
Using Linear Systems Theory to Study Nonlinear Dynamics of
Relay Cells
Rahul Agarwal and Sridevi V. Sarma
Institute for Computational Medicine, Johns Hopkins University, 3400 N Chrles Street, Baltimore, U.S.A.
Keywords:
Relay Neurons, Thalamus, Reliability, Hodgkin Huxley Type Models.
Abstract:
Relay cells are prevalent throughout sensory systems and receive two types of inputs: driving and modulating.
The driving input contains receptive field properties that must be relayed while the modulating input alters
the reliability of this relay. In this paper, we analyze a biophysical based nonlinear model of a relay cell
and use systems theoretic tools to construct analytic bounds on how well the cell transmits a driving input
as a function of the neuron’s electrophysiological properties, the modulating input, and the driving signal
parameters. Our analysis applies to both 2
nd
& 3
rd
order model as long as the neuron does not spike without
a driving input pulse and exhibits a refractory period. Our bounds suggest, for instance, that if the frequency
of the modulating input increases and the DC offset decreases, then reliability increases. Our analysis also
shows how the biophysical properties of the neuron (e.g. ion channel dynamics) define the oscillatory patterns
needed in the modulating input for appropriately timed relay of sensory information.
1 INTRODUCTION
Relay neurons are found in various brain nuclei in-
cluding the thalamus (Samuel et al., 2004; Hirsch
et al., 1983; Seki et al., 2003). Experiments have
suggested that the inputs to a relay neuron can be
divided into two categories: driving and modulat-
ing. The driving input is made up of few synapses
on the proximal dendrites whereas the modulating in-
put comprises all other synapses (Guillery and Sher-
man, 2002) (see Figure 1). The relay neuron pro-
cesses and relays information in the driving input,
conditioned on the modulating input.(Sherman and
Guillery, 1998; Sherman, 2007). For example, the
lateral geniculate nucleus (LGN) in the thalamus re-
ceives the driving input from the retina and modu-
lating input from cortex. The function of the LGN
is to selectively relay information from the retina to
primary visual cortex (Guillery and Sherman, 2002;
O’Connor et al., 2002).
In this study we rigorously analyze biophysical
based models of relay neurons and characterize the
nonlinear electro-physiological dynamics of a single
relay cell as a function of the cell type and the inputs.
Various attempts to study relay neurons are made in
(Rubin and Terman D., 2004; Masson et al., 2002;
Guo et al., 2008; Wolfart et al., 2005; Rubin and Josic
K, 2007). These studies also suggest that the relay
neuron’s reliability is governed by the modulating in-
put combined with the intrinsic properties of the neu-
ron, however the results were shown to be true for
only few realizations of modulating and driving input.
The work presented here is an extension of the work
we presented in (Agarwal and Sarma, 2011) where
we employ systems theoretic tools to obtain an ex-
plicit analytical bounds on reliability for a 2
nd
order
model. In this work we showed that same method-
ology can also be employed to obtain bounds on 3
rd
order model as long as the neuron does not spike with-
out a pulse in the driving input and exhibits a refrac-
tory period. Consequently, our analysis is relevant for
relay cells whose electrophysiological dynamics, in-
cluding bursting, may be governed by several differ-
ent ion channels and is more rigorous than previous
works. Our bounds predict the dependence of relay
reliability as a function of the neuron’s electrophysio-
logical properties (i.e., model parameters), the modu-
lating input signal, and the driving signal parameters.
Our lower and upper bounds suggests, for example,
that if the frequency of the modulating input increases
and/or its DC offset decreases, then relay reliability
increases.
433
Agarwal R. and V. Sarma S..
Using Linear Systems Theory to Study Nonlinear Dynamics of Relay Cells.
DOI: 10.5220/0003987504330438
In Proceedings of the 9th International Conference on Informatics in Control, Automation and Robotics (ICINCO-2012), pages 433-438
ISBN: 978-989-8565-21-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
2 MATERIALS AND METHODS
In this section, first we describe a biophysical model
of a relay neuron, and then use systems theoretical
tools to compute bounds on relay reliability.
2.1 A Relay Neuron Model
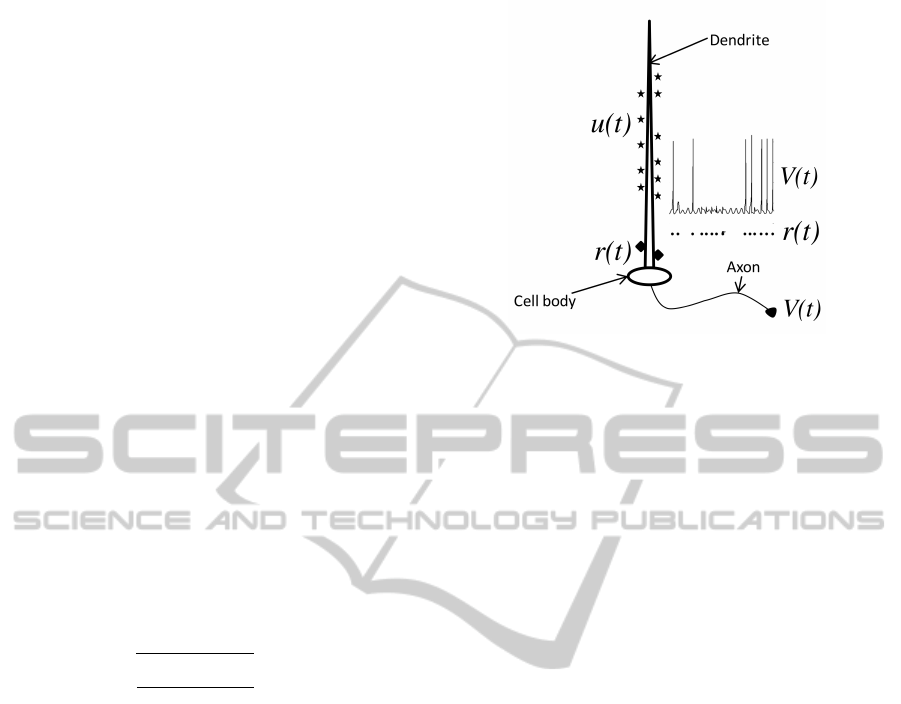
A relay neuron receives two kinds of inputs: a driving
input, r(t) and a modulating input u(t), and generates
one output, V (t), as shown in Figure 1.
Below is a state space representation of 3
rd
order
Hodgkin-Huxley type biophysical model of a relay
neuron. This model was used in (Guo et al., 2008;
Rubin and Terman D., 2004), which is a simplifica-
tion of model used in (Sohal and Huguenard, 2002;
Sohal et al., 2000). The response of the model for
an oscillating modulating input and a Poisson driver
input is also shown in Figure 1.
˙
x = f(x) +
−u(t) 0 0
0 0 0
0 0 0
x +
r(t)
0
0
, (1)
where f(x) =
−(I
L
+ I
Na
+ I
K
+ I
T
) + I
ext
(h
∞
(X
1
+V
syn
)−X
2
)
(τ
h
(X
1
+V
syn
))
(r
∞
(X
1
+V
syn
)−X
2
)
(τ
r
(X
1
+V
syn
))
. (2)
Here I
L
= g
L
(V − V
L
) , I
Na
= g
Na
m
3
∞
h(V − V
Na
),
I
K
= g
L
(0.75(1 − h))
4
(V − V
L
) are the leak current,
sodium and potassium current respectively. I
T
=
g
T
p
2
∞
(V )r(V − V
T
) and I
ext
are the low threshold
potassium current and external current respectively.
All the parameters used are same as those in (Guo
et al., 2008) and are given in the table 1.
In general, a state space representation takes the
form
˙
x = f(x,r,u), however, there is more structure
in (1). From (1), one can see that f(x) is only a func-
tion of the system’s internal states. The modulating
input, u(t), multiplies the first component of the state
x, while the driving input, r(t), is an exogenous input
to the system.
2.1.1 Inputs and Outputs
For our analysis, we assume that the driving input
belongs to class of delta pulse train with Poisson ar-
rivals, i.e. r(t) = I
0
n
∑
i=1
δ(t − τ
i
). The τ
0
i
s are generated
randomly such that τ
i+1
= τ
i
+ T
0
+ τ
0
, where T
0
∈ R
is a constant that represents the refractory period of
driving input, and τ
0
∈ R
+
is exponentially distributed
Figure 1: A Relay Neuron - Illustrating a relay neuron.
Ensemble activity of all the distal synapses (stars) is modu-
lating input u(t). The proximal synapses (diamonds) form
driving input r(t). The output is axonal voltage V (t). The
inset plots the voltage profile obtain from the model in re-
sponse to pulses in r(t). Note that each pulse in r(t) may or
may not generate a spike.
with rate λ. Therefore, the average inter-pulse interval
is T = E(τ
i+1
− τ
i
) = T
0
+ 1/λ. We assume that the
modulating input belongs to set of sinusoidal func-
tion i.e. u(t) = c
1
+c
2
sin(ωt). The output of the relay
neuron is membrane voltage V (t) = x
1
(t) +V
syn
. De-
tails of all the parameters and reasons for the chosen
classes for driving and modulating input can be found
in (Agarwal and Sarma, 2011).
2.1.2 Properties of the Model
Finally, the function f(x) is assumed to have the fol-
lowing properties but is otherwise general:
1. Stable Neuron. Consider (1) with u(t) = c
1
and
r(t) = 0,. In general, this system may have multiple
equilibria with different stability properties. But for
our purposes, we choose f(x) such that (1) with u(t) =
c
1
,r(t) = 0 has only one globally stable equilibrium
point,
¯
x, for all pragmatic c
1
. Such a neuron is called
a stable neuron (Manor et al., 1997). This condition
ensures that the neuron does not have any limit cycle,
therefore, the neuron does not spike without a pulse
in r(t).
This further implies that if a small periodic mod-
ulating input is applied to a stable neuron (1), u(t) =
c
1
+c
2
sin(ωt),r(t) = 0, then after a sufficient amount
of time the system’s state vector will lie within a small
neighbourhood of the equilibrium point. However,
the state vector never reaches
¯
x due to the time vary-
ing modulating input. The trajectory of the state in
this neighbourhood can be solved using linearization
methods and is periodic (Agarwal and Sarma, 2011).
We define this periodic trajectory as the steady state
ICINCO2012-9thInternationalConferenceonInformaticsinControl,AutomationandRobotics
434
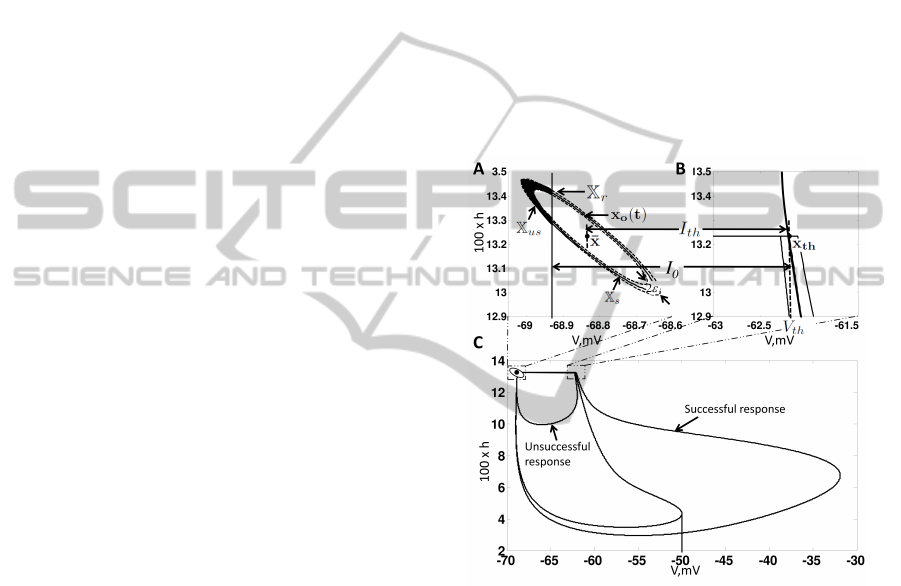
orbit of a stable neuron, x
o
(t). See Figure 2 A.
Next, we define X
∞
as the collection of all points
in the steady state orbit. If the initial state of the sys-
tem x(t = 0) /∈ X
∞
then X
∞
is not achievable in finite
time. Therefore, we relax our definition to the collec-
tion of all points inside a tube of ε thickness around
the steady state orbit, and define this tube as the set
X
r
, i.e.
X
r
= {x ∈ R
n
| kx −vk ≤ ε for a v ∈ X
∞
}. (3)
2. Threshold Behaviour. To define threshold be-
haviour of a neuron, we first define a successful re-
sponse. A successful response at time t is a change
in V such that V (t) > −50mV & V (t − τ) ≤ −50mV
∀τ ∈ (0, L)ms. Note that both a single spike or a burst
of spikes, with intra burst interval less than L ms, are
counted as a successful response under this definition.
We use this definition so that we can extend our anal-
ysis to bursty neurons characterized by higher order
models.
Almost all stable neurons show a threshold be-
haviour (Platkiewicz and Brette R, 2010; Lodish
et al., 2000).
Now, we define x
th
= V
th
−V
syn
, where V
th
is the
traditional threshold voltage for generating a success-
ful response (Platkiewicz and Brette R, 2010; Lodish
et al., 2000; FitzHugh, 1955). In (Platkiewicz and
Brette R, 2010) it has been shown that spike threshold
is influenced by ion channels activation and synap-
tic conductance. In our case we assume a constant
threshold for simplicity. We show in results section
that our analytical bounds calculated using this as-
sumption contained the relay performance obtained
by doing simulations on the original model. Fi-
nally, we define the threshold current, I
th
, such that
¯x
1
+I
th
= x
th
. Note, by definition both I
th
and V
th
have
the same units and hence can be added.
Illustrations of a successful response, unsuccess-
ful response, I
th
, V
th
, x
th
X
∞
, X
r
are shown in Figure
2 A B, C, for a second order system. Note that, I
th
and V
th
are functions of c
1
, since different values of
c
1
result in different F(x). This dependence is a lin-
ear function i.e I
th
(c
1
) ' I
th
(0) + mc
1
.
2.2 Response to Pulses in r(t)
When a reference pulse arrives, there are 2 possible
responses of system in (1). The neuron either gives
a successful response or unsuccessful response. In
Figure 2 C, we have plotted these two types of re-
sponses.
It is straightforward to see how these two re-
sponses occur. The reference pulse causes the state
vector to “ jump” to x(t
i
) = x(t
−
i
) + [I
0
,0]
T
. This is
easy to show by direct integration of (1), on the time
interval lim
∆t→0
[t
i
− ∆t,t
i
]. If the dynamics of x
2
,x
3
,
are slow and x
1
(t
i
) > x
th
− I
0
(see Figure 2 A,B) the
neuron will generate a successful response, otherwise
it will return back to the equilibrium point generat-
ing unsuccessful response. Now, we define set X
s
as the collection of points in X
r
whose first compo-
nent x
1
> x
th
− I
0
. These points result in successful
response after a pulse in r(t). Similarly we define
X
us
⊆ X
r
as the collection of points whose first com-
ponent x
1
≤ x
th
− I
0
, hence these points result in un-
successful spikes after a pulse in r(t). These sets are
illustrated in Figure 2 A. Note that if the reference
pulse does not occur for a T
r
time interval i.e, the sys-
tem state will move into X
r
.
Figure 2: Properties of f(x) - (A) Illustrates the equilib-
rium point
¯
x, the steady state orbit x
o
(t) and the orbit tube,
X
r
, for f(x) for a second order neuron. (B) Illustrates x
th
,
the threshold voltage V
th
and threshold current I
th
. Note
that these parameters are defined by the undriven system
u(t) = c
1
,r(t) = 0. (C)Illustrates a successful response tra-
jectory and an unsuccessful response trajectory.
2.3 Relay Reliability
We define relay reliability as:
R , Pr(Successful spike due to a reference pulse ).
(4)
Using (4) and definition of X
s
, we can write:
R = Pr
X(t
−
) ∈ X
s
|r(t) = I
0
δ(0)
(5a)
= Pr (X(t
i
) ∈ X
s
) for any i = 1,2,··· . (5b)
UsingLinearSystemsTheorytoStudyNonlinearDynamicsofRelayCells
435

Noting X
s
⊆ X
r
, we break (5b) as:
R = Pr (X(t
i
) ∈ X
s
&X
r
) (6a)
= Pr (X(t
i
) ∈ X
r
)Pr (X (t
i
) ∈ X
s
|X(t
i
) ∈ X
r
) (6b)
, P
pulse
× P
spike
. (6c)
Here we have used the definition of conditional
probability (Greenstied and Snell, 2003) to go from
(6b) to (6c). Although not explicit in (6), relay re-
liability is a function of the driver input parameters,
I
0
and T, the modulating input parameters, c
1
,c
2
, and
ω, and the neuron’s dynamics (i.e. model parame-
ters) denoted by H. In the next section, we com-
pute closed-form approximations of lower and upper
bounds of reliability as a function of I
0
,T, c
2
,c
1
,ω and
H, by computing P
spike
and bounds on P
pulse
.
2.4 Calculation of P
spike
We have already calculated P
spike
for a second or-
der model and is same as R in (Agarwal and Sarma,
2011). The exact analysis can be repeated for the 3
rd
order models and P
spike
can be estimated as the ra-
tio of time spent by the state trajectory in X
s
and X
us
given it is in X
r
. This gives:
P
spike
=
π + 2sin
−1
I
0
−I
th
c
2
|H
1
( jω)| ¯x
1
2π
. (7)
Here H
1
( jω) =
1
2π
R
h
1
(t)e
− jωt
dt is the transfer
function of the neuron x
1
(t) − ¯x
1
= h
1
∗ u(t). Details
of the above expression can be found in (Agarwal and
Sarma, 2011).
2.5 Calculation of P
pulse
& R
In this section, we compute P
pulse
to ultimately obtain
an expression for R. Since a driver pulse that arrives
at time t
i
can only result in either a successful spike or
an unsuccessful spike, we can equivalently write the
definition of P
pulse
as:
P
pulse
=Pr(x(t
i
) ∈ X
r
| SR at t
i−1
) · Pr(SR at t
i−1
)
+ Pr(x(t
i
) ∈ X
r
| USR at t
i−1
) · Pr( USR t
i−1
).
(8a)
Here, we have used the law of total probability and
the definition of conditional probability (Greenstied
and Snell, 2003) to arrive at (8a). We know that after
a successful spike at t
i−1
, the system state X (t) /∈ X
r
,
only for t ∈ (t
i−1
,t
i−1
+ T
r
). Therefore, we see that
Pr(X (t
i
) ∈ X
r
| SR at t
i−1
) = Pr(t
i
−t
i−1
≥ T
r
). (9)
Similarly, if T
us
r
denotes time to resting tube after un-
successful spike, then we get:
Pr(X (t
i
) ∈ X
r
| USR at t
i−1
) = Pr(t
i
−t
i−1
≥ T
us
r
).
(10)
As T
us
r
has complex dependence upon input and
model parameters it is hard to calculate Pr(t
i
−t
i−1
≥
T
us
r
). However, it is certain that T
us
r
≤ T
r
. This implies
that Pr(t
i
−t
i−1
≥ T
r
) ≤ Pr(t
i
−t
i−1
≥ T
us
r
), by prop-
erties of cumulative distributive functions (Greenstied
and Snell, 2003). Therefore, we get the following
bounds:
Pr(t
i
−t
i−1
≥ T
r
) ≤ Pr(t
i
−t
i−1
≥ T
us
r
) ≤ 1 (11)
Putting together (8a),(9),(10) and (11), we get:
P
pulse
≥R · Pr(t
i
−t
i−1
≥ T
r
) (12a)
+(1 − R) · Pr(t
i
−t
i−1
≥ T
r
) (12b)
and P
pulse
≤R · Pr(t
i
−t
i−1
≥ T
r
) + (1 − R). (12c)
Now we calculate Pr(t
i
− t
i−1
≥ T
r
). Recall that
the inter pulse intervals of r(t), t
i
−t
i−1
= τ + T
0
, here
τ is generated from an exponential distribution and T
0
is refractory period. Therefore:
Pr(t
i
−t
i−1
≥ T
r
) = Pr(T
0
+ τ ≥ T
r
) (13a)
= Pr(τ ≥ T
r
− T
0
) (13b)
=
Z
∞
T
r
−T
0
f
τ
(τ)dτ , α. (13c)
It can be easily shown that:
α =
(
e
−(T
r
−T
0
)
T −T
0
T
r
− T
0
≥ 0
1 T
r
− T
0
< 0
. (14)
Here T is the average inter pulse interval, E(t
i
−t
i−1
).
Finally, by combining (12c) and (13) we get:
α ≤P
pulse
≤ 1 − R(1 − α). (15a)
Now we compute bounds on relay reliability i.e
R
l
≤ R ≤ R
u
as:
P
spike
1 + (1 − α)P
spike
≥ R ≥ α · P
spike
. (16)
From (16) and (13), one can see that if T >> T
r
,
then R
l
→ R
u
→ P
spike
. This result is intuitive because
if pulses in r(t) occur at a slow rate, then the solution
of (1) has enough time to return to the orbit tube after
each pulse. Therefore, P
pulse
, P(X(t) ∈ X
r
) → 1 and
hence R → P
spike
.
3 RESULTS
In this section, we will apply (16) to (1).
ICINCO2012-9thInternationalConferenceonInformaticsinControl,AutomationandRobotics
436
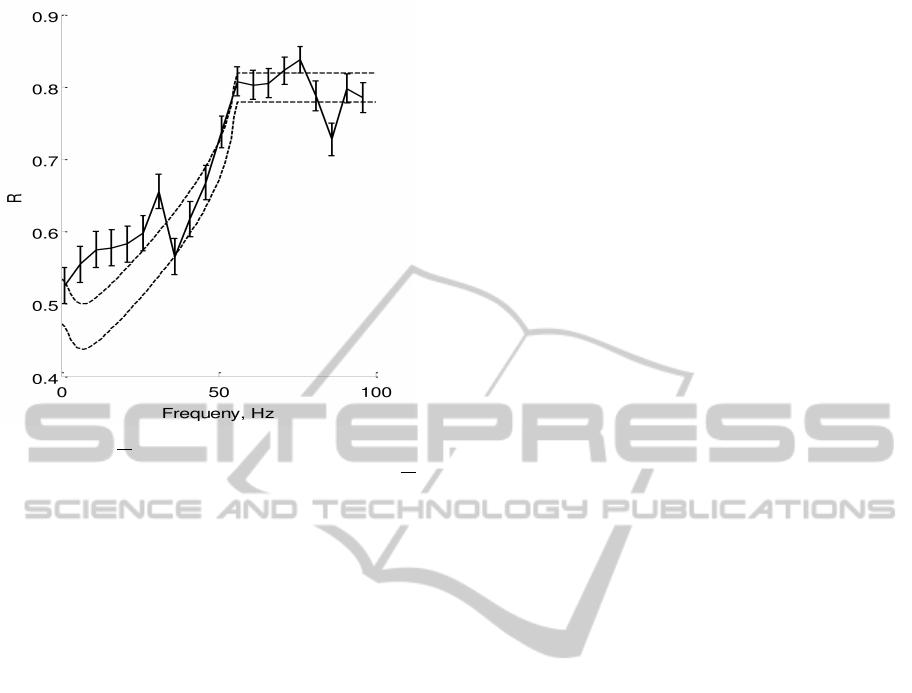
Figure 3: R vs
ω
2π
for the 3
rd
order model - Plots the-
oretical and numerically computed reliability versus
ω
2π
,
with c
2
= 0.015, c
1
= 0.075, I
0
= 6.5, T
r
= 105ms, T
0
=
80ms,T = 180ms. Dotted lines are lower and upper bounds
on reliability from the (16). Solid line is plotting R
emp
cal-
culated by running simulation of (1), error bars show ±std.
We estimated I
th
= 6 as the minimum hieght of a r(t) pulse,
that make the neuron spike.
In Figure 3, we plot our reliability bounds (16)
along with reliability computed numerically through
simulation of the 3rd order model. We see that our
bounds predict the reliability well except for some er-
rors in the low frequency range. This is due to the fact
that we did not consider higher order dynamics of the
3
rd
order model. However, our bounds qualitatively
predict the trend of reliability well even for the higher
order model.
In general, our analytical bounds are applicable to
higher dimensional models as long as the model 1.
does not generate a spike if there is no pulse in r(t),
and 2. has a threshold behavior. The second condi-
tion is true for most of neurons that satisfy the first
condition. Our analysis may also be extended to in-
clude neurons that spike without any driver input, but
in this manuscript we neglect such dynamics.
4 DISCUSSION
In this manuscript, we studied the reliability of a relay
neuron. A relay neuron receives two inputs: a driving
input, r(t), and a modulating input, u(t). The neuron
generates one output, V (t), which relays r(t) condi-
tioned on u(t). Our goal was to precisely determine
how the modulating input impacts relay reliability. To
calculate relay reliability, we used LTI systems theo-
retic tools to derive the analytical bounds (16) on relay
reliability as a function of different input and model
parameters. Specifically, (16) implies that if the mod-
ulating input is of the form u(t) = c
1
+c
2
sin(ωt), then
increasing c
1
or c
2
decreases reliability. However, in-
creasing ω increases reliability. In addition, our reli-
ability curve (see Figure 3) suggests that on increas-
ing ω, reliability first increases slowly and then in-
creases rapidly and plateaus. (16) is powerful as it
characterizes the multiple dependencies of reliability
on u(t), r(t) and relay neuron model parameters. Fur-
thermore, analytic bounds from (16) contain results
obtained through simulation of the 3
rd
order models
of a relay neuron. Our bounds captured reliability
under both the depolarized and hyperpolarized (not
shown due to limited space) states of the 3rd order
neuron and shows the generality of our analysis.
REFERENCES
Agarwal R. and Sarma S. V. (2011). The effects of dbs
patterns on basal ganglia activity and thalamic relay. J
Comput Neurosci, (accepted for publication).
Bekisz M. and Wrobel A. (1999). Coupling of beta and
gamma activity in corticothalamic system of cats at-
tending to visual stimuli. NeuroReport, 10:3589–94.
Erwin E., Baker F. H., Busen W. F., and Malpeli J. G.
(1999). Relationship between laminar topology and
retinotopy in the rhesus lateral geniculate nucleus: re-
sults from a functional atlas. J.Comp neurol, 407:92–
102.
FitzHugh R. (1955). Mathematical models of threshold phe-
nomena in the nerve membrane. Bulletin of Mathe-
matical Biology, 17(4):257–278.
Greenstied Charles M. and Snell J. Laurie (2003). Introduc-
tion to Probability. American Mathematical Society.
Guillery R. W. and Sherman S. M. (2002). Thalamic relay
functions and their role in corticocortical communica-
tion: Generalizations from the visual system. Neuron,
33:163–176.
Guo Y., Rubin J. E., McIntyre C. C., Vitek J. L., and Ter-
man D. (2008). Thalamocortical relay fidelity varies
in deep brain stmulation protocols in data-driven com-
putational models. J. Neurophysiol., 99:1477–1492.
Hirsch J. C., Fourment A., and Marc M. E. (1983). Sleep-
related variations of membrane potential in the lateral
geniculate body relay neurons of the cat. Brain Re-
search, 259(2):308–312.
Hughes S. W., Lorincz M., Cope D. W., Blethyn K. L.,
Kekesi K. A., Parri H. R., Juhasz G., and Crunelli V.
(2004). Synchronized oscillations at α and θ frequen-
cies in the lateral geniculate nucleus. Neuron, Vol. 42,
253268, April 22, 2004,.
Kastner S., Schneider K. A., and Wunderlich K. (2006).
Chapter 8 beyond a relay nucleus: Neuroimaging
UsingLinearSystemsTheorytoStudyNonlinearDynamicsofRelayCells
437

views on the human lgn. Progress in Brain Research,
155 Part B:125–143.
Lagier Samuel , Carleton Alan, and Lledo Pierre-Marie
(2004). Interplay between local gabaergic interneu-
rons and relay neurons generates γ oscillations in
the rat olfactory bulb. The Journal of Neuroscience,
24(18):4382–4392.
Lodish H., Berk A., and Zipursky S. L. (2000). Molecular
Cell Biology. 4th edition. W. H. Freeman.
Logothetis N. K. (2002). The neural basis of blood oxygen
level dependent functional magnetic resonance imag-
ing signal. Phil. Trans R. Soc. Lond B, 357(1003-37).
Lorincz M. L., Kekesi K. A., Juhasz G., Crunelli V., and
Hughes S. W. (2009). Temporal framing of thalamic
relay-mode firing by phasic inhibition during the al-
pha rhythm. Neuron, 63:683–96.
Manor Y., Rinzel J., Segav I., and Yarom Y. (1997). Low
amplitude oscillations in inferior olive: A model based
on electrical coupling of neurons with heterogeneous
channel densities. J. Neurophysiol.
Masson G. L., Masson S. R. L., D., and Bal T. (2002). Feed-
back inhibition controls spike transfer in hybrid thala-
mic circuits. Nature, 417:854–858.
O’Connor D. H., Fukui M. M., Pinsk M. A., and Kastner
S. (2002). Attention modulates responses in the hu-
man lateral geniculate nucleus. nature neuroscience,
5(11):1203–1209.
Platkiewicz J. and Brette R. (2010). A threshold equa-
tion for action potential initiation. PloS Comput Biol,
6(7):1000850.
Reinagel P., Godwin D., Sherman M., and Koch C. (1999).
Encoding of visual information by lgn bursts. Journal
of Neurophysiology, 81:2558–69.
Rubin J. and Josic K. (2007). The firing of an excitable
neuron in the presence of stochastic trains of strong
synaptic inputs. Neural Computation, 19:1251–1294.
Rubin J. E. and Terman D. (2004). High frequency stimula-
tion of the subthalamic nucleus eliminates pathologi-
cal thalamic rhythmicity in a computational model. J.
Comput. Neurosci., 16(3):211–35.
Seki Kazuhiko, Perlmutter Steve I., and Fetz Eberhard E.
(2003). Sensory input to primate spinal cord is presy-
naptically inhibited during voluntary movement. Na-
ture Neuroscience, 6:1309–1316.
Sherman S. M. (2007). The thalamus is more than just a
relay. Current Opinion in Neurobiology, 17(4):417–
422.
Sherman S. M. and Guillery R. W. (1998). On the actions
that one nerve can have on another: Distinguishing
”drivers” from ”modulators”. PNAS, 95(12):7121–26.
Sherman S. M. and Guillery R. W. (2002). The role of the
thalamus in the flow of information to the cortex. Phil.
Trans. R. Soc. Lond. B., 357(1428):1695–1708.
Sohal V. and Huguenard J. (2002). Reciprocal inhibition
controls the oscillatory state in thalamic networks.
Neurocomp, 44:653–659.
Sohal V., Huntsman M., and Huguenard J. (2000). Recipro-
cal inhibitory connections regulate the spatiotemporal
properties of intrathalamic oscillations. J Neurosci,
20:1735–1745.
Wolfart J., Debay D., Masson G. L., Destexhe A., and
Bal T. (2005). Synaptic background activity controls
spike transfer from thalamus to cortex. Nature Neuro-
science, 8(12):1760–1767.
Zhan X., Cox C., Rinzel J., and Sherman S. (1999). Current
clamp and modeling studies of lowthreshold calcium
spikes in cells of the cats lateral geniculate nucleus. J.
Neurophysiol, 81:2360–2373.
APPENDIX
Table 1: Parameters and functions for (1).
m
∞
(V )
1
(1+exp(−(V+37)/7))
p
∞
(V )
1
(1+exp(−(V+60)/6.2))
τ
h
(V )
1
0.128exp(−
46+V
18
)+4/(1+exp(−
23+V
5
))
h
∞
(V )
1
(1+exp((V+41)/4))
r
∞
(V )
1
(1+exp((V+84)/4))
τ
r
(V ) 0.4(28 + exp((V + 25)/(−10.5)))
V
syn
,V
Na
−85,50mV
V
K
,V
L
,V
T
−90,−70, 0mV
g
Na
,g
K
3,5
g
L
,g
T
0.05,5
ICINCO2012-9thInternationalConferenceonInformaticsinControl,AutomationandRobotics
438
