
Development of a Process Assessment Model for Assessing Security of
IT Networks Incorporating Medical Devices against ISO/IEC 15026-4
Anita Finnegan, Fergal Mc Caffery and Gerry Coleman
Regulated Software Research Group, Dundalk Institute of Technology & Lero, Dundalk, Co Louth, Ireland
Keywords: Medical Device Security, Process Assessment, ISO/IEC 15026-2, ISO/IEC 15026-4, IEC 80001-2-2, IEC
62443-3-3, ISO/IEC 15504, ISO 27799, ISO/IEC 27001, ISO/IEC 27002.
Abstract: Advancements in medical device design over the last number of years have allowed medical device
manufacturers to add more complex functionality particularly through the use of software. Such
advancements include the ability for devices to communicate wirelessly across networks, from device to
device and over the Internet. However, with such advancements comes additional risks; these are security
risks, vulnerabilities and threats. In the past twelve months, concern within the medical device community
has led to the US Government calling upon the FDA to take responsibility of medical device security. In
support of this, this position paper details a research proposal to address medical device security issues
through the development of a Process Reference Model (PRM) and a Process Assessment Model (PAM) to
assess the capability of the processes used to develop medical devices intended to be incorporated onto
healthcare networks and also determine the product security capability through the development of security
assurance cases created following the lifecycle process. Further, in support of IEC 80001-2-2, the output
from this PRM will be an assurance case with a security assurance level, which will be used to communicate
the security capabilities of the product between Medical Device Manufacturers (MDMs) and Healthcare
Delivery Organisations (HDOs). The intent is to build a better awareness of vulnerability types, threats and
related risks to assist in reducing the likelihood of harm resulting from a security risk.
1 INTRODUCTION
Medical devices have made substantial innovative
design improvements over the last number of years
due to the growing incorporation of software in the
devices. Following on from the addition of software
came the introduction of interoperable medical
devices bringing with it, a new set of security risks
for consideration. Although there have been no
reports of malicious attacks on interoperable medical
devices, there have been quite a number of
controlled hacking demonstrations. One such
demonstration was at the Black Hat Security
Conference in Las Vegas in 2011. A security
researcher, Jeremy Radcliffe, manipulated the
settings of his own insulin pump during a
presentation. The ease with which the insulin pump
was hacked created a lot of concern within the
medical device community. Then in October 2012,
Barnaby Jack, a researcher at IOActive, presented
research results at the Breakpoint Conference in
Australia, which looked at the security of
pacemakers. Jack’s research identified a bug in
several manufacturers’ pacemakers that allowed an
attacker to deliver a potentially lethal shock to a
patient’s heart by sending a signal to the pacemaker
by simply using a laptop. The pacemaker contained
a coding error that allowed it to send a wireless
command to the device that returned the model and
serial number of the device.
Consequently, in a letter to the US Office of
Management and Budget, the Information Security
and Privacy Advisory Board (ISPAB) called upon
the government to assign the responsibility of
medical device security to a federal entity such as
FDA. In August 2012, the Government
Accountability Office (GAO) released a report of an
audit carried out on the FDA for the assessment of
two implantable medical devices that highlighted
shortfalls in the assessment of intentional and
unintentional risk (Government Accountability
Office, 2012).
In addition to this quite a number of medical
device security information and guidance documents
250
Finnegan A., Mc Caffery F. and Coleman G..
Development of a Process Assessment Model for Assessing Security of IT Networks Incorporating Medical Devices against ISO/IEC 15026-4.
DOI: 10.5220/0004327502500255
In Proceedings of the International Conference on Health Informatics (HEALTHINF-2013), pages 250-255
ISBN: 978-989-8565-37-2
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

have been recently released. One example, the US
Department of Homeland Security (DHS) issued a
bulletin “Attack Surface: Healthcare and Public
Health Sector”(DHS, 2012) which discusses security
related risks associated with using medical devices
on IT networks, including those risks to patient
safety and theft or loss of medical information.
Addressing this recent concern, the aim of this
research is to build security assurance into the
product lifecycle through a set of strategic processes,
activities and tasks and to establish and
communicate the product security capabilities of a
medical device between Medical Device
Manufacturers, vendors and Healthcare Delivery
Organisations. This will create awareness for both,
the Medical Device Manufacturers in understanding
the needs of the Healthcare Delivery Organisation,
and also for Healthcare Delivery Organisation IT
admin staff, to understand the capabilities of the
medical device that could potentially be acquired for
use on their IT network.
Section two of this paper outlines the most
relevant standards and documents currently used
within the software industry in relation to process
assessment, software lifecycle assurance and
security. Section three looks at related work in the
area of process assessment models and security.
Section four then discusses the future work planned
for this research, including the validation of the
model and also the impact this research is expected
to have in the medical device domain for regulators,
Medical Device Manufacturers, IT vendors and
Healthcare Delivery Organisations.
2 CURRENT LANDSCAPE WITH
STANDARDS
With the substantial transformation in medical
device design over the last few years to incorporate
software and communication functionality, the
medical device communities around the world have
published many standards and guidance documents
to assist with the development of safe medical
devices. This has resulted in an abundance of
published security guidance documents and a sense
of confusion among Medical Device Manufacturers
and Healthcare Delivery Organisations with regards
which guidelines and best practices to follow.
This section outlines the most applicable
standards and guidance documents which are
deemed most relevant in establishing and controlling
security risks. A broad range of standards have been
reviewed for this research, with the most
fundamental being discussed here. Additional
standards identified and incorporated into this
research to date are discussed in Section three.
2.1 ISO/IEC 15026-4
ISO/IEC 15026-4 (IEEE, 2011) provides
recommendations and guidelines for implementing
software and systems development processes that
require additional assurance for a particular property
of that system or software; in this case the critical
property is security. This standard presents a list of
recommended processes, activities and tasks
required in order to achieve a claim in relation to
that critical property of a system or software.
Through the development of an assurance case
(further discussed in section 2.2), the entire
development and maintenance of the product is
addressed which also includes project-planning
considerations. It is intended that this standard be
used coupled with an already defined life cycle
model.
2.2 ISO/IEC 15026-2
ISO/IEC 15026-2 (IEEE, 2011) defines
requirements for the structure and content of an
assurance case. An assurance case is a body of
evidence organised into an argument demonstrating
some claim that a system holds i.e. ‘Is Assured’. An
assurance case is needed when it is important to
show that a system exhibits some complex property
such as safety, security, or reliability (Goodenough
et al., 2012).
A security assurance case is often compared with
a legal case where there are two elements to the
case, the argument and the evidence to support a
claim. For an assurance case to be effective it must
satisfy the following points:
Must make a claim or set of claims about a
property of a system;
Provide a set of arguments;
Make clear the assumptions and judgements
underlying the arguments;
Associate different viewpoints and levels of detail.
Produce the supportive evidence;
2.3 IEC 80001-2-2
IEC 80001-2-2 (IEC, 2011c) is a technical report
which provides a framework for the communication
and disclosure of medical device needs, risks and
controls which are to incorporated onto a IT
DevelopmentofaProcessAssessmentModelforAssessingSecurityofITNetworksIncorporatingMedicalDevicesagainst
ISO/IEC15026-4
251

network. This technical report presents 20 security
capabilities (Table 1) e.g. Authorization, Audit
Controls etc. These capabilities provide Healthcare
Delivery Organisations, Medical Device
Manufacturers and IT vendors with information
regarding user needs, security related requirements
and required security control types.
This information is critical prior to development,
acquisition, installation and use of medical devices
for IT networks. Each of these security capabilities
represents a potential security risk control. These
are addressed in the requirement goals, which
identify the risks that can be mitigated against
through the use of that particular security capability.
The user need section develops this further by
detailing particular specific environmental
requirements.
Table 1: IEC 80001-2-2 Security Capabilities.
Security Capability Code
1 Automatic Logoff ALOF
2 Audit Controls AUDT
3 Authorization AUTH
4 Configuration of Security Features CNFS
5 Cyber Security Product Upgrades CSUP
6
Data Backup and Disaster
Recovery
DTBK
7 Emergency Access EMRG
8 Health Data De-Identification DIDT
9
Health Data Integrity and
Authentication
IGAU
10
Health Data Storage
Confidentiality
STCF
11 Malware Detection/Protection MLDP
12 Node Authentication NAUT
13 Person Authentication PAUT
14 Physical Locks on Device PLOK
15 Security Guides SGUD
16 System and Application Hardening SAHD
17
Third-Party Components in
Product Lifecycle Roadmaps
RDMP
18 Transmission Confidentiality TXCF
19 Transmission Integrity TXIG
20 Unique User ID UUID
The security disclosure is a summary statement
provided by the Medical Device Manufacturer
and/or IT vendor that details the security capability
of the medical device. This security disclosure is
then reviewed by the Healthcare Delivery
Organisation to establish the security integrity of the
product and prompt further discussion prior to
acquisition. The risk management team within the
Healthcare Delivery Organisation utilise this further
to perform a risk analysis based on the known
security capabilities.
2.4 IEC 62443-3-3
IEC 62443-3-3 (IEC, 2011a) defines security system
requirements based on a combination of system
functional requirements and a risk assessment. Key
inputs into the development of this document are
security standards ISO/IEC 27002 (ISO/IEC, 2005)
and NIST SP800-53(NIST, 2009). The standard is
adopted by allowing the asset owner for the system
so dictate the target Security Assurance Level (SAL-
T). The standard details seven Foundational
Requirements (FRs) as listed in Table 2. The seven
FRs listed are the baseline for the system Security
Assurance Levels (SALs). Within each of the seven
FRs are applicable System Requirements (SRs).
These are further broken down detailing the
requirement of that SR, the rationale and
supplemental guidance, the Requirement
Enhancements (REs) and guidance for selection of
REs relating to the SR dependent on the chosen SAL
level for establishment of the achieved Security
Assurance Level (SAL-A).
Table 2: IEC 62443-3-3 Foundational Requirements.
Foundational Requirement Code
1 Identification and Authentication Control IAC
2 Use Control UC
3 Data Integrity DI
4 Data Confidentiality DC
5 Restricted Data Flow RDF
6 Timely Response to Events TRE
7 Resource Availability RA
3 SOLUTION DEVELOPMENT
3.1 Related Work
ISO/IEC 15504-2 (ISO/IEC, 2003) provides a
measurement framework for process capabilities and
defines the requirements for performing the
assessment, building the Process Reference Model
(PRMs), Process Assessment Models (PAMs) and
verifying conformity of process models and the
process assessment. Existing generic Software
Process Improvement (SPI) models are available
which include the Capability Maturity Model
Integration (CMMI®) (SEI, 2010) and ISO 15504-
5:2006 (ISO/IEC, 2006) (SPICE), but these models
were not developed to provide sufficient coverage
for regulatory compliance for the security of IT
Networks incorporating medical devices.
While this relates to the development of the PRM
HEALTHINF2013-InternationalConferenceonHealthInformatics
252

and the PAM for establishment of process assurance,
it does not specifically address medical device
product quality. One difference here is that, in
addition to assessing the Medical Device
Manufacturers software processes and practices, the
key to this research is to also address product
capabilities in relation to security of the
interoperable medical devices.
To address the requirement for a PAM for
assessing the security of IT networks incorporating
medical devices, we undertook extensive research in
this area assisted by leading members from the
international standards IEC SC62A JWG7 working
group looking at a medical devices specific SPI
model. This work is being developed in
collaboration with the SPICE User Group. The
approach taken here is in line with the approach
taken for both the development of Automotive
SPICE (Automotive SIG) a domain specific SPI
model for the automotive industry, and Medi SPICE
(Fergal McCaffery and Dorling, 2010).
3.2 Proposal
ISO/IEC 15026-4 (IEC, 2011a) is a process lifecycle
standard and provides a solid foundation for the
PRM. It details processes for risk management
which will be extended to include relevant security
standards and requirements such as ISO/IEC 27002,
ISO 27799 (ISO, 2008), IEC 62443, IEC 80001-2-2,
NIST SP 800-53 and NIST SP 800-23 (NIST, 2009).
All security controls and capabilities from these
named sources will be crossed referenced and
mapped to develop a comprehensive set of security
capabilities which will need to be addressed when
conducting a risk assessment and establishing
relevant risk controls. For example, Automatic Log
Off from IEC 80001-2-2 (Table 1) would use the
requirements of SR.1.10 Session Lock in IEC
62443-3-3 as they relate to each other. All relevant
controls/requirements from guidance docs and
security standards included in the research will
follow a similar mapping.
The PRM will provide a description of the
processes and characterise these in terms of their
purpose and outcome. This Process Assessment
Model will be developed in compliance with
ISO/IEC 15504-2 (ISO/IEC, 2003) which outlines
what is required in the Process Assessment Model.
This will be developed along with a measurement
framework and ISO/TR 24774 (IEC, 2010) will
provide the guidelines for process definition.
These steps take care of the processes to be
addressed for the development of a product.
Establishing process assurance or maturity has many
benefits for both the medical device manufacturers
and third party assessors in terms of meeting
regulatory compliance and also determination of
process quality. However, considering the security
risks associated with interoperable medical devices
consisting of software, a major objective is to
establish a method for the communication of the
final product quality in relation to security
capabilities between the Medical Device
Manufacturer, the IT vendor and the Healthcare
Delivery Organisation. Communication of a security
assurance level to Healthcare Delivery Organisations
will provide a simple and meaningful method for
establishing suitability of the device for the users
need and its environment. To do this, IEC 62443-3-
3 will be used as a guide for establishing the system
security assurance level (SAL) by the Medical
Device Manufacturers. The Healthcare Delivery
Organisation will determine the appropriate security
capabilities from within IEC 80001-2-2, along with
any other validated capabilities from other standards.
With regards the different types of SAL, the critical
property is the achieved SAL (SAL-A) since this is
most valuable to the Healthcare Delivery
Organisation and FDA when establishing the
security capability of the product. A SAL vector
will be developed by the Medical Device
Manufacturer post product development for the
achieved SAL (SAL-A), which will be based on the
target SAL (SAL-T) level (0-4) as determined by the
Healthcare Delivery Organisation as the start of the
acquisition process. The SAL vector that details the
assurance level and security capabilities is presented
here:
SAL-A = ({FR,} domain) = {AC UC DI DC RDF TRE RA}
SAL-A = ({FR,} domain) = {3 3 3 3 2 1 0}
For each of the parameters (refer to table 2 for FR
descriptions) within the vector, a value of zero to
four will be used to represent the SAL level for that
particular requirement. Following on from this, the
Medical Device Manufacturer will then verify the
selected SAL level through the use of the SAL
Mapping Matrix as shown in Annex B of IEC
62443-3-3 (IEC, 2011a), which will also be
included in the PRM.
To further build upon the communication and
disclosure of security capabilities, an assurance case,
compliant with IEC/ISO 15026-2 (IEEE, 2011) will
be developed by the Medical Device Manufacturer.
Delivering the actual product assurance level will be
achieved through the utilisation of a tool. This tool
will be used for the development of the risk
DevelopmentofaProcessAssessmentModelforAssessingSecurityofITNetworksIncorporatingMedicalDevicesagainst
ISO/IEC15026-4
253
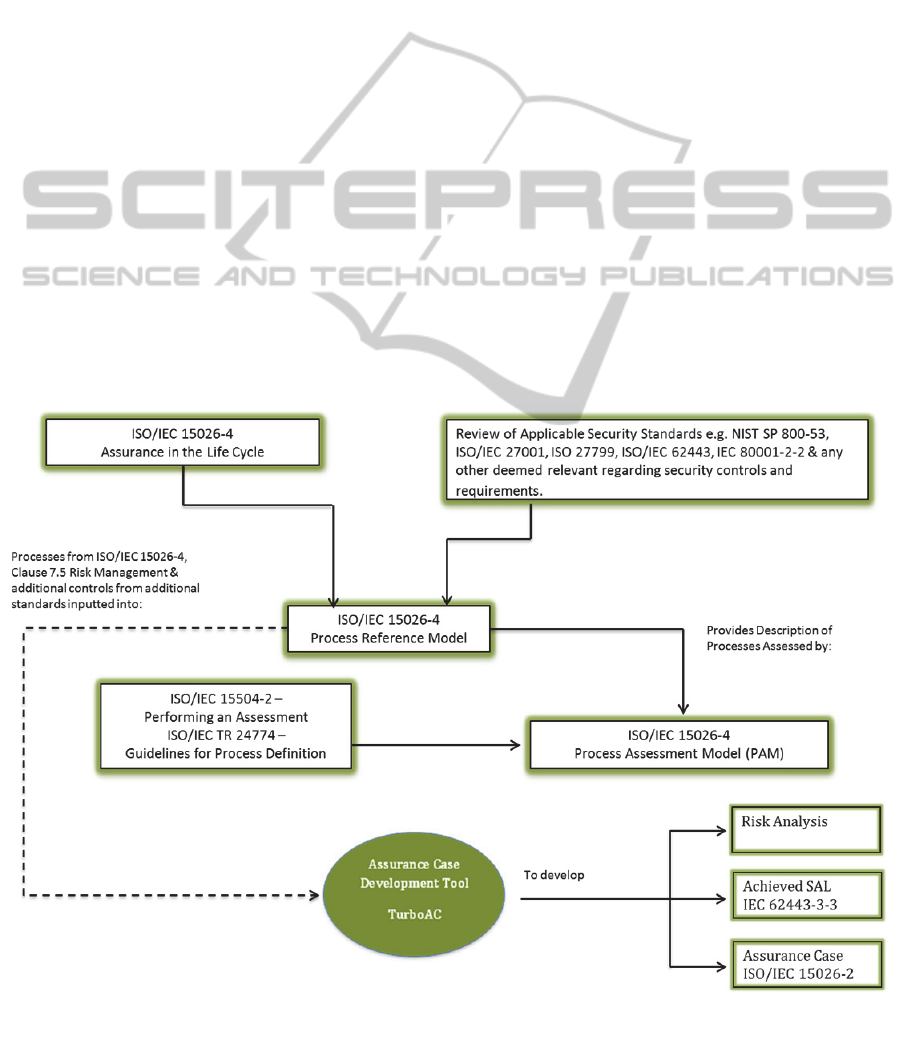
assessment and will in turn automatically build the
assurance case and outline in detail the evidence
gathered to support the achievement of each SAL
level.
The outcome of the PRM will be the
development and communication of:
1. A process maturity level for the development of
the product;
2. A security assurance case detailing in-depth the
arguments and evidence supporting the
security/safety claim of the medical device;
3. An achieved security assurance level (SAL-A)
for the product.
4 SOLUTION DEVELOPMENT
It is planned that this model will be trialled in
industry for validation purposes. This will be done
at manufacturing facilities and Healthcare Delivery
Organisations both in Ireland and the U.S. In
addition to this, it is intended that the model will
also be validated by experts from the International
Standards Committee of IEC SC62A.
At present, there is much concern in the area of
medical devices particularly related to security
vulnerabilities, threats and risks of devices with
communication abilities or those incorporated onto
IT networks. Over the past 12 months, this concern
has been highlighted through many federal body
guidance publications and reports, security
researchers’ demonstrations, publications and also
the development of new standards such as IEC
80001-1(IEC, 2011b).
With the publication of the GAO (Government
Accountability Office, 2012) report in August, it is
clearly indicated here that future strategies are
required in order to sufficiently address medical
device security. As medical devices become more
advanced with software and complex wireless
capabilities, it is feared that security vulnerabilities,
threats and related risks will grow with this
development. The GAO has recommended that the
FDA work on addressing these issues following their
assessment of FDA approved implantable
defibrillators and insulin pumps.
IEC 80001-2-2 defines the security capabilities
that a Medical Device Manufacturer or IT vendor
must communicate to the Healthcare Delivery
Organisation in order to enhance knowledge of
security risks and controls the Healthcare Delivery
Organisation IT admin staff should consider. This
research sets out to support this from both a process
Figure 1: Research approach.
HEALTHINF2013-InternationalConferenceonHealthInformatics
254

and a product point of view.
Developing a model to assess the Medical
Device Manufacturer’s development process
maturity coupled with guidance for the
establishment of a security assurance level addresses
development and output. The product output is the
medical device achieved security assurance level
(SAL-A) reflecting the security capabilities for that
device and also the security controls incorporated.
This output, in the form of a security assurance case,
will be the communication from Medical Device
Manufacturers to Healthcare Delivery Organisations
prior to acquisition. Managing the lifecycle process
and the product security capability between the
Medical Device Manufacturer and the Healthcare
Delivery Organisation would greatly improve the
current processes in the industry at the moment.
Currently there is no methodology to address both
the development processes and the product
capabilities for security in medical devices. This is
the primary focus of this research.
Hence, it is envisaged that the output of this
research will positively impact the medical device
domain by building awareness of security
vulnerabilities, threats and related risks between the
Healthcare Delivery Organisation and the Medical
Device Manufacturer.
ACKNOWLEDGEMENTS
This research is supported by the Science
Foundation Ireland (SFI) Stokes Lectureship
Programme, grant number 07/SK/I1299, the SFI
Principal Investigator Programme, grant number
08/IN.1/I2030 (the funding of this project was
awarded by Science Foundation Ireland under a co-
funding initiative by the Irish Government and
European Regional Development Fund), and
supported in part by Lero - the Irish Software
Engineering Research Centre (http://www.lero.ie)
grant 10/CE/I1855.
REFERENCES
DHS 2012. Attack Surface: Healthcare and Public Heath
Sector.
Fergal McCaffery & Dorling, A. 2010. Medi SPICE
Development. Software Process Maintenance and
Evolution: Improvement and Practical Journal. 255-
268.
Goodenough, J., Lipson, H. & Weinstock, C. 2012.
Arguing Security - Creating Security Assurance Cases.
Government Accountability Office 2012. Medical
Devices, FDA Should Expland Its Consideration of
Information Security for Certain Types of Devices. In:
GAO (ed.).
IEC 2010. IEC/TR 24774 Systems and software
engineering. Life cycle management. Guidelines for
process description.
IEC 2011a. IEC 62443-3-3 Ed. 1.0, Security for industrial
automation and control systems - Network and system
security.
Part 3-3: System security requirements and security
assurance levels Introductory Note. International
Electrotechnical Committee.
IEC 2011b. IEC/TR 80001-1 - Application of risk
management for IT-networks incorporating medical
devices.
IEC 2011c. IEC/TR 80001-2-2 Ed. 1.0 - Draft Technical
Report - Application of risk management for IT-
networks incorporating medical devices. Part 2-2:
Guidance for the disclosure and communication of
medical device security needs, risks and controls.
International Electrotechnical Committee.
IEEE 2011. ISO/IEC 15026-2: 2011 Systems & Software
Engineering, Systems & Software Assurance, Part 2:
Assurance Case.
ISO 2008. EN ISO 27799:2008 Health informatics.
Information security management in health using
ISO/IEC 27002.
ISO/IEC 2003. ISO/IEC 15504-2: 2003 Software
Engineering - Process Assessment - Performing an
Assessment.
ISO/IEC 2005. ISO/IEC 27002:2005 Information
Technology - Security Techniques - Code of Practice
for Information Security Management.
ISO/IEC 2006. ISO/IEC 15504-5: 2006 Information
technology — Process Assessment — Part 5: An
exemplar Process Assessment Model.
NIST 2009. 800-53 Recommended Security Controls for
Federal Information Systems and Organisations. In:
COMMERCE, U. S. D. O. (ed.) Revision 3 ed.
SEI 2010. CMMI-DEV, CMMI for Development.
DevelopmentofaProcessAssessmentModelforAssessingSecurityofITNetworksIncorporatingMedicalDevicesagainst
ISO/IEC15026-4
255
