
Modeling Workflow for Study of Functional Electrical Stimulation
in Peripheral Nerves
Fábio Rodrigues
1
, Marian Bartek
2
and Paulo Mendes
1
1
Centro Algoritmi, University of Minho, Guimarães, Portugal
2
Dimes/ECTM, Delft University of Technology, Delft, the Netherlands
Keywords: Modeling, Selective Stimulation, Multipolar Cuff Electrode.
Abstract: Urinary dysfunctions are among the most devastating consequences of spinal cord injuries (SCI).
Neurostimulation of intact sacral nerve roots innervating bladder is potentially a good alternative to the
treatments using drugs and catheterization. A finer control over the electrical stimulation of sacral roots can
be an enabling technology for developing advanced neurostimulation matching the patient needs. In this
paper a modeling workflow to study axon behaviour in sacral nerve roots is presented. Simulation results
show that the width and the amplitude of the stimulation electric pulses can be tuned to selectively recruit
axons in sacral roots. A selective recruitment of axons innervating bladder is shown to be possible for pulse
widths above 9 ms.
1 INTRODUCTION
Spinal cord injuries (SCIs) are estimated to affect
330,000 people (with about 11,000 new cases every
year) in Europe (Social and F.A.C, 2002), and about
300,000 in the USA (with also about 12,000 new
cases every year) (National Institute of Neurological
Disorders and Stroke, 2012). Among the most
traumatic consequences of an SCI event are the
urinary dysfunctions (e.g., incontinence) due to
lesions above the level of sacral nerve roots (bundle
of nerves innervating bladder and urethral
sphincter). In the conservative medical approach,
drugs and catheters are used to manage the lower
urinary system in cases of SCIs. However, side
effects of medication and the unwilling of patients to
use catheters have driven research in the pursuit of
electrical devices for neurostimulation of sacral
roots innervating bladder.
Sacral nerve roots have become a preferential
site to elicit bladder voiding by neurostimulation
(Rijkhoff et al., 1997); (Brindley, 1977). The sacral
roots are made out of several sub-units, known as
fascicles. Each fascicle is derived from neural cells
in the spinal cord with certain degree of specificity
for their function (Probst et al., 1997). Somatic and
parasympathetic are the two fiber types present in
the fascicles of sacral roots - the parasympathetic
fibers are the smaller fibers innervating bladder,
while the somatic fibers are the larger fibers
innervating sphincter.
Even there are differences between individuals in
terms of histology of sacral roots, still fascicular
trends in specificity are observed. This fact raises the
potential that a focal stimulation can selectively
stimulate the neural unit (fiber/nerve) for a certain
function, e.g., parasympathetic stimulation for
bladder contraction (Hauck et al., 2009). Hence, the
steering of electric current applied in
neurostimulation of sacral roots can yield a selective
recruitment of parasympathetic fibers resulting in
bladder voiding. The selective control of muscles
activation can be achieved using a multipolar nerve
cuff electrode (Schiefer et al., 2010). In terms of
design and optimization of multipolar electrodes for
functional electrical stimulation (FES) of peripheral
nerves two factors are crucial: 1) anatomical trends
and 2) nerves response. Indeed, modeling nerve
response to a given external stimulation parameters
(e.g. pulse width, pulse amplitude) allows to predict
whether a neuron will or will not be activated. In this
work, axonal response in sacral roots is studied -
axon is the most excitable part of a peripheral nerve.
The hypothesis of this study is that a multipolar cuff
electrode placed proximally on the sacral root with a
fixed number of contacts can selectively stimulate
smaller parasympathetic axons that are preferentially
grouped by specialized fascicles. Stimuli is defined
178
Rodrigues F., Bartek M. and Mendes P..
Modeling Workflow for Study of Functional Electrical Stimulation in Peripheral Nerves.
DOI: 10.5220/0004250001780183
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2013), pages 178-183
ISBN: 978-989-8565-34-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
a priori as a simple monopolar, square and cathodic
waveform. Through the use of finite element models
and nonlinear axonal models, the presented study
investigates selectivity for given combinations of
pulse width/pulse amplitude.
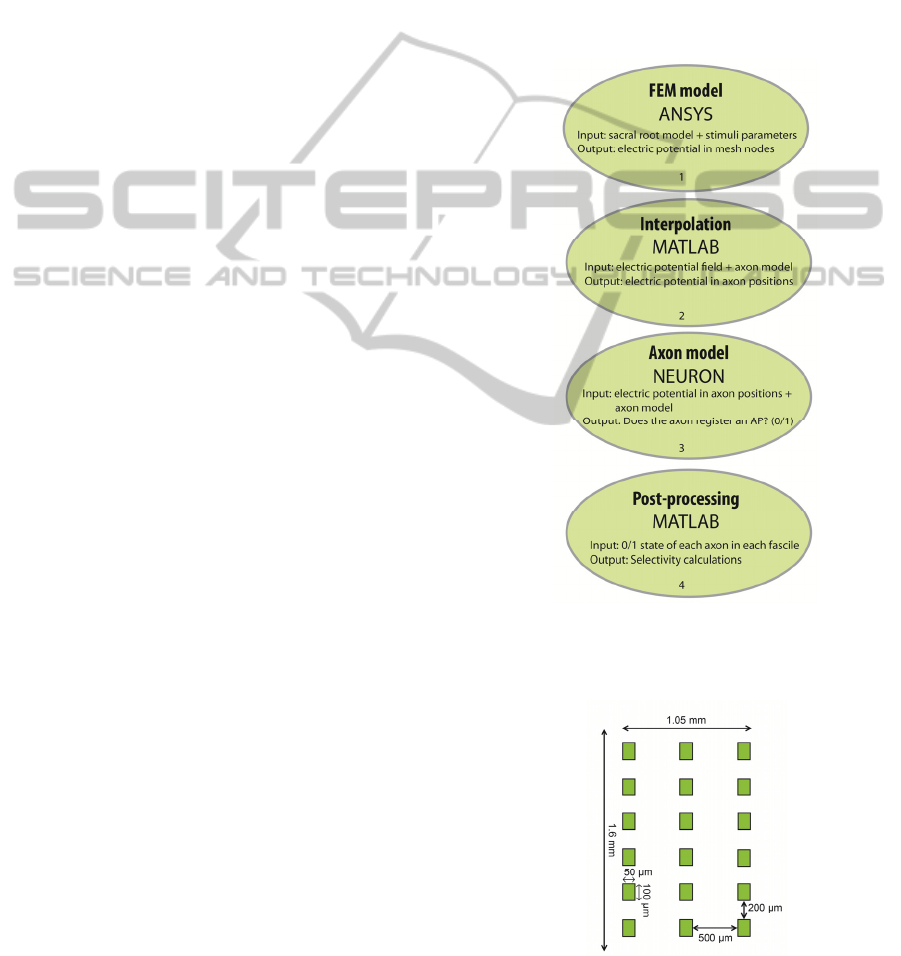
2 METHODS
Selective stimulation effect of a multipolar cuff
electrode on sacral roots was investigated using a
modeling workflow based on 3 different software
tools: ANSYS Multiphysics, MATLAB and
NEURON. First, a finite element model (FEM) of a
sacral root together with a multipolar cuff electrode
was developed and implemented in ANSYS
Multiphysics. Steady-state electric potential field
was calculated in ANSYS for several electrode
configurations. The potentials were exported to
MATLAB. In MATLAB, ordered pairs (x, y) were
randomly generated inside a circle with radius of
300 µm and centered at the origin – this circle
defines the contour of the sacral root. Longitudinal
points representing different segments of each axon
were randomly generated. Voltages were
interpolated at each (x, y, z) position representing a
specific anatomical compartment of each axon. As
its output MATLAB returned a matrix of [M x N],
where M is the number of axons inside each fascicle
and N is the number of axon’s segments (nodes of
Ranvier + internodal positions). In NEURON,
electric potential at each axon position is applied as
an extracellular field to an axon model representing
the mammalian motor axon. Each node of Ranvier is
checked for action potential (AP) events indicating
that the extracellular field invoked axonal activation.
Results from simulations in NEURON were
analyzed in MATLAB to determine selectivity. The
modeling workflow to determine selectivity is
schematically shown in Fig. 1.
In the presented design, the multipolar electrode
is made out of 18 electrode contacts (six tripoles)
that were placed in direct contact with the sacral
root. Each tripole is positioned around the sacral root
at positions {30, 90, 150, 210, 270, and 330 deg}.
The stimulating surface of each electrode is 100 µm
x 50 µm (length x width) and its thickness is 2 µm.
Thickness of the electrodes is derived from the
fabrication process which was used to fabricate the
first prototype of a flexible multipolar cuff
(Rodrigues et al., 2012). Figure 2 shows a schematic
of the contact spacing in the multipolar electrode in
its unrolled state. The simulated sacral root was
600 µm in diameter and 16 mm in length (z axis).
Part of the model is shown in Fig. 3. It comprises
sacral root, 18 metal contacts, insulating cuff,
cephalo raquidian fluid and a highly resistive
boundary layer. Geometric and electric properties of
the various elements within the model were reported
previously (Rodrigues et al., 2012). The electrode
contacts are modeled as current sources and a zero
voltage boundary condition is added to the outer
surfaces of the boundary layer. Steady-state
simulations were performed for several electrode
configurations. The two electrode configurations
presented here are shown in Fig. 4 and Fig. 5.
Figure 1: The modeling workflow used to determine
selectivity of multipolar cuff electrode in sacral roots
stimulation.
Figure 2: Schematic of the multipolar electrode in its
unrolled state.
ModelingWorkflowforStudyofFunctionalElectricalStimulationinPeripheralNerves
179
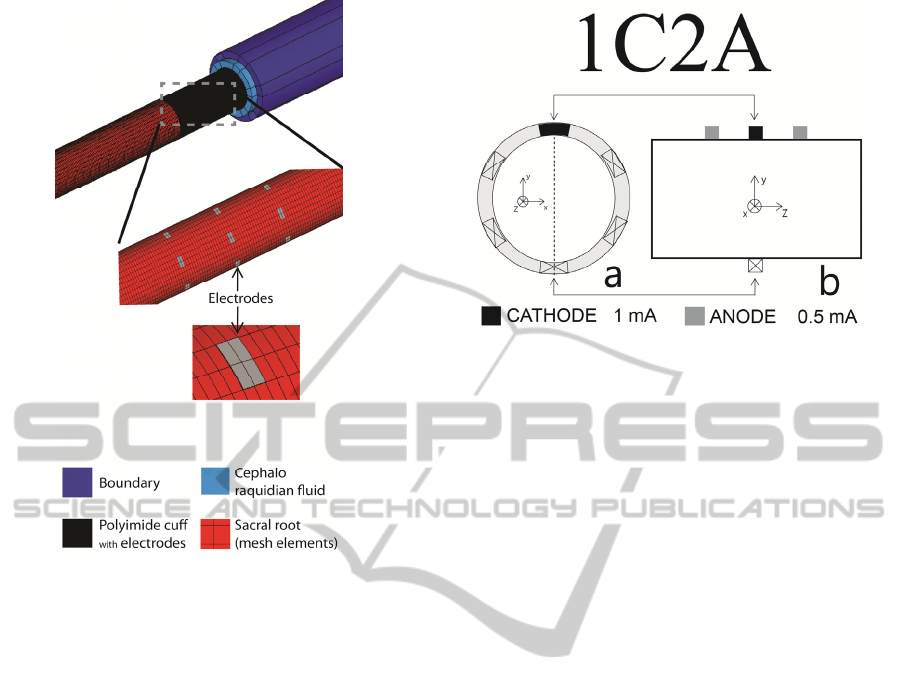
Figure 3: Parts of the FEM model. Note that the mesh
elements in the sacral root are half width of the electrode.
To shorten the time of modeling iterations it is
favourable to introduce a pulse amplitude factor
(PAF). This factor is multiplied by electric potential
field solution in each axonal position. So, assuming
V=1 as the electric potential at a given
node/internode position of the axon, if PAF = 0.1,
then V=0.1 will be the electric potential solution at
the same coordinate.
Indeed, simulations were run in NEURON for all
combinations of pulse widths (PW) of 0.01, 0.05,
0.1, 0.2, 0.5, 1, 2, 5, 7, 8, 8.33, 8.66, 9, 10 and 11 ms
and pulse amplitude factors of 0.1, 0.2, 0.5 and 1.
Axonal results from NEURON simulations were
analyzed to determine which stimulation case
(electrode configuration vs. PW vs. PAF) produced
the greatest selectivity. Adapted from Choi (Choi et
al., 2001), muscular selectivity, S, was defined as the
fraction of axons activated within all fascicles
innervating a target muscle (the “recruitment
benefit” or “RB”) minus the fraction of axons not
innervating the target muscle that were activated (the
“recruitment cost” or “RC”) – Eq. 1.
Figure 5: Electrode configuration 1C2A: (a) a transverse
cross section under the central row of electrodes; (b) a
longitudinal cross section with tripole at the top (position
90 deg). Cathode drains 1 mA and each anode injects
0.5 mA.
SRBRC
(1)
In the case of targeting bladder muscle to elicit
bladder voiding, S will be higher when
parasympathetic fibers (innervating bladder) will be
recruited in larger ratio (higher RB) than somatic
fibers innervating the sphincter (lower RC).
For the anatomy of sacral root presented in Fig.
6, selectivity for bladder muscle (activation of
parasympathetic fibers in red color) was calculated
for the considered electrode configurations: 1C2A
and 2C6A.
2.1 Anatomical and Physiological
Considerations
2.1.1 Anatomy of Sacral Nerve Roots
Sacral roots have a mixed population of fibers with
diameters ranging from 1 µm (smaller fibers) to
14 µm (larger fibers). In the present study, a ventral
sacral root, known as S3 is selected to analyze the
effect of the multipolar electrode on axonal
selectivity. S3 has a bimodal axon distribution with
peaks at 4 and 14 µm (Anon, 1996). Inside
peripheral nerves (e.g. sacral nerve roots) the sub-
structures giving support to the axons/fibers are
known by fascicles. In the terminology of Hauck
(Hauck et al., 2009), fascicles mostly carrying
parasympathetic fibers are called vegetative fascicles
and fascicles with predominance of somatic fibers
are called somatic fascicles. In our analysis, 21
fascicles were considered, which is in agreement
with the reported number of fascicles in S3 sacral
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
180
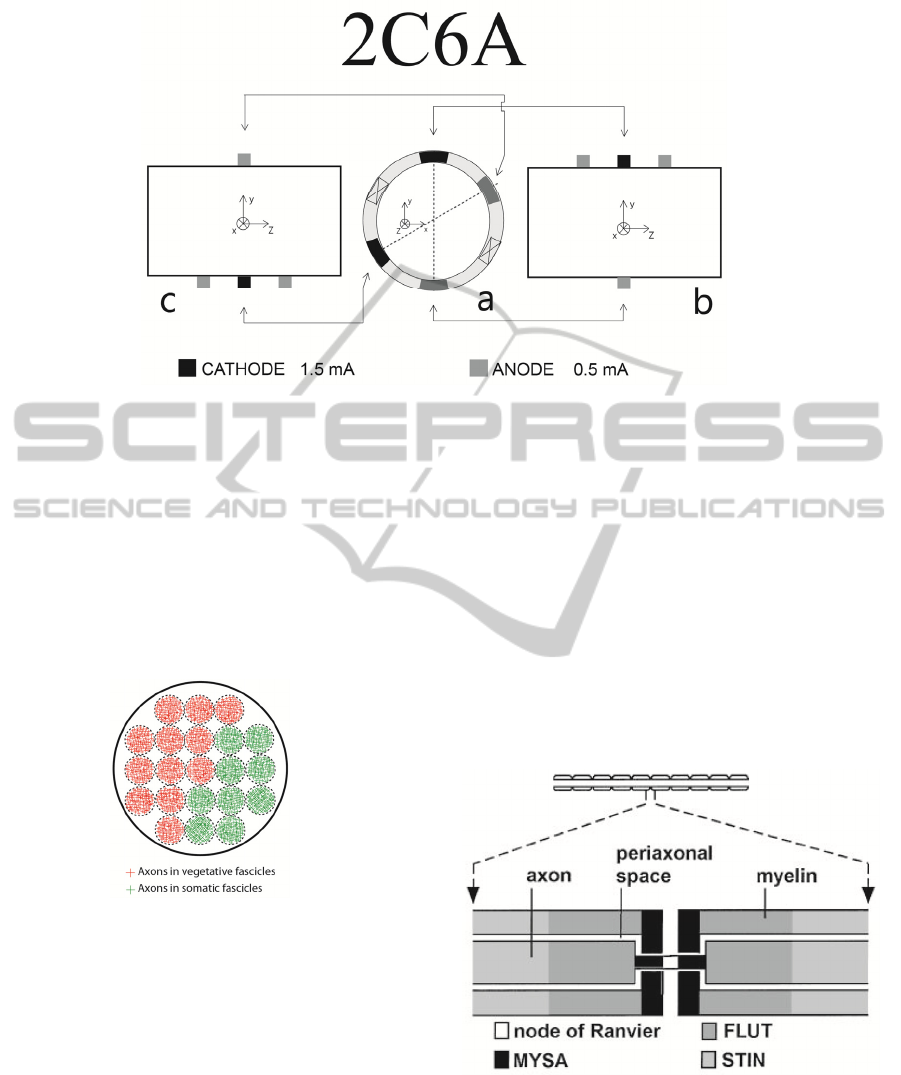
Figure 4: Electrode configuration 2C6A: (a) a transverse cross section under the central row of electrodes; (b) a longitudinal
cross section with tripole at the top (position 90 deg) and steering anode at the bottom (position 270 deg); (c) longitudinal
cross section with steering anode at the top (position 30) and tripole in the bottom (position 210 deg). Each cathode drains
1.5 mA and each anode injects 0.5 mA.
nerve root (Hauck et al., 2009). Distribution in
vegetative and somatic fascicles was previously set
and it is represented in Fig. 6. Each somatic fascicle
contains 50 axons of 14 µm in diameter (somatic
axons innervating sphincter) and each vegetative
fascicle has 50 axons of 4 µm in diameter
(parasympathetic axons innervating bladder).
Figure 6: Sacral nerve roots anatomy considered in the
presented study.
2.1.2 Physiology of the Axon
Myelinated axons are modeled using an active
electrical network to simulate the dynamics of each
node of Ranvier and of internodal sections of the
axon (McIntyre et al., 2002). A simulation procedure
was developed in NEURON to integrate the
NEURON’s open source axon model of McIntyre
(McIntyre et al., 2002). Axonal excitation is
generated by extracellular potentials (potentials
generated outside node of Ranvier and outside
myelinated internodes). These potentials are
assumed to be unaffected by the fiber response and
thus determined only by the stimulus electrodes and
the conductivity of the extracellular space.
Axon model from McIntyre is shown in Fig. 7.
This model used 10 segments between successive
nodes with an explicit representation of the myelin
attachment segment (MYSA), paranode main
segment (FLUT), and internode segment (STIN)
regions of the fiber. Extracellular potentials
interpolated in MATLAB and exported to NEURON
were assigned to these compartments.
Figure 7: Multi-compartment axon model. Axon and
nodes of Ranvier are at the top. Physiological
compartments are in the bottom. Figure adapted from
McIntyre (McIntyre et al., 2002).
ModelingWorkflowforStudyofFunctionalElectricalStimulationinPeripheralNerves
181
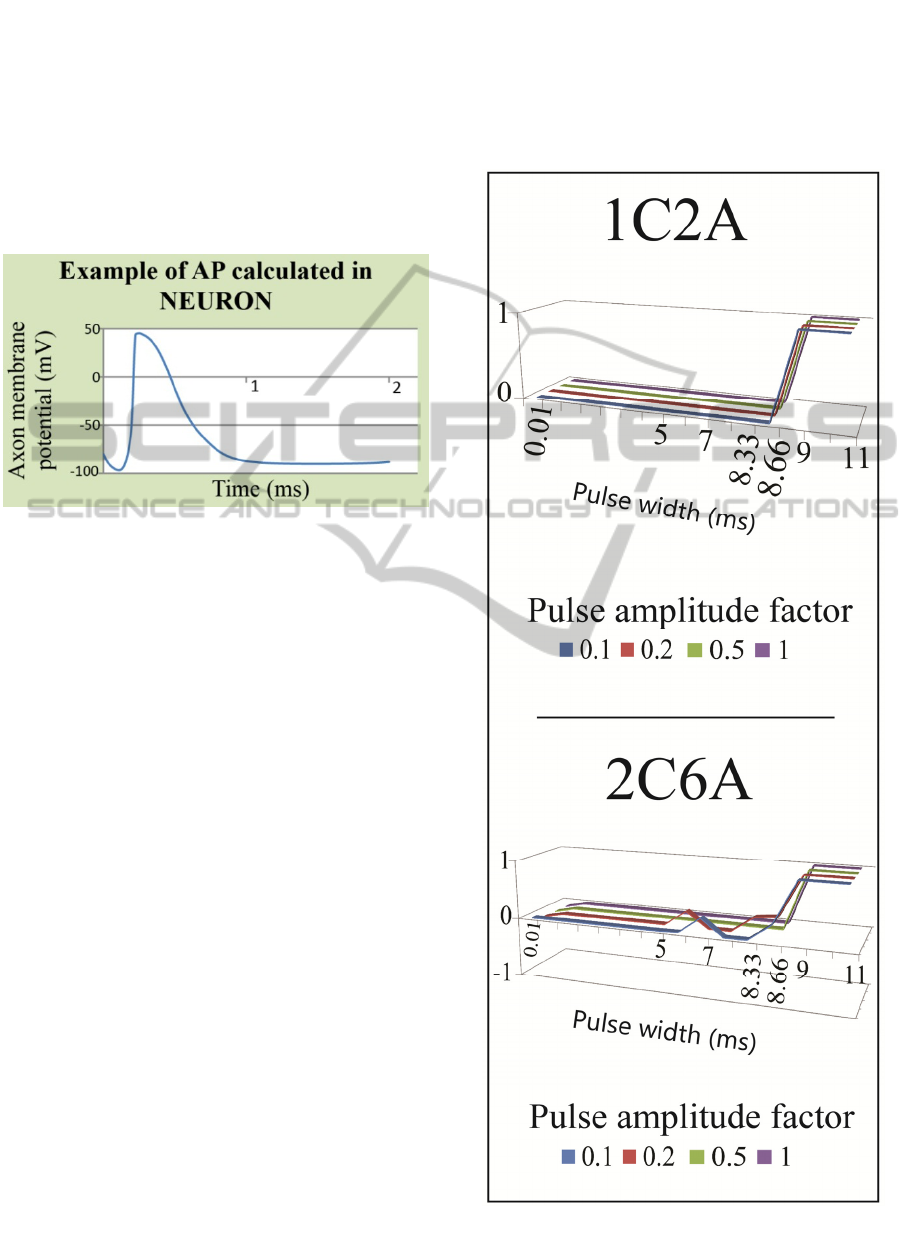
3 RESULTS
In NEURON, action potentials (APs) were used as a
measure to evaluate if the axon has fired for a given
combination of “electrode configuration / PW /
PAF”. It was assumed that if each axon has
registered more than 11 APs (1 AP / 1 node of
Ranvier), axon activation would be assumed. An
example of an action potential registered in
NEURON is plotted in Fig. 8.
Figure 8: Plot of action potential from NEURON.
Selectivity results are shown in Fig. 9. For each
electrode configuration – 1C2A and 2C6A –
selectivity index is plotted for 4 different pulse
amplitude factors (PAF), along the different pulse
widths (PWs).
In case of 1C2A, the activation of any fiber
(parasympathetic or somatic) only occurs for
PW>9 ms. A hypothesis can be that because of the
limitation of electric current (1 mA @ PAF=1), the
pulse width required to inject a sufficient charge is
high. For PW>9 ms, the selectivity shifts from 0 to
1. This means that all the parasympathetic fibers
(4 µm in diameter) were activated and none of the
somatic fibers (14 µm) was. As suggested by
McIntyre (McIntyre et al., 2002), the smaller
diameter fibers have longer chronaxies (i.e. smaller
fibers may need longer time to be electrically
stimulated) than the larger diameter ones. This can
explain the observation that after a 9 ms long pulse,
only the 4 µm fibers are depolarized (i.e. only the
parasympathetic fibers register an action potential).
In case of 2C6A, there is also complete
selectivity (S=1) for PW>9 ms. However, for lower
PWs intermediate selectivity values are registered.
These intermediate values of selectivity were due to
the activation of smaller parasympathetic as well as
to the activation of larger somatic. As it could be
expect, for larger input current levels (3 mA for
2C6A) it is possible to lower the pulse width
required for activation (activation was registered for
PW = 5 ms).
Lowering pulse width is a trade-off. If on the one
side, the lowering of pulse width also lowers the
required power to achieve axonal stimulation; on the
other side, it matches the chronaxie values for larger
somatic fibers, which contributes to lower the
selectivity.
Figure 9: Selectivity for electrode configurations – 1C2A
and 2C6A. Selectivity varies between -1 and 1.
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
182

Figure 10 shows the complete selectivity of axons
for PW > 9 ms. One can observe that only the
smaller parasympathetic fibers are active.
Figure 10: Activated axons for PW > 9 ms.
4 CONCLUSIONS
In this work design and testing of a multipolar cuff
electrode for peripheral nerve is presented. For that
purpose, a modeling workflow to study selectivity in
sacral nerve roots was implemented using ANSYS
Multiphysics, MATLAB and NEURON. Anatomical
studies were used to define a representative
distribution of fascicles in the sacral root. These
anatomical features were used to carry a study on the
effect of a multipolar cuff electrode on selective
stimulation of different fiber diameters. For pulse
widths higher than 9 ms, the selectivity is maximum
(most probably because of a “chronaxie effect”).
However to lower the need for electric power in the
electrical stimulation of sacral roots, further studies
have to be done in order to achieve selectivity for
lower pulse widths – e.g. increasing number of
electrode contacts, increasing number of rows of
electrodes in order to “reshape” the external
stimulation potential on each axon.
Modeling workflow showed to be effective for
the element size used in ANSYS. However, further
simulations are required for finer meshes in order to
study on effectiveness and stability of the workflow.
For that, a real anatomic mesh derived from a
histological cross section of the sacral roots will be
used.
ACKNOWLEDGEMENTS
This work was supported by the Portuguese
Foundation for Science and Technology
(SFRH/BD/62608/2009).
REFERENCES
Anon, 1996. Morphometric data of canine sacral nerve
roots with reference to electrical sacral root
stimulation. Neurourology Urodynamics, 15, pp.235–
248.
Brindley, G. S., 1977. An implant to empty the bladder or
close the urethra. Journal of Neurology, Neurosurgery,
and Psychiatry, 40, pp.358–369.
Choi, A., Cavanaugh, J. & Durand, D., 2001. Selectivity
of Multiple-Contact Nerve CuffElectrodes: A
Simulation Analysis. IEEE Transactions on
Biomedical Engineering, 48, pp.165–172.
Hauck, E. F. et al., 2009. Measurements and mapping of
282,420 nerve fibers in the S1-5 nerve roots. Journal
of neurosurgery. Spine, 11(3), pp.255–63. Available
at: http://thejns.org/doi/full/10.3171/
2009.3.SPINE17684 [Accessed November 2, 2011].
McIntyre, C., Richardson, A. & Grill, W., 2002. Modeling
the Excitability of Mammalian Nerve Fibers: Influence
of Afterpotentials on the Recovery Cycle. Journal
Neurophysiology, 87, pp.995–1006.
McIntyre, C. et al, 2002. No Title. Available at:
http://senselab.med.yale.edu/ModelDb/.
National Institute of Neurological Disorders and Stroke,
2012. Spinal Cord Injury: Hope Through Research.
Available at: http://www.ninds.nih.gov/disorders/sci/
detail_sci.htm [Accessed August 20, 2012].
Probst, M. et al., 1997. Neurostimulation for bladder
evacuation: is sacral root stimulation a substitute for
microstimulation? British Journal of Urology, 79,
pp.554–566.
Rijkhoff, N. et al., 1997. Selective Detrusor Activation by
Electrical Stimulation of the Human Sacral Nerve
Roots. Artificial Organs, 21, pp.223–226.
Rodrigues, F. et al., 2012. Flexible Multipolar Cuff
Microelectrode For FES Of Sacral Nerve Roots. In
Smart Machines – Neural Evolution. Banff, Canada:
IFESS .
Schiefer, A. et al., 2010. Selective stimulation of the
human femoral nerve with a flat interface nerve
electrode. Journal Neural Engineering, 7.
Social, H. and F. A. C., 2002. Towards concerted efforts
for treating and curing spinal cord injury. Available at:
http://assembly.coe.int/ASP/Doc/XrefViewHTML.asp
?FileID=17004&Language=EN [Accessed August 20,
2012].
ModelingWorkflowforStudyofFunctionalElectricalStimulationinPeripheralNerves
183
