defect depth of
stimuli locations in a cluster, etc.) are used to enrich
the original perimetric result. The enriched feature
vector of a perimetric result is x ∈ X contains the
following features:
• the set M of 192 three-dimensional vectors repre-
senting the stimuli (see Equation 1)
• the number n
d
of defects in the perimetric exami-
nation result
• the number n
c
of clusters derived by the SOM-
based clustering
• the set of cluster means {µ
1
, ..., µ
n
c
}
• the set of cluster sizes {S
1
, ..., S
n
c
}
• the set of the numbers of stimuli locations in each
cluster {sc
1
, ..., sc
n
c
}
• the set containing the average defect depth in each
cluster {dd
1
, ..., dd
n
c
}
1
Matlab SOM Toolbox, http://www.cis.hut.fi/projects/
somtoolbox/
(a) Perimetric result showing a central visual field
defect
(b) The normalized perimetric data from (a) (red
circles) and a mapped SOM with a lattice of 12x6
units (black circles)
(c) U-Matrix visualizing
the distances between
neighboring units of the
SOM from (b)
(d) Dendrogram and clusters
resulting from HAC with
centroid-linkage on the SOM
from (b)
Figure 2: An example of SOM usage for clustering perimet-
ric results.
Such an enriched perimetric result becomes the in-
put of the classifier in Step 3, by which it is mapped
to one of the eight visual field defect classes depicted
in Figure 1.
Step 3: Recognition of Visual Field Defect
Types
The classifier has been designed as a function
f : X → C , C = {C
1
,C
2
, . . . ,C
8
}, that maps an input
x ∈ X to one of the 8 classes in C in a way that is
similar to how an expert ophthalmologist would de-
cide, namely based on rules derived from frequently
Rule-basedClassificationofVisualFieldDefects
37

occurring observations. Hence, the classification
method consists of a collection of rule-based binary
classifiers that operate based on a one-versus-all
scheme. For each of the 8 classes, a first-order logic
rule has been manually assembled by considering
decision rules used by ophthalmologists and also
by empirically analyzing a hand-labeled data set
of perimetric results of quality levels 3 and 4, see
Table 1. This data set was recommended by an expert
ophthalmologist and included 20 representatives of
each scotoma class, except for the classes C
5
and C
7
,
which were represented by 10 perimetric results each.
In order to identify appropriate values for some of the
input features (i.e., for values that are prone to uncer-
tainty), a correlation analysis was run on this data set
with the goal of increasing the accuracy of the sco-
toma classifier. To this end, the Matthews Correlation
Coefficient, MCC, was computed on the number of
true positives (TP), true negatives (TN), false posi-
tives (FP), and false negatives (FN) for a given class:
MCC =
T P×TN−FP×FN
√
(T P+FP)(T P+FN)(T N+FP)(T N+FN)
(Baldi
et al., 2000). The characterization of each class is
based on the TSCS from Figure 1.
Normal Visual Field (C
1
). The normal visual field
is characterized by only one defect cluster that corre-
sponds to the physiological blind spot and few spo-
radic defects that occur due to the patient’s inatten-
tion. The correlation analysis on the data revealed a
maximum number of 10 defects. Note that this thresh-
old is in accordance with typical thresholds used by
expert ophthalmologists, which range between 8 and
11. This number includes the failed stimuli loca-
tions within the blind spot cluster c
blindspot
with cen-
troid µ
blindspot
and size S
blindspot
, and few sporadic de-
fects. The values µ
blindspot
and S
blindspot
stem from the
TSCS.
(n
d
≤ 10 ∧ n
c
= 1 ∧ µ ≈ µ
blindspot
∧
S ≈S
blindspot
) →C
1
(5)
Central Scotoma (C
2
). A central scotoma is char-
acterized by absolute or relative defects that do not
respect the vertical or horizontal meridian in the cen-
tral visual field. A perimetric result is assigned to this
class if it fulfills the rule (RC ∨RCC) →C
2
, where RC
and RCC are defined in the Formulas 6 and 7, respec-
tively.
The RC part of the rule stems entirely from expert
knowledge: All 13 stimuli locations within 2
◦
eccen-
tricity are checked; if more than 50% of these loca-
tions, i.e., more than 6, are defects, the presence of a
central scotoma is assumed.
[RC] :
∑
s
i
∈M
(
k
(x
s
i
, y
s
i
)
k
2
≤ 2)(dd
s
i
≥ 1)) ≥ 6 (6)
RCC stems also to a large extent from expert
knowledge: A paracentral scotoma is assumed when-
ever there exists a central cluster of defects with cen-
troid within 10
◦
eccentricity, containing more than 5
stimuli. The minimum number of stimuli locations
within the central cluster was derived from correla-
tion analysis (i.e., MCC analysis) on the data. The
value 5 was revealed as a reliable threshold.
[RCC] : ∃c
i
:
k
µ
i
k
2
≤ 10 ∧ sc
i
≥ 5 ∧ dd
i
≥ 3 (7)
Concentric Constriction (C
3
). This defect type is
manifested by an intact central visual field and a pe-
ripheral field constriction. Thus, we consider a central
(within 15
◦
eccentricity) and a peripheral defect clus-
ter and check their positions. In addition, the average
defect depths in the clusters are compared. The de-
fect depth in the peripheral defect cluster is expected
to be at least 1.3 times larger than the defect depth in
the central cluster. The values 15 and 1.3 were estab-
lished by means of MCC analysis on the data.
(∃c
i
, c
j
: µ
i
≈ (0, 0) ∧ ∀s
k
∈ c
i
:
(x
s
k
, y
s
k
)
2
≤ 15
∧ µ
j
≈ (0, 0) ∧ dd
j
≥ dd
i
∗1.3) →C
3
(8)
Glaucoma (C
4
). This class is characterized by
arcuate-shaped defects. However, the perimetric re-
sults for this class only rarely showed well-separated
defect clusters as depicted in the schematic view of
Figure 1. In reality, the defect clusters were often
distorted. Furthermore, the five stages of glaucoma
reveal different cluster shapes. A perimetric exami-
nation is classified as glaucomatous if there exists a
cluster with at least 15 defects spanning over the right
and left hemifield according to the rule in Equation 9.
While a minimum average defect depth of dd
i
= 3
is a typical value observed by experts, the remaining
thresholds were established by means of MCC analy-
sis.
(∃c
i
: sc
i
≥ 15 ∧ dd
i
≥ 3 ∧
∃s
j
, s
k
∈ c
i
: x
s
j
> 10 ∧ x
s
k
< −10) →C
4
(9)
Diffuse Visual Field Defects (C
5
). These are de-
fects that are spread across the visual field, see Fig-
ure 1. Clustering typically yields one large cluster
encompassing more than half of the visual field (i.e.,
≥0.5*VF). Experts also expect to observe an average
defect depth of at least 2 in this cluster.
(∃c
i
: S
i
≥ 0.5 ∗V F ∧ dd
i
≥ 2) →C
5
(10)
HEALTHINF2014-InternationalConferenceonHealthInformatics
38

Blind Spot Enlargement (C
6
) These are defects
that occur due to the enlargement of the blind spot.
Such cases are typically assumed when there is a de-
fect cluster close to the blind spot area, for which the
centroid or the size does not correspond to the cen-
troid or the size of the normal physiological blind spot
cluster. The following is a decision rule used by ex-
pert ophthalmologists for this class of defects.
∃c
i
: µ
i
6= µ
blindspot
∨ S > S
blindspot
→C
6
(11)
Sector- or Wedge-shaped Defects (C
7
). These are
defect areas that are wedge-shaped. A perimetric re-
sult is assigned to this class, if there is a defect cluster
with the following features: (i) it contains more than 8
defect stimuli, (ii) it is not the blind spot, and (iii) the
angle between the sides of the wedge-shaped cluster
(l
l
and l
r
) is between 30
◦
and 90
◦
. The values for the
minimum number of defect stimuli in (i) and the min-
imum average defect depth in (ii) were established by
means of MCC analysis. The angle between the clus-
ter sides stems from expert knowledge.
(∃c
i
: µ
i
6= µ
blindspot
∧ S
i
6= S
blindspot
∧ sc
i
≥ 8 ∧
dd
i
≥ 2 ∧ 30 ≤ ∠(l
l
(c
i
), l
r
(c
i
)) ≤ 90) →C
7
(12)
Hemianopic Defect (C
8
). These defects expand
over one hemifield and respect the vertical meridian
of the visual field. The corresponding perimetric
results are typically characterized by an impaired left
or right visual field side, where most of the stimuli
were not recognized. The other half of the visual
field is intact, except for the blind spot area and few
relative or absolute defects, e.g., due to patient’s
inattention. To detect this defect type, the algorithm
counts the number of defects in each half of the visual
field. Correlation analysis on the data, by means of
MCC, revealed that a left-sided hemianopic defect is
found, if at least 60% of the stimuli locations of the
left side (i.e., with x coordinate x ≤ 0) of the visual
field are absolute defects (i.e., stimuli locations with
defect depth dd ≥ 3). The threshold 3 for the defect
depth of a stimulus is based on expert knowledge.
The right side of the visual field is considered intact,
if there are less than 10% of relative defects (i.e.,
stimuli locations with defect depth dd ≤2). The rule
for the detection of the left-sided hemianopic defect
is defined by RHL in Formula 13. The detection
of a right-sided hemianopic defect is defined by
RHR in the Formula 14 and is symmetric to RHL.
In summary, a perimetric examination result is
assigned to this defect class if it fulfills the rule
(RHL ∨RHR) →C
8
.
[RHL] :
∑
s
i
∈M
((x
s
i
≤ 0)(dd
s
i
≥ 3))
∑
s
i
∈M
(x
s
i
≤ 0)
≥ 0.6 ∧
∑
s
j
∈M
((x
s
j
> 0)(dd
s
j
≤ 2))
∑
s
j
∈M
(x
s
j
> 0)
≤ 0.1 (13)
[RHR] :
∑
s
i
∈M
((x
s
i
≥ 0)(dd
s
i
≥ 3))
∑
s
i
∈M
(x
s
i
≥ 0)
≥ 0.6 ∧
∑
s
j
∈M
((x
s
j
< 0)(dd
s
j
≤ 2))
∑
s
j
∈M
(x
s
j
< 0)
≤ 0.1 (14)
Note that in general more than one of the presented
decision rules may fire for the same input. Under
the assumption that each perimetric examination re-
sult belongs to one and only one defect class, we iter-
ate through the rules in the order they were introduced
and the class corresponding to the first rule that fires.
4 EXPERIMENTAL RESULTS
The presented algorithm was evaluated on all 8, 848
perimetric examination results of quality levels 4, 3,
and 2 from Table 1, in a one-against-all scheme. The
classification performance was measured by means of
accuracy (ACC) and specificity (SP). The evaluation
results of the classification method are presented in
Table 2. For each scotoma class and the correspond-
ing quality level, the ACC and SP values are reported.
Furthermore, weighted average values for ACC and
SP are shown per defect type, as well as for the entire
data set.
The overall classification ACC and SP of the pre-
sented rule-based classification method were 83% and
85%, respectively. For examination results of qual-
ity level 4, the average (weighted) ACC and SP val-
ues were 92% and 98%, respectively. Considering
the three defect classes with the largest number of in-
stances, namely normal visual field, glaucoma, and
hemianopic defect, the algorithm shows very good
performance, i.e., ACC and SP values of above 90%.
With decreasing data quality (from examination
results of quality level 4 to examination results of
quality level 2), which corresponds to increasing un-
certainty in classification by the ophthalmologists, we
found that the performance of our algorithm also de-
creases, see Table 2. For examination results of qual-
ity levels 3 and 2, there was a decrease in the average
ACC and SP values to 87% and 85% (for quality level
3) and 79% and 78% (for quality level 2), respec-
tively. This effect could be observed for each scotoma
class. The main reason for this effect is the high diver-
sity of manifestations of a scotoma type, which may
Rule-basedClassificationofVisualFieldDefects
39

Table 2: Evaluation of the accuracy (ACC) and specificity (SP) of the rule-based classifier on 8, 848 hand-labeled perimetric
examination results.
Weighted avg. Quality 4 Quality 3 Quality 2
Visual field defect class ACC
(%)
SP
(%)
ACC
(%)
SP
(%)
ACC
(%)
SP
(%)
ACC
(%)
SP
(%)
C
1
: Normal visual field 91 97 91 98 91 97 90 92
C
2
: Central scotoma 83 82 91 91 86 85 80 78
C
3
: Concentric constriction 88 89 97 98 89 90 84 85
C
4
: Glaucoma 76 78 93 95 77 79 74 73
C
5
: Diffuse visual field defects 88 88 95 95 88 88 86 86
C
6
: Blind spot enlargement 83 84 94 95 85 85 81 82
C
7
: Sector- or wedge-shaped defect 88 88 97 97 88 89 87 87
C
8
: Hemianopic defect 91 92 97 98 90 91 89 90
Overall 83 85 92 98 87 85 79 78
lead to uncertainty in the classification result by the
physician or a miss-classification by the algorithm.
The largest loss in ACC (i.e., from 93% for data
of quality level 4 to 74% for data of quality level
2) is found in the recognition of glaucoma. Indeed,
the recognition of glaucoma is considered particularly
challenging (Nouri-Mahdavi et al., 2011), mainly be-
cause of its manifestation in 5 Aulhorn-stages, as de-
picted schematically in Figure 1. In the clinical rou-
tine, the classification of glaucoma is not only based
on the perimetric result, but also under considera-
tion of patient-related features, such as the intraocular
pressure. Such features, however, are often difficult
to quantify and were thus not considered in our rule-
based classification method.
In some cases, especially for data of low quality,
(e.g., quality level 2) more than one of the presented
decision rules might fire. Under the assumption that
each perimetric examination result belongs to one and
only one defect class, the presented rule iteration or-
der interferes with the classification performance.
Comparison to Related Work
The accuracy achieved by our method regarding the
detection of glaucoma varied from 74% (for data of
quality level 2) to 93% (for data of quality level 4), see
Table 2. The overall weighted average accuracy for
glaucoma recognition was 76%, which is well in line
with other approaches (Boden et al., 2007; Brigatti
et al., 1997; Goldbaum, 2005; Goldbaum et al., 2009;
Goldbaum et al., 2002; Goldbaum et al., 1994; Hen-
son et al., 1996; Mutlukan and Keating, 1994). How-
ever, note that these approaches were evaluated on
relatively small data sets consisting of a few hun-
dred perimetric examination results, whereas our al-
gorithm was evaluated on 4,080 perimetric results of
glaucomatous visual field defect cases.
An approach that has considered a larger subset of
visual field defect classes was presented in (Keating
et al., 1993). The achieved accuracy reported there
varied from 74% to 96% on simulated perimetric re-
sults of the following defect types: (1) central vi-
sual field defects, (2) concentric constriction, and (3)
hemianopic defects. Our algorithm outperforms this
approach for all the mentioned defect types and all
data quality levels.
In comparison to the classification accuracy of
80% that was achieved by (Fink, 2004), where a
Hopfield-attractor network was used to recognize the
visual field defect classes, our algorithm performs
better when considering the overall accuracy on data
of quality levels 4 and 3. For data of quality level
2, our approach yields similar accuracy results as the
approach of (Fink, 2004).
In comparison to the approach presented in
(J
¨
urgens et al., 2001) that achieves a classification
accuracy of 95%, our algorithm shows similar perfor-
mance on data of quality level 4, where about 1,000
perimetric results were classified. It is important
to note that it is unclear how the above approaches
would have performed on large data sets of varying
quality levels.
In summary, the main advantage of our rule-based
scotoma classifier is the integration of expert knowl-
edge into the automated classification method. Fur-
thermore, the rules can be easily adapted and new fea-
tures can be included.
5 CONCLUSIONS
We presented a rule-based classification method for
the automated recognition of the visual field defect
types from visual field measurements. This approach
was evaluated on a large set of visual field measure-
ments of different quality levels and showed a high
HEALTHINF2014-InternationalConferenceonHealthInformatics
40

classification accuracy. The presented method can
also be used beyond the classification purpose. More
specifically, the two-level approach can be employed
to automatically extract areas of reduced perception
in the visual field assessed with methods other than
perimetry, e.g., with EFOV (Tafaj et al., 2013), which
measures the visual exploration capability of a subject
based on the online analysis of eye movements (Tafaj
et al., 2012). Besides its usage in local diagnostic pro-
cesses, e.g., assisting the clinical routine, the method
could also be used in tele-medicine. Further improve-
ments of the presented algorithm include: (1) the re-
finement of the decision rules and the investigation
of further features, such as features related to the pa-
tient’s general health condition, with the focus on the
glaucoma defect type, (2) the development of a user-
friendly interface for individual threshold adaptation,
and (3) integration with other software tools for vision
research, such as Vishnoo (Tafaj et al., 2011b).
REFERENCES
Aulhorn, E. and Karmeyer, H. (1977). Frequency distribu-
tion in early glaucomatous visual field defects. Doc-
umenta Ophthalmologica Proceedings Series, 14:75–
83.
Baldi, P., Brunak, S., Chauvin, Y., Andersen, C. A. F., and
Nielsen, H. (2000). Assessing the accuracy of predic-
tion algorithms for classification: an overview. Bioin-
formatics, 16(5):412–424.
Bengtsson, B., Bizios, D., and Heijl, A. (2005). Effects
of input data on the performance of a neural net-
work in distinguishing normal and glaucomatous vi-
sual fields. Investigative Ophthalmology & Visual Sci-
ence, 46(10):3730–3736.
Bizios, D., Heijl, A., and Bengtsson, B. (2007). Trained
artificial neural network for glaucoma diagnosis using
visual field data: a comparison with conventional al-
gorithms. Journal of Glaucoma, 16(1):20–28.
Boden, C., Chan, K., Sample, P. A., Hao, J., Lee, T.,
Zangwill, L. M., Weinreb, R. N., and Goldbaum,
M. H. (2007). Assessing Visual Field Clustering
Schemes Using Machine Learning Classifiers in Stan-
dard Perimetry. Investigative Ophthalmology & Visual
Science, 48(12):5582–5590.
Brigatti, L., Nouri-Mahdavi, K., Weitzmann, M., and Capri-
oli, J. (1997). Automatic Detection of Glaucoma-
tous Visual Field Progression with Neural Networks.
Archives of Ophthalmology, 115(6):725–728.
Duda, R. O., Hart, P. E., and Stork, D. G. (2000). Pat-
tern Classification (2nd Edition). Wiley-Interscience,
2 edition.
Fink, W. (2004). Neural attractor network for application
in visual field data classification. Physics in Medicine
and Biology, 49(13):2799–2809.
Flach, P. (2012). Machine Learning: The art and science of
algorithms that make sense of data. Cambridge Uni-
versity Press.
Goldbaum, M. H. (2005). Unsupervised learning with in-
dependent component analysis can identify patterns of
glaucomatous visual field defects. Transactions of the
Americal Ophthalmological Society, 103:270–280.
Goldbaum, M. H., Jang, G. J., Bowd, C., Hao, J., Zangwill,
L. M., Liebmann, J., Girkin, C., Jung, T. P., Wein-
reb, R. N., and Sample, P. A. (2009). Patterns of
glaucomatous visual field loss in SITA fields automat-
ically identified using independent component analy-
sis. Transactions of the Americal Ophthalmological
Society, 107:136–144.
Goldbaum, M. H., Sample, P. A., Chan, K., Williams, J.,
Lee, T. W., Blumenthal, E., Girkin, C. A., Zangwill,
L. M., Bowd, C., Sejnowski, T., and Weinreb, R. N.
(2002). Comparing machine learning classifiers for di-
agnosing glaucoma from standard automated perime-
try. Investigative Ophthalmology & Visual Science,
43(1):162–169.
Goldbaum, M. H., Sample, P. A., White, H., Colt, B.,
Raphaelian, P., Fechtner, R. D., and Weinreb, R. N.
(1994). Interpretation of automated perimetry for
glaucoma by neural network. Investigative Ophthal-
mololgy & Visual Science, 35(9):3362–3373.
Henson, D. B., Spenceley, S. E., and Bull, D. R. (1996).
Spatial classification of glaucomatous visual field
loss. British Journal of Ophthalmology, 80(6):526–
531.
Henson, D. B., Spenceley, S. E., and Bull, D. R. (1997).
Artificial neural network analysis of noisy visual field
data in glaucoma. Artificial Intelligence in Medicine,
10(2):99–113.
Jain, A. K., Murty, M. N., and Flynn, P. J. (1999). Data
clustering: a review. ACM Computing Surveys,
31(3):264–323.
J
¨
urgens, C., Koch, T., Burth, R., Schiefer, U., and Zell, A.
(2001). Classification of perimetric results and reduc-
tion of number of test locations using artificial neural
networks [arvo abstract nr. 4539]. Investigative Oph-
thalmology & Visual Science, 42(4):846.
Kaski, S. (1997). Data exploration using self-organizing
maps. In Acta Polytechnica Scandinavica: Mathe-
matics, Computing and Management in Engineering
Series No 82.
Keating, D., Mutlukan, E., Evans, A., McGarvie, J., and
Damato, B. (1993). A back propagation neural net-
work for the classification of visual field data. Physics
in Medicine and Biology, 38(9):1263.
Kohonen, T. (1990). The self-organizing map. Proceedings
of the IEEE, 78(9):1464–1480.
Mangiameli, P., Chen, S. K., and West, D. (1996). A com-
parison of som neural network and hierarchical clus-
tering methods. European Journal of Operational Re-
search, 93(2):402–417.
Mutlukan, E. and Keating, D. (1994). Visual field interpre-
tation with a personal computer based neural network.
Eye, 8(3):321–323.
Nevalainen, J., Krapp, E., Paetzold, J., Mildenberger, I.,
Besch, D., Vonthein, R., Keltner, J. L., Johnson, C. A.,
Rule-basedClassificationofVisualFieldDefects
41

and Schiefer, U. (2007). Visual field defects in acute
optic neuritis - distribution of different types of defect
pattern, assessed with threshold-related supraliminal
perimetry, ensuring high spatial resolution. Graefe’s
Arch Clin Exp Ophthalmol, 246(4):599–607.
Nouri-Mahdavi, K., Nassiri, N., Giangiacomo, A., and
Caprioli, J. (2011). Detection of visual field progres-
sion in glaucoma with standard achromatic perime-
try: A review and practical implications. Graefe’s
Archive for Clinical and Experimental Ophthalmol-
ogy, 249(11):1593–1616.
Tafaj, E., Dietzsch, J., Bogdan, M., Schiefer, U., and Rosen-
stiel, W. (2011a). Reliable classificatin of visual field
defects in automated perimetry using clustering. In
Proceedings of the 8th IASTED International Confer-
ence on Biomedical Engineering, BIOMED ’11.
Tafaj, E., Hempel, S., Heister, M., Aehling, K., Schaef-
fel, F., Dietzsch, J., Rosenstiel, W., and Schiefer, U.
(2013). A New Method for Assessing the Exploratory
Field of View (EFOV). In Stacey, D., Sol-Casals, J.,
Fred, A. L. N., and Gamboa, H., editors, HEALTHINF
2012, pages 5–11. SciTePress.
Tafaj, E., Kasneci, G., Rosenstiel, W., and Bogdan, M.
(2012). Bayesian online clustering of eye movement
data. In Proceedings of the Symposium on Eye Track-
ing Research and Applications, ETRA ’12, pages
285–288, New York, NY, USA. ACM.
Tafaj, E., K
¨
ubler, T., Peter, J., Schiefer, U., Bogdan, M.,
and Rosenstiel, W. (2011b). Vishnoo - an open-
source software for vision research. In Proceedings of
the 24
th
IEEE International Symposium on Computer-
Based Medical Systems, CBMS’ 11, pages 1–6. IEEE.
Tan, P. N., Steinbach, M., and Kumar, V. (2005). Introduc-
tion to Data Mining. Addison Wesley.
HEALTHINF2014-InternationalConferenceonHealthInformatics
42
