
EEG AND HUMAN LOCOMOTION
Descending Commands and Sensory Feedback should be Disentangled
from Artifacts Thanks to New Experimental Protocols
Position Paper
Thierry Castermans
1
, Matthieu Duvinage
1
, Guy Cheron
2
and Thierry Dutoit
1
1
TCTS lab, Faculty of Engineering, Universit´e de Mons, 31 Boulevard Dolez, 7000, Mons, Belgium
2
LNMB lab, Universit´e Libre de Bruxelles, CP 168, 50 Av F. Roosevelt, Brussels, Belgium
Keywords:
EEG Analysis, Human Locomotion, Artifacts, Brain-computer Interfaces.
Abstract:
The main challenge when studying EEG signals related to human walk control comes from the fact that
signals of many different origins are mixed up. Indeed, descending commands from the brain are generated,
while ascending sensorimotor information coming from the feet is sent to the brain. In addition to the inherent
complexity of the human control mechanism, experimental investigation of the cerebral activity elicited during
walk is highly challenging: electrode movements are produced by movements of the head, but also by the
shocks undergone by the whole body at each step, which – albeit significantly attenuated – are transmitted to
the head and degrade the quality of EEG signals. Recently, different EEG studies of human locomotion have
been published. These are based on different hypotheses and/or produce results that are contradictory. After
reviewing and describing the discrepancies between the different approaches, we propose new experimental
protocols which should help to solve important issues.
1 INTRODUCTION
Human locomotion is known to be based on a very
complex hierarchical system which includes several
control networks located at spinal and supraspinal
levels (Hanakawa, 2006). Basically, high-level mo-
tor commands are sent by the brain to a spinal net-
work composed of central pattern generators (CPG)
and, at the same time, each level of motor control re-
ceives peripheral sensory information (sensory feed-
back) which is used to modify the motor output at that
level.
The central pattern generators network consists
in coupled antagonist oscillators specifically dedi-
cated to extensor or flexor muscles acting at the dif-
ferent joints. Their mechanism allows to generate
simple and coordinated rhythmic movements such as
those involved in steady walk (Kiehn, 2006). Ahead
of the CPG, supra-spinal networks (i.e. the brain-
stem, cerebellum and cortex) are of crucial impor-
tance in the control of walking. Indeed, as summa-
rized in (Presacco et al., 2011) and references therein,
significant changes in motor and cognitive demands
(i.e. spatial attention) have been observed in the con-
text of bipedal walking in unknown or cluttered dy-
namic environments. Functional neuroimaging stud-
ies have shown that the primary motor cortex is re-
cruited during rhythmic foot or leg movements. Ad-
ditionally, the technique of functional near-infrared
spectroscopy (fNIRS) has allowed to detect involve-
ment of frontal, premotor and supplementary motor
areas during walking (Harada et al., 2009), (Suzuki
et al., 2008).
Electroencephalography (EEG) represents an in-
teresting complementary technique for investigating
neural processes governing walk. Indeed, while stan-
dard functional imaging is characterized by a good
spatial resolution, EEG is the only wearable and non-
invasive measurement technique which offers a tem-
poral resolution good enough in order to study the dy-
namics of brain. However, electrophysiologicalinves-
tigation of the cerebral activity elicited during walk
is highly challenging. Indeed, head and body move-
ments constitute an important source of mechanical
artifacts strongly affecting the EEG signals quality.
This explains why very few papers have been writ-
ten on the subject.
Recently, EEG studies of human locomotion have
309
Castermans T., Duvinage M., Cheron G. and Dutoit T..
EEG AND HUMAN LOCOMOTION - Descending Commands and Sensory Feedback should be Disentangled from Artifacts Thanks to New Experimental
Protocols Position Paper.
DOI: 10.5220/0003871403090314
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2012), pages 309-314
ISBN: 978-989-8425-89-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

been undertaken but give incompatible results. In this
context, the objective of this paper is twofold: (1) re-
view and discuss the new available results, empha-
sizing contradictory aspects as well as discrepancies
with independent experimental results; (2) propose
new experimental protocols to resolve the ambigui-
ties.
2 EEG STUDIES OF HUMAN
LOCOMOTION
2.1 Static vs Dynamic Approaches
EEG signals are by essence noisy and difficult to mea-
sure (a few microvolts only). This is due to the fact
that each EEG scalp electrode is only able to measure
the combination (superposition) of the electrical po-
tentials generated by thousands of neurons, which are
weakened and smeared by the volume conduction ef-
fect of the skull (Nunez et al., 1997). Moreover, EEG
signals may be affected by different kinds of artifacts
generated either by extracerebral physiological activ-
ity (blinks, eye movements, muscle or cardiac activ-
ity), by interference with power line, or by record-
ing electrodes and equipment. On top of this, typical
artifacts related to gait further degrade EEG signals
quality when the measurements are made in ambula-
tory conditions (see for instance (Castermans et al.,
2011) for a review). The combination of these mul-
tiple effects renders EEG study of human locomotion
extremely complex.
Consequently, the main strategy generally used to
overcome these experimental difficulties consists in
focusing on simplified foot or leg movements which
imply common cerebral processes with gait. In these
experimental protocols, subjects are mainly static and
produce only limited lower limb movements. A
strong advantage of this approach is of course that
motion artifacts are drastically limited. In this case,
however, the full neural activity related to walk is
not available and, for instance, cerebral processes
involved in posture and balance control are miss-
ing. Recording EEG signals of subjects walking on a
treadmill include of course all these aspects but then
requires a powerful analysis technique to discriminate
the different artifact contributions from the real corti-
cal signal. Analysis results of these two approaches,
static on the one hand and dynamic on the other hand,
are reviewed hereinafter.
2.2 Electrocortical Potentials related to
Lower Limb Activation in Static
Condition
The cortical activity associated to bilateral anti-phase
and in-phase rhythmic foot movements produced by
subjects sitting on a chair was investigated in (Raeth-
jen et al., 2008). In this study, the authors found sig-
nificant corticomuscular coherence between EEG sig-
nals and the anterior tibial muscles, at the stepping
frequencies in the central midline region, extending
further to the frontal mesial area. During isometric
cocontractionof the calf muscles, coherence appeared
between 15 and 30 Hz, concentrated on the central
midline area. This is the first study demonstrating that
there exists a representation of rhythmic foot motor
patterns in the cortex, transmitted to the muscles and
fed back to the cortex with delays compatible with
fast corticospinal transmission, which may be impor-
tant for gait control.
Assisted lower limb movements have also been
investigated using electroencephalography (Wieser
et al., 2010). In this study, subjects performed stan-
dardized, assisted stepping movements (i.e. mim-
icking walk) in an upright position, while being se-
cured to a tilt table. Electrocortical sources associ-
ated to the movement-related potential were local-
ized in the primary motor cortex, the premotor cor-
tex, the supplementary motor cortex, the cingulate
cortex, the primary somatosensory cortex and the so-
matosensory association cortex (i.e. in accordance
with the findings of functional brain imagery). The
authors demonstrated that a clear succession of activa-
tions and deactivations was present in the movement-
related potential, in direct relationship with specific
phases of the gait-like leg movements. In particu-
lar, it was shown that cortical activity was the greatest
during transition between flexion and extension of the
legs and vice versa.
Given the obvious possibility to detect electrocor-
tical potentials related to lower limb activation, two
studies were undertaken in order to develop brain-
computer interfaces (BCI) for motor augmentation.
In (Gwin and Ferris, 2011), it was shown that knee
contractions could be distinguished from ankle con-
tractions (subjects performed these exercises sitting
on a bench) using an independent analysis mixture
model applied on high-density EEG, without prior
knowledge of the exercise. An inverse modeling ap-
proach indicated the presence of electrocortical cur-
rent dipoles significantly different for the knee and
ankle exercises. This finding is of course very promis-
ing for new applications in neurorehabilitation of gait
and control of robotic lower-limb exoskeletons.
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
310

In (Do et al., 2011), a non-invasive EEG-based
BCI governing a functional electrical stimulation
(FES) system for ankle movement is presented. In this
application, healthy subjects perform repetitive foot
dorsiflexions. EEG patterns underlying this action are
detected in real time, and this information is subse-
quently used to trigger the FES of the tibialis anterior
of the contralateral foot so as to achieve its dorsiflex-
ion. In fact, the trigger (or non-trigger) information
is given by a linear Bayesian classifier trained using a
vector of spatio-spectral features which optimally dis-
criminate the idling and dorsiflexion states. The au-
thors state that analysis of subject-specific prediction
models demonstrated that the EEG power changes in
the µ, β and low γ bands observed over mid-central ar-
eas (i.e. electrode Cz) were the most informative fea-
tures for classification. This likely corresponds to ac-
tivity within the primary motor cortex’s foot represen-
tation area and/or supplementary motor area (which is
not surprising from a brain anatomy standpoint) and
is in perfect agreement with prior studies (Neuper and
Pfurtscheller, 1996), (Solis-Escalante et al., 2008).
2.3 Electrocortical Potentials related to
Walk
To our knowledge, two very recent studies addressed
the dynamic approach of the problem (i.e. where sub-
jects are really walking). The first analysis of EEG
during walk on treadmill was published by (Gwin
et al., 2011). By using a method based on independent
component analysis (ICA) combined with an inverse
modeling approach, the authors claimed they could
discriminate electrocortical sources, muscle sources
and other artifacts from the raw EEG signals. They
found that cortical activity in the anterior cingulate,
posterior parietal and sensorimotor cortex exhibited
significant and smooth intra-stride changes in spectral
power. More precisely, alpha and beta band spectral
powers increased in or near the left/right sensorimo-
tor and dorsal anterior cingulate cortex at the end of
each stance phase (i.e. as the leading foot was con-
tacting the ground and the trailing foot was pushing
off). According to this study, power increases in the
left/right sensorimotor cortex were more important
for contralateral limb push-off (ipsilateral heel-strike)
than for ipsilateral limb push-off (contralateral heel-
strike). Finally, the authors reported evidence of intra-
stride high-gamma spectral power changes in anterior
cingulate, posterior parietal and sensorimotor cortex.
In parallel, (Presacco et al., 2011) showed for
the first time that the kinematics of the ankle, knee
and hip joints during human treadmill walking can
be inferred from EEG signals. Successful decod-
ing of these signals was done basically by filtering
them (0.1 – 2 Hz) and passing them through a lin-
ear autoregressive model. According to this study,
gait trajectories were inferred with accuracies com-
parable to those from neural decoders based on mul-
tiple single-unit activity recorded in non-human pri-
mates (Fitzsimmons et al., 2009). The results of
this study indicate a high involvement of a fronto-
posterior cortical network in the control of walking
and suggest that EEG signals can be used to study
in real time the cortical dynamics of walking and to
develop brain-machine interfaces aimed at restoring
human gait function.
3 DISCUSSION
3.1 About the Spatio-frequential
Characteristics of the Detected
Potentials
The results produced by the different analyses pre-
sented in previous section are in some way contra-
dictory. Table 1 summarizes the brain areas activated
during walk (or gait-like exercises) as well as the fre-
quency bands of interest, as reported by the different
authors.
From the spatial point of view, all the studies
found activations of the brain globally compatible
with the primary motor cortex’s foot representation
area and/or supplementary motor area, except one.
Surprisingly, (Presacco et al., 2011) report the acti-
vation of a complex, distributed and sparse cortical
network, in which scalp areas over anterior, right lat-
eral and right anterior-occipital scalp areas seem to
equally contribute (at least to their decoding of the
kinematics of the right leg, for subjects walking on a
treadmill).
From the frequential point of view, spectral power
variations were generally found from alpha to gamma
bands but, astonishingly, a successful neural decod-
ing of treadmill walk was realized by (Presacco et al.,
2011) using EEG signals band-pass filtered between
0.1 and 2 Hz. This is particularly surprising, because
it was shown in another study, conducted to assess
EEG signal quality in motion environments (Kerick
et al., 2009), that EEG spectra in the walking (or
jogging) condition exhibit frequency peaks consistent
with the fundamental stride frequency as well as its
harmonics. The authors also state that motion arti-
facts affect signal integrity most prominently at low
frequencies (i.e. the delta band) during steady walk.
In their analysis protocol, (Presacco et al., 2011) do
EEG AND HUMAN LOCOMOTION - Descending Commands and Sensory Feedback should be Disentangled from
Artifacts Thanks to New Experimental Protocols Position Paper
311
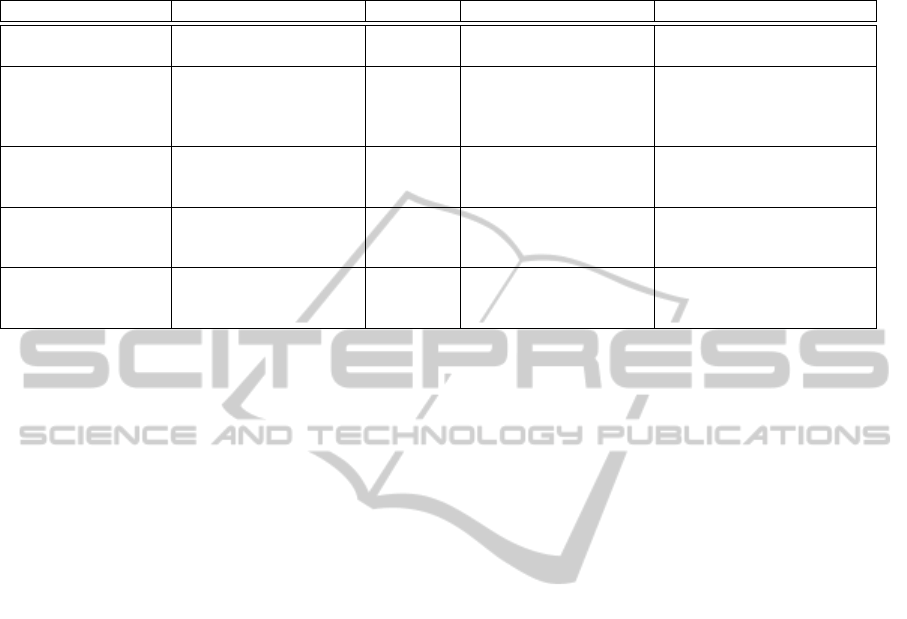
Table 1: EEG studies of human locomotion: a schematic view of recent results obtained with static and dynamic experimental
protocols. M1 is the primary motor cortex, PMC is the premotor cortex, SMA is the supplementary motor cortex, CC is the
cingulate cortex, S1 is the primary somatosensory cortex and SA is the somatosensory association cortex.
Publication Aim of the study Approach Activated brain areas Frequency bands of interest
Raethjen et al., 2008 Rhythmic foot move-
ments
Static Central midline region
+ frontal mesial area
Stepping frequency + β
band (15 – 30 Hz)
Wieser et al., 2010 Assisted lower-limb
movements
Static M1, PMC, SMA, CC,
S1, SA
No frequency analysis. Ac-
tivations are directly related
to specific phases of the
gait-like movements
Do et al., 2011 BCI dedicated to a FES
system for ankle move-
ment
Static Mid-central areas (elec-
trode Cz)
µ, β and low-γ bands
Gwin et al., 2011 EEG activity during
treadmill walking
Dynamic Anterior cingulate, pos-
terior parietal and sen-
sorimotor cortex
α and β bands + clear evi-
dence of high-γ intra-stride
spectral power changes
Presacco et al., 2011 Neural decoding of
treadmill walking from
EEG signals
Dynamic Involvement of a
broad fronto-posterior
cortical network
Delta band (0.1 – 2 Hz)
not mention any pre-processing method aiming at ei-
ther correcting or discriminating these motion arti-
facts from the real cortical signals. The only way
for them to make the choice of this frequency band
legitimate is the fact that good results are obtained
and, moreover, other studies exploited the same por-
tion of the EEG spectrum to decode upper limb move-
ments. We strongly emphasize the fact that, in the
latter studies, no motion artifacts due to gait are pro-
duced. Consequently, this might suggest that the de-
coding of kinematics of walk – periodical movement
– on the basis of the EEG signals is done by (Pre-
sacco et al., 2011) with a linear autoregressive model
exploiting the periodical motion artifacts present in
the EEG recordings. This option is furthermore sup-
ported by the fact that no spectral information is given
under 3 Hz in the study of (Gwin et al., 2010).
3.2 About the Origin of the Detected
Signals
Among all the works described in section 2, only
(Raethjen et al., 2008) try to determine the origin
of the information flux contained in the studied sig-
nals (descending commands from the brain or sen-
sory feedback sent to the brain). This is done by
computing time delays between EEG time series and
electromyographicactivity of the involved lower limb
muscles by means of the “maximising coherence
method” (Govindan et al., 2005). Actually, other
studies presented in section 2 do not consider this as-
pect and give no indication on the direction of the
brain-muscle interaction (i.e. if it is up-going or
down-going). It is therefore unknown, for instance,
if the intra-strides spectral power variations found by
(Gwin et al., 2010) are due to voluntary movements
or sensory feedback (or a combination of both). The
same question arises concerning the EEG decoding
presented by (Presacco et al., 2011). Resolving this
ambiguity is particularly crucial, though, for the de-
velopment of gait rehabilitation systems. Indeed, if
the information detected in the EEG signals is purely
due to a sensory feedback of the gait-related move-
ments, it would be unusable to drive any device, given
that no valid prediction of a movement can be done
exploiting sensory information resulting from it.
Most importantly, studying EEG signals in tread-
mill walking also requires the need to exclude gait-
related artifacts. Only one study tackles this issue
(Gwin et al., 2011). Using an ICA analysis cou-
pled with an inverse solution approach, these authors
claim that they could disentangle muscular contribu-
tions and other artifacts from real cortical signals.
However, in a previous study, the very same authors
(Gwin et al., 2010) clearly stated that:
“Unlike more spatially stationary artifacts in EEG
signals arising from eye movements, scalp muscles,
fMRI gradients, etc., which may be resolved by ICA
decomposition into a subspace of one or more in-
dependent components, we found that gait-related
movement artifact remained in many if not most of
the independent components. This prevented us from
removing only a small subset of components captur-
ing the movement artifacts.”
For this reason, they considered the removal of
motion artifacts from EEG during walking and run-
ning on treadmill using an artifact template subtrac-
tion method. Such method allowed to enhance the de-
tection of P300 potentials in ambulatory conditions.
Nevertheless, the study of cerebral processes involved
in human locomotion is not possible using a subtrac-
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
312
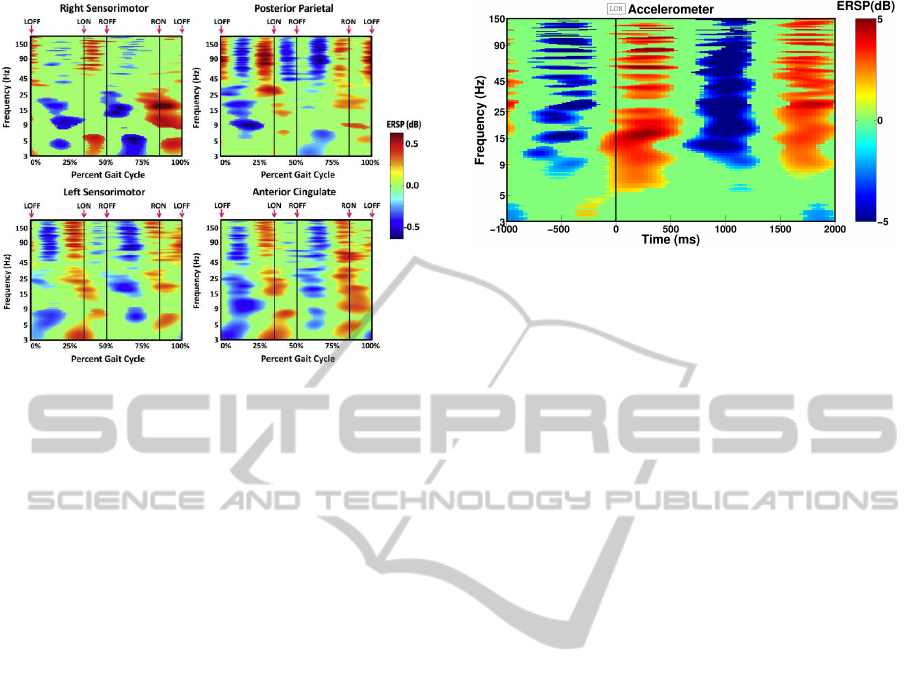
Figure 1: Gait event-related spectral perturbation (ERSP)
plots showing average changes in spectral power during the
stride cycle relative to the full gait cycle baseline for differ-
ent brain areas (Figure from (Gwin et al., 2011)).
tion method, as it would undoubtedly remove inter-
esting signal from the EEG recordings. For this rea-
son, the authors used only the ICA approach to clean
the EEG signals (Gwin et al., 2011). In this study,
the issue of motion artifacts was completely eluded
and no mention was made of any appropriate treat-
ment to reject them. Thus, it can be doubted that the
time-frequency analysis plots shown in that paper do
not contain any motion artifact contribution (see Fig-
ure 1). Figure 2 shows, for instance, a time-frequency
analysis of the signal of an accelerometer placed on
the head of a subject walking at 1.67 m/s on a tread-
mill. Periodic power spectral changes over large fre-
quency bands can be observed, in a similar way to the
results obtained after ICA (see Figure 1) by (Gwin
et al., 2011).
4 FUTURE WORK
Several contradictory results coming from different
recent EEG studies of human locomotion have been
discussed. We have shown that the discrepancies con-
cern the spatio-frequential characteristics of the de-
tected potentials and that the presence of motion arti-
facts is not to be excluded in certain studies.
In this context, it seems necessary to define sev-
eral experimental protocols in order to disentangle the
different signals (are they associated to descending or
ascending pathways, are they artifacts, ...).
First, we propose to characterize the descending
brain commands which are involved in human walk
control in a static approach (inspired by (Raethjen
Figure 2: Representative ERSP plot obtained with the out-
put signal of an accelerometer placed on the head (thus
undergoing the same shocks as the EEG electrodes) of a
subject walking at 1.67 m/s on a treadmill. The horizon-
tal axis is time (and not a percentage of gait cycle like in
Figure 1) as no time-warping analysis was done. Reference
time (t=0) corresponds to the left heel strike (LON) instant.
The same alternating spectral power changes are also ob-
served at other speeds.
et al., 2008)), in order to ensure absence of EEG me-
chanical artifacts. To this aim, the EEG signals of sub-
jects sitting on a chair will be recorded. The subjects
will then be asked to produce voluntary rhythmic foot
movements, staying at the same tempo. The feet will
not be in contact with the ground, to ensure a mini-
mal sensorimotor feedback. Several tempos will be
produced. We will also record EEG when the sub-
ject is sitting and not moving the feet, to define a
baseline, necessary when using brain imagery tools
like LORETA (Pascual-Marqui, 2002). To assess the
presence (or absence) of mechanical artifacts, an ac-
celerometer will be placed on his neck. A complete
characterization of these data will then be realized,
by analysing the event-related spectral perturbations
(ERSP) combined with a time-warping transforma-
tion (Gwin et al., 2011), and by computing cortico-
muscular coherence and in particular delays between
EEG and EMG time series (to assess the information
flow direction).
Then, we will characterize EEG signals caused by
somatosensory information coming from the feet of
the subject when sitting (again, to prevent any me-
chanical artifacts). More precisely, the same experi-
ment as above will be realized, with the feet in contact
with ground, this time. By comparing the two states
(contact/no contact), it will be possible to emphasize
the contribution of sensory feedback. Alternatively,
we intend to use special tactors to stimulate the feet,
mimicking the sensation of walk and will study the
properties of the EEG signals that are phase-locked
with this stimulation.
Additionally, in order to characterize the motion
artifacts contribution in an independent way, we pro-
EEG AND HUMAN LOCOMOTION - Descending Commands and Sensory Feedback should be Disentangled from
Artifacts Thanks to New Experimental Protocols Position Paper
313

pose to use an EEG test setup analog to the one de-
scribed in (Nonclercq and Mathys, 2010), compris-
ing a generator that produces cerebral-like waves, a
dummy head, an electrode/gel/skin interface model,
electrodes, and leads. Placing this setup in the ref-
erence frame of a subject walking on a treadmill
would produce realistic gait-related motion artifacts
(it would indeed undergo the same shocks as the EEG
electrodes) and should give valuable information to
subsequently reject them.
Finally, if we correctly reject motion artifacts, pro-
vided we know the signals due to descending com-
mands (voluntary rhythmic movements) and those
due to tactile stimulation (tactors, mimicking the sen-
sation of walk), we should be able to disentangle the
contribution of posture and balance control when the
subject is standing and walking.
ACKNOWLEDGEMENTS
This work was funded by the FEDER support (BIO-
FACT). M. Duvinage is a FNRS (Fonds National de
la Recherche Scientifique) Research Fellow. This
paper presents research results of the Belgian Net-
work DYSCO (Dynamical Systems, Control, and Op-
timization), funded by the Interuniversity Attraction
Poles Programme, initiated by the Belgian State, Sci-
ence Policy Office. The scientific responsibility rests
with its author(s).
REFERENCES
Castermans, T., Duvinage, M., and Dutoit, T. (2011). Op-
timizing the performances of a P300-based Brain-
Computer Interface in ambulatory conditions. IEEE
Journal on Emerging and Selected Topics in Circuits
and Systems (JETCAS), Special Issue on ”Brain Ma-
chine Interfaces” (in Press).
Do, A.-H., Wang, P.-T., King, C.-E., Abiri, A., and Nenadic,
Z. (2011). Brain computer interface controlled func-
tional electrical stimulation system for ankle move-
ment. Journal of NeuroEngineering and Rehabilita-
tion, 8(49).
Fitzsimmons, N. A., Lebedev, M. A., Peikon, I. D., and
Nicolelis, M. A. L. (2009). Extracting kinematic pa-
rameters for monkey bipedal walking from cortical
neuronal ensemble activity. Frontiers in integrative
neuroscience, 3(March):19.
Govindan, R., Raethjen, J., Kopper, F., Claussen, J., and
Deuschl, G. (2005). Estimation of time delay by co-
herence analysis. Physica A: Statistical Mechanics
and its Applications, 350(2-4):277 – 295.
Gwin, J. and Ferris, D. (2011). High-density EEG and in-
dependent component analysis mixture models distin-
guish knee contractions from ankle contractions. In
Proc. of the 33rd Annual International Conference of
the IEEE EMBS, Boston, Massachussets, USA, pages
4195 – 4198.
Gwin, J., Gramann, K., Makeig, S., and Ferris, D. (2010).
Removal of movement artifact from high-density EEG
recorded during walking and running. Journal of Neu-
rophysiology, 103(6):3526–3534.
Gwin, J., Gramann, K., Makeig, S., and Ferris, D. (2011).
Electrocortical activity is coupled to gait cycle phase
during treadmill walking. NeuroImage, 54(2):1289 –
1296.
Hanakawa, T. (2006). Neuroimaging of standing and walk-
ing: Special emphasis on parkinsonian gait. Parkin-
sonism and Related Disorders, 12(Supplement 2):S70
– S75. Proceedings of the 1st International Sympo-
sium on Dopaminergic and Nondopaminergic Mecha-
nisms in Parkinson’s Disease (ISDNMPD).
Harada, T., Miyai, I., Suzuki, M., and Kubota, K. (2009).
Gait capacity affects cortical activation patterns re-
lated to speed control in the elderly. Experimental
Brain Research, 193(3):445–454.
Kerick, S. E., Oie, K. S., and McDowell, K. (2009). Assess-
ment of EEG signal quality in motion environments.
Army Research Laboratory Report.
Kiehn, O. (2006). Locomotor circuits in the mammalian
spinal cord. Ann. Rev. of Neuroscience, 29:279 – 306.
Neuper, C. and Pfurtscheller, G. (1996). Post-movement
synchronization of beta rhythms in the EEG over
the cortical foot area in man. Neuroscience Letters,
216(1):17 – 20.
Nonclercq, A. and Mathys, P. (2010). Quantification of the
movement artifact reduction in EEG recording. IEEE
trans. on Biom. Eng., 57(11):2746 – 2752.
Nunez, P., Srinivasan, R., Westdorp, A., Wijesinghe, R.,
Tucker, D., Silberstein, R., and Cadusch, P. (1997).
EEG coherency. Electroencephalography and Clini-
cal Neurophysiology, 103(5):499–515.
Pascual-Marqui, R. (2002). Standardized low-resolution
brain electromagnetic tomography (sloreta): techni-
cal details. Methods Find. Exp. Clin. Pharmacol.,
24(D):5–12.
Presacco, A., Goodman, R., Forrester, L., and Contreras-
Vidal, J. (2011). Neural decoding of treadmill walking
from non-invasive, electroencephalographic (EEG)
signals. Journal of Neurophysiology. in press.
Raethjen, J., Govindan, R., Binder, S., Zeuner, K. E.,
Deuschl, G., and Stolze, H. (2008). Cortical represen-
tation of rhythmic foot movements. Brain Research,
1236:79 – 84.
Solis-Escalante, T., M¨uller-Putz, G., and Pfurtscheller, G.
(2008). Overt foot movement detection in one single
laplacian EEG derivation. Journal of Neuroscience
Methods, 175(1):148 – 153.
Suzuki, M., Miyai, I., Ono, T., and Kubota, K. (2008). Ac-
tivities in the frontal cortex and gait performance are
modulated by preparation. An fNIRS study. Neuroim-
age, 39(2):600 – 607.
Wieser, M., Haefeli, J., B¨utler, L., J¨ancke, L., Riener, R.,
and Koeneke, S. (2010). Temporal and spatial pat-
terns of cortical activation during assisted lower limb
movement. Exp. Brain Res., 203:181–191.
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
314
