
Automatic Detection of Single Slow Eye Movements and Analysis of
their Changes at Sleep Onset
Filippo Cona
1
, Fabio Pizza
2
, Federica Provini
2
and Elisa Magosso
1
1
Department of Electrical, Electronic and Information Engineering “Guglielmo Marconi”, University of Bologna,
Via Venezia 52, 47521, Cesena, Italy
2
Department of Biomedical and Neuromotor Sciences, University of Bologna, Bellaria Hospital,
Via Altura 3, 40139, Bologna, Italy
Keywords: Slow Eye Movements, Sleep Onset, Automatic Detection, Template Matching.
Abstract: An algorithm that can automatically identify slow eye movements from the electro-oculogram is presented.
The automatic procedure is trained using the visual classification of an expert scorer. The algorithm makes
use of both the spectral and morphological signal information to detect single slow eye movements. On the
basis of this detection some parameters that characterize the slow eye movements (amplitude, duration,
velocity and number) are extracted. A few possible applications of the algorithm are shown by means of a
preliminary study: the average patterns of slow eye movements parameters at sleep onset are evaluated for
healthy volunteers and for patients affected by obstructive sleep apnea syndrome. Finally, general
considerations are drawn regarding the clinical interest of the study.
1 INTRODUCTION
Eye movements – controlled by a wide neural
network involving the cerebellum, brain stem and
cerebral cortex – may convey important information
on the state and activity of the central nervous
system. Eye movements vary from wakefulness to
sleep and during the different sleep stages. Since
1968, with the recommendation for visual sleep
scoring (Rechtschaffen and Kales, 1968), inspection
of eye movements in electro-oculogram (EOG) is
routinely performed in clinical polysomnography
(PSG) to increase the accuracy and reliability of
sleep stage categorization.
In this work we focus on slow eye movements
(SEMs), which are pendular, predominantly
horizontal eye movements (Aserinsky and Kleitman,
1955); (Värri et al., 1996). SEMs are characteristic
of drowsy wakefulness and light sleep (stages 1 and
2), and occur at the beginning and end of sleep
(Aserinsky and Kleitman, 1955). In recent years,
rising attention has been devoted to SEMs. First,
SEM activity at sleep onset has been investigated in
relation to other physiological and behavioural
measures in order to shed light on the mechanisms
underlying wake-sleep transition (Santamaria and
Chiappa, 1987); (Ogilvie et al., 1988); (De Gennaro
et al., 2000). Furthermore, several studies have
specifically investigated SEMs with the aim of
identifying early predictor of sleep onset to be used
in clinical and occupational settings (Torsvall and
Akerstedt, 1987); (Torsvall and Akerstedt, 1988);
(Marzano et al., 2007).
The growing interest in SEMs has led to
development of various algorithms for automated
SEMs detection in EOG, based on different
techniques and with different aims. However, some
of these algorithms do not identify single SEMs
(Värri et al., 1995); (Virkkala et al., 2007); others
identify single SEMs, but the validation procedure
either exhibits moderate performance (48%
sensitivity) (Värri et al., 1996), or is not examined in
depth (consisting only in the autodetection/visual
scoring ratio) (Hiroshige, 1999); (Suzuki et al.,
2001), or is absent (Shin et al., 2011). Moreover, so
far none of these studies has characterized SEMs in
terms of their parameters (such as amplitude or
duration) nor has investigated how SEMs parameters
evolve across the sleep onset period.
In recent years, we developed an automatic
method for off-line detection of SEM activity in
EOG. The method is based on the wavelet transform
of two unipolar EOG channels; SEM activity is
identified on the basis of EOG power redistribution
474
Cona F., Pizza F., Provini F. and Magosso E..
Automatic Detection of Single Slow Eye Movements and Analysis of their Changes at Sleep Onset.
DOI: 10.5220/0004551304740481
In Proceedings of the 5th International Joint Conference on Computational Intelligence (NCTA-2013), pages 474-481
ISBN: 978-989-8565-77-8
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

towards higher scales (i.e. lower frequencies) of
wavelet decomposition (Magosso et al., 2006). The
method was validated against visual scoring on both
8 h and 24 h PSG recordings acquired in a
laboratory setting (Magosso et al., 2006); (Magosso
et al., 2007); (Magosso et al., 2009). The automatic
method for detection of SEM activity was proven to
perform reliably in detecting sleep onset in
obstructive sleep apnea syndrome (OSAS) patients
(Fabbri et al., 2009); (Fabbri et al., 2010). In a
further study (Pizza et al., 2011), the algorithm was
applied to quantify SEMs distribution during the
different sleep stages and across the sleep cycle.
Despite the promising applications of this
method, it suffers from some drawbacks that may
limit its future use. The main weakness is that the
method was conceived, developed and trained to
detect SEM activity periods – that may consist of an
isolated SEM, a few consecutive SEMs or a long
burst of consecutive SEMs – identifying the initial
and final instants of each period, but without
distinguishing the single eye movements within each
SEM period. Hence, the method is not suitable to
count the number of single SEMs, nor to extract
parameters characterizing each single SEM.
The aim of the present work is to present an
advanced version of the algorithm that overcomes
the previous limitations and a potential application
with some preliminary results. In particular, the new
version of the algorithm improves the previous one
as to the following points: i) it allows the detection
of single slow eye movements in the EOG,
segmenting each identified SEM activity period into
single movements; ii) more importantly, it is able to
extract parameters characterizing each detected slow
eye movement.
The proposed algorithm, being able to extract
and quantify the parameters of SEMs, may have
important clinical implications. In particular,
determination of SEMs parameters (number,
amplitude, duration, velocity) and analysis of their
evolution at the wake-sleep transition may be of
value to characterize – by means of quantitative and
objective measures – the process of falling asleep in
normal, healthy sleepers. This may contribute to a
better description and comprehension of the
complex process of falling asleep. Moreover, the
algorithm can be used to investigate abnormalities of
SEMs parameters in patients suffering from sleep-
related disorders (such as insomnia, OSAS,
narcolepsy), in order to identify potential different
SEMs signatures related to different pathologies,
which may be of clinical significance. In this regard,
the algorithm has been used to assess the evolution
of SEM parameters (amplitude, duration, velocity,
number) at the wake-sleep transition in healthy
volunteers and in OSAS patients, and the obtained
results are critically discussed.
2 METHOD
The new version of the algorithm consists of two
steps. In the first step, the algorithm identifies SEM
activity periods in the EOG: the original version of
the algorithm (Magosso et al., 2006), based on EOG
wavelet decomposition, has been refined in order to
improve its performances. In the second step, the
algorithm segments each identified SEM period into
single SEMs and extracts some fundamental
parameters from the detected movements. The
algorithm was trained and validated on the basis of
visual identification of SEMs performed by a sleep
medicine expert on EOG tracings.
2.1 Data Acquisition
12 healthy subjects and 8 OSAS patients participated
in the study. All subjects gave their written informed
consent for participation in the study which was
conducted with the approval of the local Ethics
Committee. The study consisted in a 24 hours PSG
recording performed in real-life conditions with a
portable digital polygraph (Trex by XLTeck).
Volunteers and patients came to the laboratory for
about 2 hours in the early morning for device
setting, then they left the laboratory, performed their
normal life activities for 24 hours and slept at home.
The next morning returned to the laboratory for
device removing. Recordings included three EEG
derivations (F3-A2, C3-A2, O1-A2; filters: 0.5-70
Hz), one submentalis EMG (filters: 30-100 Hz), two
EOG derivations (E1-A1, E2-A2; filters: 0.1-15 Hz),
and one ECG derivation (filters 1-70 Hz). Each
recording was scored by an expert for sleep staging,
according to the standard visual criteria
(Rechtschaffen and Kales, 1968). Stages were
evaluated in 30-s epochs and labeled as wakefulness
(W) or as one of the five sleep stages (1, 2, 3, 4
NREM, and REM). Signals are sampled at 512 Hz
and resampled at 64 Hz before the processing.
2.2 Visual SEM Scoring
An expert scorer recognized the SEMs on the EOG
traces, in particular in a time window around the
sleep onset (from 15 minutes before stage 1 to 10
minutes after the beginning of stage 2) and the
awakening (from 10 minutes before the end of stage
AutomaticDetectionofSingleSlowEyeMovementsandAnalysisoftheirChangesatSleepOnset
475

2 to 20 minutes after the first wake epoch), since
these are the moments in which the SEMs are more
frequent and distinguishable from other
superimposed activity.
An eye movement was identified as an SEM by
the expert if it met the following criteria: i) single
period of an almost sinusoidal excursion (0.1–1 Hz),
beginning and ending at near-zero velocity; (ii)
amplitude between 20 and 200 µV; (iii) binocular
synchrony with opposed-phase deflections in the
two channels; (iv) onsets of the right and left eye
movement occur within 300 ms of one another; (v)
absence of artefacts (such as blinks, EEG/EMG
artefacts). All the parts of the examined EOG
portions not identified as SEMs, were defined as
Non-SEM (NSEM) activity.
2.3 Identification of SEM Epochs
Following visual detection of single SEMs, the
inspected EOG traces were split into 0.5 s epochs:
one epoch was defined as an SEM epoch according
to the visual classification if at least 50% of the
epoch was covered by an SEM marked by the
expert; otherwise it was classified as an NSEM
epoch. On the basis of this classification we trained
a classifier that categorizes each 0.5 s epoch of EOG
as belonging to SEM or NSEM periods.
To this end, we calculated the discrete wavelet
transform of ΔEOG(t) = EOG
R
(t) - EOG
L
(t) (eye
movements give opposite contributions to the two
electrodes) using Daubechies order 4 as wavelet
function, and evaluated 8 scales that approximately
cover the frequency bands 16-32 Hz, 8-16 Hz, 4-8
Hz, 2-4 Hz, 1-2 Hz, 0.5-1 Hz, 0.25-0.5 Hz and
0.125-0.25 Hz respectively. From the wavelet
coefficients we generated another set of 8 time series
pc(t) = [pc(1,t),…,pc(8,t)]: in particular we
performed the principal component analysis (PCA)
of the logarithm to base 10 of the squared wavelet
coefficients. The aim of this processing is to extract
power measures, to make their distribution “more
normal” and then make them uncorrelated through a
change in coordinates.
The 8 quantities pc(n,t) (n = 1,…,8) are
resampled with a time resolution of 0.5 s and
represent the features used in the classifier. Using
the classification of the human scorer we have
generated the distributions P
n,SEM
(pc(n,t)) and
P
n,NSEM
(pc(n,t)) that represent the probability that a
given value of pc(n,t) is observed during SEM and
NSEM epochs, respectively. As the features are
continuous quantities, the range covering the 98% of
each feature distribution was uniformly divided into
20 bins. Figure 1 shows the frequency distributions
of the features on visually classified SEM and
NSEM epochs (in grey and black respectively).
Figure 1: Distributions P
n,SEM
(pc(n,t)) in gray and
P
n,NSEM
(pc(n,t)) in black.
The information carried by the 8 values of pc(t)
is then composed into two functions
SEM
t
P
,
pc
n,t
(1)
NSEM
t
P
,
pc
n,t
(2)
that represent the likelihood that an EOG epoch at
the time t belongs to an SEM or NSEM period
respectively. So the EOG epoch at time t is
classified as an SEM epoch if SEM(t) > NSEM(t),
and as an NSEM epoch otherwise.
The final outcome of this first step is the
identification of SEM activity periods in the EOG
consisting of a single SEM epoch or consecutive
SEM epochs. This first step of the algorithm may be
viewed as a refinement of the algorithm previously
developed and validated by Magosso and colleagues
(Magosso et al., 2006); (Magosso et al., 2007).
Indeed, that algorithm was tested only on EOG
recorded in clinical settings and showed reduced
-5 0 5
0
0.2
0.4
P
1,SEM
and P
1,NSEM
pc(1,t)
-2 0 2 4
0
0.5
1
P
2,SEM
and P
2,NSEM
pc(2,t)
-2 0 2
0
0.5
1
P
3,SEM
and P
3,NSEM
pc(3,t)
-1 0 1
0
1
2
P
4,SEM
and P
4,NSEM
pc(4,t)
-0.5 0 0.5 1
0
1
2
P
5,SEM
and P
5,NSEM
pc(5,t)
-0.5 0 0.5
0
1
2
P
6,SEM
and P
6,NSEM
pc(6,t)
-0.5 0 0.5 1
0
2
4
P
7,SEM
and P
7,NSEM
pc(7,t)
-0.4-0.2 0 0.2 0.4
0
5
P
8,SEM
and P
8,NSEM
pc(8,t)
c
a
d
e
hg
f
b
IJCCI2013-InternationalJointConferenceonComputationalIntelligence
476

performances (results not presented) on EOG
acquired in real-life environments.
2.4 Identification of Single SEMs
The next step of the algorithm is devoted to obtain
an ideal prototype waveform of SEM, φ(t), to be
used as a template to detect single SEMs in each
SEM period identified according to the previous
step. To generate the SEM prototype all the SEMs
identified by the experts have been preprocessed by
i) removing biases and slow trends; ii) normalizing
them in time (all SEMs have been interpolated to
have the same number of time samples) and in
amplitude (each SEM has been divided by its
standard deviation). In this way, only morphological
information is left (figure 2). The generation of this
prototype corresponds to training the algorithm for
the identification of single SEMs.
Figure 2: SEM prototype. The grayscale image on the
background represents the overall distribution of all the
visually scored SEMs (some example are drawn in gray),
while the black plot represents their mean, φ(t).
To identify the single SEMs, we implemented an
“SEM transform” which is very similar to a
continuous wavelet transform, in which the wavelet
function is φ(t). The wavelet functions at different
scales have been obtained by resampling φ(t): for
each length L (in samples) spanning from the
shortest to the longest SEM classified by the expert
(L
min
< L < L
max
) we have created a φ
L
(k), where
k∈
1,…,L
is the resampling index. After the
resampling, φ
L
(k) is treated as if it had the same
sampling time as the EOG signal, so the smaller L
the smaller the scale of φ
L
(k).
For each SEM period identified in the first step,
we can evaluate the correlation coefficient between
the EOG differential mode and each φ
L
(k), thus
obtaining a map of similarity in the time-scale
domain. In particular, we computed the correlation
coefficient ρ(L,i) between each φ
L
(k) and
ΔEOG(i+k-L/2)
ρ
L,i
c
L,i
∑
∆EOG
ik
L
2
∙
∑
φ
k
(3)
c
L,i
∆EOGik
L
2
∙φ
k
(4)
where c(L,i) represents the correlation. ρ(L,i) has
values between -1 and 1, where ±1 indicate perfect
match (the concavity of the SEM is not relevant),
while 0 indicates complete uncorrelation. A high
value of |ρ(L,i)| suggests the presence of an SEM
that begins at the time index i-L/2 and ends at time
index i+L/2.
Then a path of points (L
n
,i
n
) is found along this
map - where each i
n
corresponds to the centre of a
SEM of length L
n
- that satisfies the following
conditions: i) the mean of ρ(L
n
,i
n
) is maximized; ii)
each SEM begins within half second the end of the
previous one; iii) the union of all of these SEMs
covers completely the SEM period analysed. For the
sake of brevity, we will not discuss here the
procedure to find the best path that we used in
particular, but any optimization algorithm can
reasonably work.
At the end of this procedure, each SEM period is
subdivided into single SEMs of length L
n
and
centred in i
n
. An example of application is illustrated
in figure 3. A 45 s portion of the EOG was
recognized by the algorithm as belonging to a SEM
period; the SEM period was fragmented into single
SEMs on the basis of the similarity with the SEM
prototype at different scales and time shifts.
Note that for each of these SEMs, we can easily
derive the peak-to-peak amplitude in µV, which is
proportional to the correlation c(L
n
,i
n
), and the
duration in seconds, which is proportional to L
n
.
2.5 SEMs Parameters Extraction
The present algorithm, being able to identify single
SEMs, can extract parameters that characterize the
SEM activity. In this work we focused on the
amplitude, duration, velocity and the number of the
identified SEMs. The algorithm automatically
supplies the amplitude and the duration, while the
other two parameters can be easily derived.
The n
th
SEM detected by the algorithm, of length
L
n
and centred in i
n
, can be modelled as
φ
k
c
L
,i
φ
ki
(5)
(see for example the panel c of figure 3). It is worth
noting that φ
fit
(k) fits the differential EOG.
0 0.2 0.4 0.6 0.8 1
-4
-3
-2
-1
0
1
2
SEM prototype
time (normalized)
amp
li
tu
d
e
(
norma
li
ze
d)
AutomaticDetectionofSingleSlowEyeMovementsandAnalysisoftheirChangesatSleepOnset
477
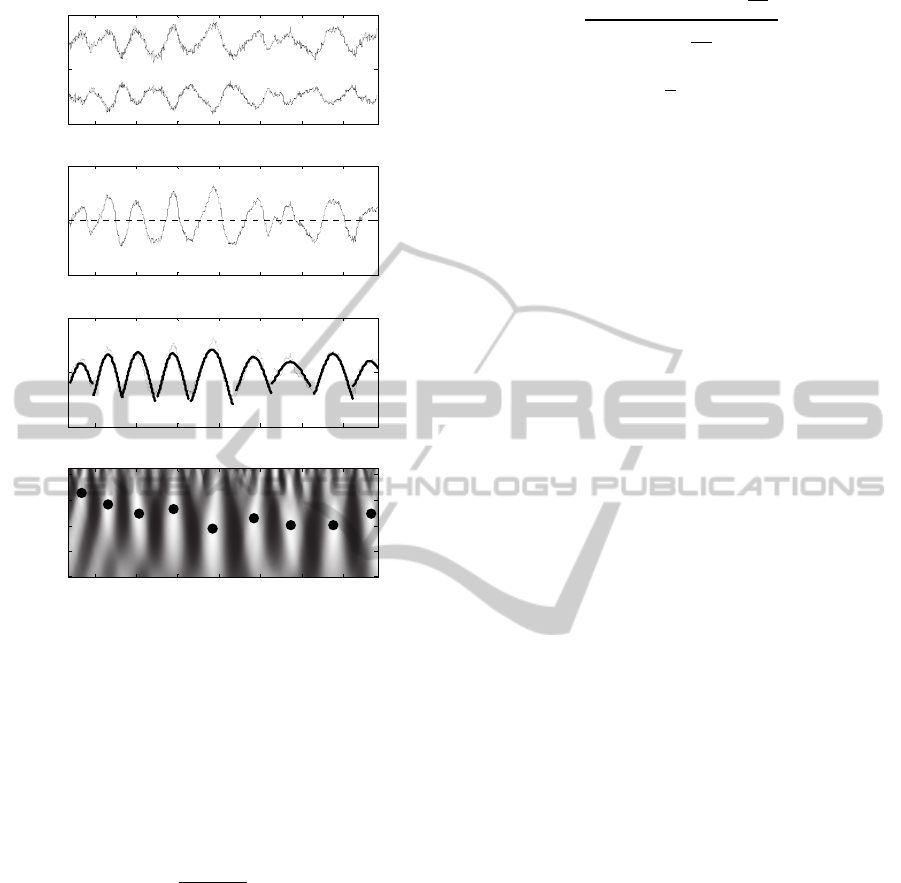
Figure 3: Example of detection of single SEMs in a 45 s
EOG trace. Panel a shows the 2 EOG channels; panel b
shows the differential mode ΔEOG(t); panel c shows the
recognized SEM superimposed to ΔEOG(t); panel d shows
the scale frequency map of the correlation coefficient
ρ(L,i); the black dots indicate the coordinate (L
n
,i
n
) of the
SEMs detected.
The amplitude (Amp) in μV and duration (Dur)
in seconds of the SEM are computed as follows:
Amp G
c
L
,i
2
(6)
DurL
⋅Δt
(7)
where G is the ratio between the peak-to-peak
amplitude and the standard deviation of φ(t), and Δt
is the sampling period of the EOG signal (= 1/64 s).
In amplitude computation, the correlation value has
been divided by 2, to obtain measures relative to the
single EOG channels, rather than their difference
(during SEMs the EOG channels have opposite
phases, so ΔEOG(t) has double amplitude with
respect to the single channels).
The velocity (expressed in μV/s) is taken as the
highest mean velocity of the waveform from its
beginning, according to the following formulas:
v
max
φ
k
φ
i
L
2
ki
L
2
⋅Δt
(8)
Vel
v
2
(9)
where i
n
–L
n
/2 is the initial time index of the
analysed SEM and k is a general time index. As in
amplitude computation, the quantity v is divided by
2, to obtain a measure relative to the single EOG
channels.
Finally, we consider the number (Num) of SEMs
that are recognized in a given time window (e.g. in
Sections 3.2 and 3.3 we will consider 5 minutes time
windows, so Num will be a measure of frequency of
SEMs detected).
A calibration procedure was used to express the
values of the SEM amplitude (Amp) in deg and the
values of the SEM velocity (Vel) in deg/s.
3 RESULTS
In this section, we briefly present the algorithm
performances vs visual scoring. Then we show some
results on SEMs parameters evolution in a time
window around the sleep onset of the healthy
subjects. Finally, the values obtained for healthy
subjects are compared with those obtained for OSAS
patients. Implications of these differences will be
discussed in the Conclusions.
3.1 Validation
A leave one out cross validation has been performed
to assess the performances of the algorithm: all the
subjects but one were used to estimate the
distributions P
n,SEM
(pc(n,t)) and P
n,NSEM
(pc(n,t)) of
SEM and NSEM epochs and to construct the SEM
prototype; then, the distributions and the prototype
φ(t) were used to segment SEMs on the remaining
subject. The procedure has been applied 20 times,
once for each subject. The performances in the
identification of SEM epochs have been assessed in
terms of sensitivity (78.1%) and specificity (87.8%).
As to identification of single SEMs, only the
sensitivity was evaluated (86.0%); the specificity
could not be evaluated since the NSEM epochs are
not subdivided into single eye movements.
3.2 SEMs Parameters Pattern at Sleep
Onset: Healthy Subjects
We analysed the parameters characterizing SEM
927.4 927.5 927.6 927.7 927.8 927.9 928
-200
0
200
Identification of single SEMs within an SEM period
EOG channels (
V)
927.4 927.5 927.6 927.7 927.8 927.9 928
-200
0
200
EOG (
V)
time
(
min
)
duration (s)
927.4 927.5 927.6 927.7 927.8 927.9 928
2
4
6
8
10
927.4 927.5 927.6 927.7 927.8 927.9 928
-200
0
200
EOG (
V)
a
b
c
d
IJCCI2013-InternationalJointConferenceonComputationalIntelligence
478

activity (extracted as described in 2.5) to describe
how they evolve during the transition from
wakefulness to sleep in healthy subjects.
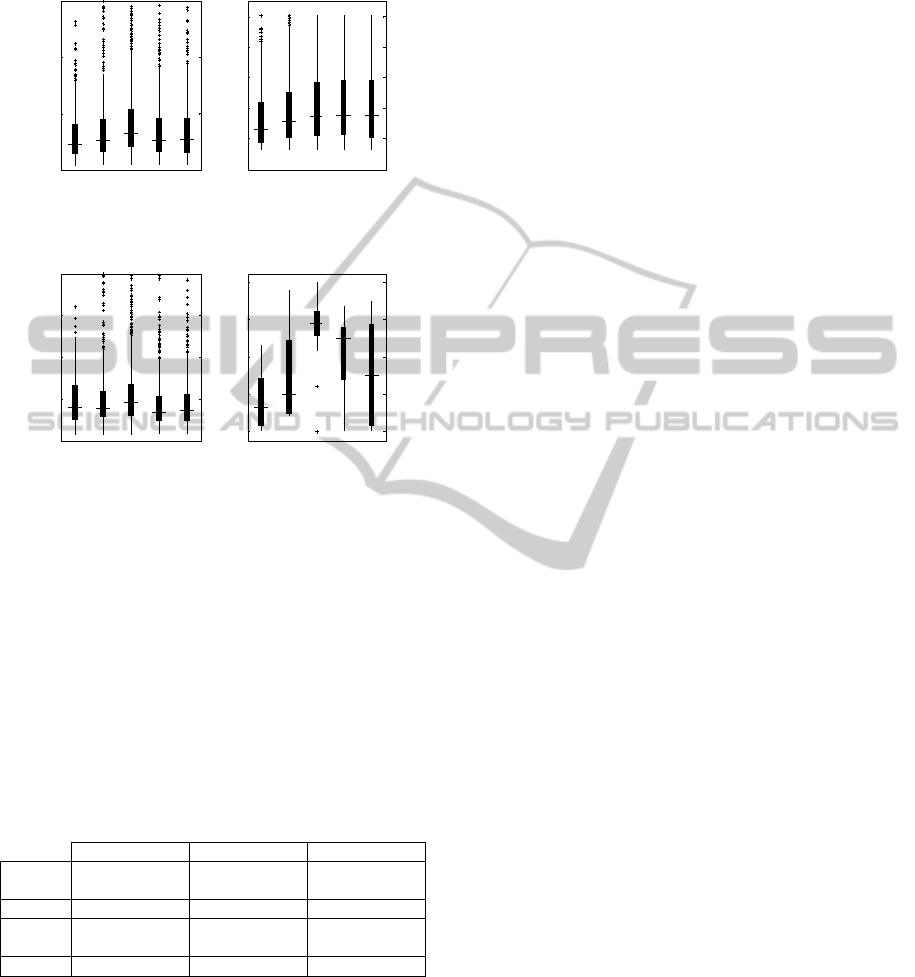
Figure 4: SEM parameters around the sleep onset for the
healthy subjects. Panels a, b, c and d show the
distributions of the values of Amp, Dur, Vel, and Num in
5 time windows of 5 minutes each.
For each healthy subject, we gathered the values
of SEM amplitude (Amp), duration (Dur), velocity
(Vel) and number (Num) of the automatically
identified SEMs during all the wake-sleep
transitions. More specifically, for each transition the
time interval from 12.5 min before to 12.5 min after
the beginning of stage 2 sleep (first epoch of stage 2
sleep) was considered, and all the wake-sleep
transitions occurring in the subjects were aligned to
stage 2 onset. The 25 minutes period at the wake-
sleep transition was subdivided into 5 bins of 5 min
each; all the values of Amp, Dur, Vel and Num of
the SEMs occurring in each bin were collected.
Then, we have generated 5 distributions for each
parameter (one per bin); each distribution is
represented by a boxplot (figure 4). Each panel in
the figure describes the evolution of the parameter
distribution in all the recorded wake-sleep
transitions. As shown in figure 4, SEMs amplitude
(Amp) has a tendency to grow before the beginning
of stage 2, and to decrease afterwards; on the
contrary, SEMs duration (Dur) keeps on increasing
as sleep deepens. Accordingly, SEMs velocity (Vel)
tends to increase before the stage 2 onset, begins to
decrease before the beginning of stage 2 and keeps
on decreasing afterwards. Finally, as shown by the
evolution of Num, SEMs become denser as stage 2
sleep approaches, and gradually become less
frequent as sleep further deepens.
To test the significance of these changes we have
used a Mann-Whitney U-test to compare the
distributions of the parameters between different
time bins. In particular, for each parameter, we
compared the first bin (-10 min) with the second (-5
min) to assess early changes, the second bin with the
third (0 min) to assess changes just before the
beginning of stage 2, and the third bin with the last
(+10 min) to assess changes that take place as sleep
deepens. The amplitude increases significantly
several minutes before stage 2 (from -10 to -5 min)
and decreases significantly during sleep (from 0 to
10 min). The duration significantly increases later
with respect to the amplitude (from -5 to 0 min)
while during the sleep it increases by a non-
significant amount. Velocity, which is proportional
to amplitude and inversely proportional to duration,
behaves accordingly: it increases significantly from -
10 to -5 minutes and decreases significantly both
from -5 to 0 minutes and from 0 to 10 minutes. The
number of SEMs also changes significantly on the
whole 25 minutes of analysis: it increases before
stage 2 and decreases afterwards. The p-values,
corrected with Bonferroni correction (24
comparisons, 12 for healthy subjects and 12 for
OSAS patients), are given in table 1.
Table 1: p-Values of the statistical analysis for the healthy
subjects.
-10 Vs. -5 -5 Vs. 0 0 Vs. 10
Amp
2.93e-8
***
5.71
6.32e-10
***
Dur 9.40
1.58e-7
***
2.71
Vel
4.14e-9
***
6.32e-7
***
4.53e-15
***
Num
2.20e-3
**
7.20e-3
**
1.01e-4
***
3.3 SEMs Parameters Pattern at Sleep
Onset: OSAS Patients
The same analysis has been performed for the OSAS
patients (figure 5). The results suggest that the
parameters for this second category of subjects have
less significant excursions. Before the sleep, the
increase in amplitude is delayed with respect to
healthy subjects, becoming significant from -5 to 0
min, rather than from -10 to -5, and the p-values are
0
10
20
30
-10 -5 0 5 10
time to stage 2 (min)
Amplit ude ( °)
0
2
4
6
8
10
-10 -5 0 5 10
time to stage 2 (min)
Duration (s)
0
5
10
15
20
-10 -5 0 5 10
time to stage 2 (min)
Veloc ity ( °/s )
0
20
40
60
80
-10 -5 0 5 10
time to stage 2 (min)
Number of SEMs
a
b
cd
AutomaticDetectionofSingleSlowEyeMovementsandAnalysisoftheirChangesatSleepOnset
479
globally larger. The duration increases continuously
but always by a non-significant amount.
Figure 5: SEM parameters around the sleep onset for the
OSAS patients. The panels show the same measures as
those of figure 4.
The velocity has a completely different evolution
in OSAS patients, since in the second bin it totally
lacks the very high values that are observed in
healthy subjects. Finally, the number of SEMs
follows qualitatively the same evolution as for the
healthy subjects, but the changes are not significant.
The p-values, corrected with Bonferroni correction
(see 3.2), are given in table 2.
Table 2: p-values of the statistical analysis for the OSAS
patients.
-10 Vs. -5 -5 Vs. 0 0 Vs. 10
Amp 6.22e-1
2.49e-4
***
8.39e-5
***
Dur 7.99e-2 1.95e-1 16.6
Vel 23.9
2.69e-2
*
1.59e-9
***
Num 8.85e-1 8.11e-1 6.30e-1
4 CONCLUSIONS
In this work we have presented an algorithm for
SEM detection which represents a substantial
improvement of our previous version (Magosso et
al., 2006; Magosso et al., 2007). Whereas the latter
was able to merely detect periods of SEM activity,
without segmenting single SEMs, the new algorithm
takes advantage of spectral and morphological
information to automatically detect single SEMs in
EOG, showing high performances vs. visual scoring.
Hence, the algorithm is able to count the single
SEMs that occurred in a given time window;
furthermore, and of great relevancy, the algorithm
extracts specific parameters from each recognized
SEM, in particular amplitude, velocity and duration.
These new features of the algorithm open
important perspectives for basic and clinical
research. As SEMs are a phenomenon typical of the
sleep onset period, quantification of SEMs
parameters and analysis of their evolution at the
wake-sleep transition can contribute to improve the
understanding of the process of falling asleep.
Indeed, this process - although widely investigated -
is still far from being fully understood and its
comprehension can benefit from a more precise
depiction of oculomotor changes. Furthermore, the
algorithm may be a valid tool to detect potential
modifications of SEMs parameters at sleep onset in
patients suffering from sleep-related disorders
(insomnia, narcolepsy, OSAS) compared to normal
sleepers, thus characterizing abnormalities in the
process of falling asleep via quantitative measures
provided by SEMs behavior. Regarding to this point,
this paper presents some preliminary results
obtained on a limited number of subjects. In
particular, SEMs parameters in the healthy subjects
and OSAS patients seem to exhibit different
evolutions at the sleep onset period. On average, in
healthy subjects, SEMs parameters change clearly:
they increase in number, amplitude and velocity
before stage 2 onset; afterwards, SEMs rapidly
decrease in number, and change their morphology
flattening and lengthening. On the contrary, in
OSAS patients, SEMs parameters change by a less
amount, exhibiting on the overall a flatter pattern
across the five temporal bins that cover the sleep
onset period. The obtained results suggest that SEMs
might signal differences in the process of falling
asleep in the patients compared to normal. The
healthy volunteers fall asleep starting from relaxed
vigilance state that consists of a few epochs of stage
1 sleep followed by stage 2 sleep and deeper stages
later on. On the other hand, the OSAS patients fall
asleep with a longer, uneven pattern of vigilance
states and they are more prone to wake up from
stage 1 and even stage 2 sleep. The absence of a
definite evolution of SEMs parameters in OSAS
patients could be a marker of the pathological route
into sleep. However, it is worth noticing that this
0
10
20
30
-10 -5 0 5 10
time to stage 2 (min)
Amplitude (°)
0
2
4
6
8
10
-10 -5 0 5 10
time to stage 2 (min)
Duration (s)
0
5
10
15
20
-10 -5 0 5 10
time to stage 2 (min)
Velocity (°/s)
0
20
40
60
80
-10 -5 0 5 10
time to stage 2 (min)
Number of SEMs
a
b
cd
IJCCI2013-InternationalJointConferenceonComputationalIntelligence
480

interpretation is far from being conclusive especially
because of the reduced size of the OSAS sample;
further and deeper analyses with a higher number of
subjects are mandatory to confirm these results and
eventually disclose other potential explanations.
In future, the algorithm will also be applied to
patients suffering from other sleep-related
disturbances to provide a depiction of the SEMs
signatures in different pathologies.
ACKNOWLEDGEMENTS
This work has been supported by a National project
funded by the Italian Ministry for the Environment,
Land and Sea “Excessive daytime drowsiness and
road accidents: Specific risks in the transportation
of waste and toxic harmful substances of significant
ecological impact.”
REFERENCES
Aserinsky, E. and Kleitman, N. (1955). Two types of
ocular motility occurring in sleep. Journal of Applied
Physiology, 8(1), pp.1–10.
Fabbri, M., Pizza, F., Magosso, E., Ursino, M., Contardi,
S., Cirignotta, F., Provini, F. and Montagna, P. (2010).
Automatic slow eye movement (SEM) detection of
sleep onset in patients with obstructive sleep apnea
syndrome (OSAS): comparison between multiple
sleep latency test (MSLT) and maintenance of
wakefulness test (MWT). Sleep Medicine, 11(3),
pp.253–257.
Fabbri, M., Provini, F., Magosso, E., Zaniboni, A., Bisulli,
A., Plazzi, G., Ursino, M. and Montagna, P. (2009).
Detection of sleep onset by analysis of slow eye
movements: a preliminary study of MSLT recordings.
Sleep Medicine, 10(6), pp.637–640.
De Gennaro, L., Ferrara, M., Ferlazzo, F. and Bertini, M.
(2000). Slow eye movements and EEG power spectra
during wake-sleep transition. Clinical
Neurophysiology, 111(12), pp.2107–2115.
Hiroshige, Y. (1999). Linear automatic detection of eye
movements during the transition between wake and
sleep. Psychiatry and Clinical Neurosciences, 53(2),
pp.179–181.
Magosso, E., Provini, F., Montagna, P. and Ursino, M.
(2006). A wavelet based method for automatic
detection of slow eye movements: a pilot study.
Medical Engineering & Physics, 28(9), pp.860–875.
Magosso, E., Ursino, M., Zaniboni, A. and Gardella, E.
(2009). A wavelet-based energetic approach for the
analysis of biomedical signals: Application to the
electroencephalogram and electro-oculogram. Applied
Mathematics and Computation, 207(1), pp.42–62.
Magosso, E., Ursino, M., Zaniboni, A., Provini, F. and
Montagna, P. (2007). Visual and computer-based
detection of slow eye movements in overnight and 24-
h EOG recordings. Clinical Neurophysiology, 118(5),
pp.1122–1133.
Marzano, C., Fratello, F., Moroni, F., Pellicciari, M.,
Curcio, G., Ferrara, M., Ferlazzo, F. and De Gennaro,
L. (2007). Slow eye movements and subjective
estimates of sleepiness predict EEG power changes
during sleep deprivation. Sleep, 30(5), pp.610–616.
Ogilvie, R.D., McDonagh, D.M., Stone, S.N. and
Wilkinson, R.T. (1988). Eye movements and the
detection of sleep onset. Psychophysiology, 25(1),
pp.81–91.
Pizza, F., Fabbri, M., Magosso, E., Ursino, M., Provini, F.,
Ferri, R. and Montagna, P. (2011). Slow eye
movements distribution during nocturnal sleep.
Clinical Neurophysiology, 122(8), pp.1556–1561.
Rechtschaffen, A. and Kales, A. (1968). A manual of
standardized terminology, techniques and scoring
system of sleep stages in human subjects. Los Angeles,
UCLA.
Santamaria, J. and Chiappa, K.H. (1987). The EEG of
drowsiness in normal adults. Journal of Clinical
Neurophysiology, 4(4), pp.327–382.
Shin, D., Sakai, H. and Uchiyama, Y. (2011). Slow eye
movement detection can prevent sleep-related
accidents effectively in a simulated driving task.
Journal of Sleep Research, 20(3), pp.416–424.
Suzuki, H., Matsuura, M., Moriguchi, K., Kojima, T.,
Hiroshige, Y., Matsuda, T. and Noda, Y. (2001). Two
auto-detection methods for eye movements during
eyes closed. Psychiatry and Clinical Neurosciences,
55(3), pp.197–198.
Torsvall, L. and Akerstedt, T. (1988). Extreme sleepiness:
quantification of EOG and spectral EEG parameters.
The International Journal of Neuroscience, 38(3-4),
pp.435–441.
Torsvall, L. and Akerstedt, T. (1987). Sleepiness on the
job: continuously measured EEG changes in train
drivers. Electroencephalography and Clinical
Neurophysiology, 66(6), pp.502–511.
Värri, A., Hirvonen, K., Häkkinen, V., Hasan, J. and
Loula, P. (1996). Nonlinear eye movement detection
method for drowsiness studies. International Journal
of Bio-Medical Computing, 43(3), pp.227–242.
Värri, A., Kemp, B., Rosa, A.C., Nielsen, K.D., Gade, J.,
Penzel, T., Hasan, J., Hirvonen, K., Häkkinen, V.,
Kamphuisen, H.A.C. and Mourtazaev, M.S. (1995).
Multi-centre comparison of five eye movement
detection algorithms. Journal of Sleep Research, 4(2),
pp.119–130.
Virkkala, J., Hasan, J., Värri, A., Himanen, S. and Härmä,
M. (2007). The use of two-channel electro-
oculography in automatic detection of unintentional
sleep onset. Journal of Neuroscience Methods, 163(1),
pp.137–144.
AutomaticDetectionofSingleSlowEyeMovementsandAnalysisoftheirChangesatSleepOnset
481
