
CHARACTERIZATION OF COARSE GRAIN MOLECULAR
DYNAMIC SIMULATION PERFORMANCE ON GRAPHIC
PROCESSING UNIT ARCHITECTURES
Ardita Shkurti
1
, Andrea Acquaviva
1
, Elisa Ficarra
1
, Mario Orsi
2
and Enrico Macii
1
1
Corso Duca degli Abruzzi 24, Department of Control and Computer Engineering, Politecnico di Torino, Torino, Italy
2
School of Chemistry, University of Southampton, SO17 1BJ, Southampton, U.K.
Keywords:
CUDA, GPU acceleration, Molecular dynamics, Coarse grain, Membrane modeling, Lipid modeling.
Abstract:
Coarse grain (CG) molecular models have been proposed to simulate complex systems with lower computa-
tional overhead and longer timescales with respect to atomistic level timescales. However, their acceleration
on parallel architectures such as Graphic Processing Units (GPU) presents original challenges that must be
carefully evaluated. The objective of this work is to characterize the impact of CG model features on parallel
simulation performance. To achieve this target, we implemented a GPU-accelerated version of a CG biomem-
brane simulator called BRAHMS, to which we apply specific optimizations for CG models, such as dedicated
data structures to handle different bead type interactions. Moreover, we explore different GPU architectures
to characterize the behavior of the optimized CG model.
1 INTRODUCTION
Most aspects of cell activity such as the conformation
of embedded proteins, the biomembranepermeability,
interaction with drugs and signaling may be perceived
observing the dynamics of cell membranes which are
composed of patterns of lipid bilayers (Orsi, 2010).
The value of such biological processes brings to
an increasing appeal for hardware and software op-
timized realistic models which may provide in silico
access to biological cell mechanisms otherwise im-
possible to achieve through real experiments.
Atomic level (AL) models introduced (Wohlert,
2006; MacCallum, 2006) require huge quantities of
computational resources due to the need of interac-
tion calculations among system atoms.
CG modeling techniques have been proposed for
handling shortcomings of atomic level models. In the
CG representation specific atom groups are organized
in clusters and each of the clusters is considered as
a unique system particle called a bead. Hence, by
diminishing the system particles number, the compu-
tational requirements are reduced as well. Figure 1
illustrates an example of AL and CG modeling for
lipid bilayers. Typically, a lipid of about 100 parti-
cles in AL approaches is modeled by approximately
10 beads in CG representations (Orsi, 2010; Marrink,
2007). While CG models are promising and may al-
Figure 1: Coarse-grain mapping of DMPC lipids from
atomistic representation.
low simulations at longer time scale, their accelera-
tion must be carefully characterized. In this work, we
accomplish this characterization dealing with a CG
lipid bilayer simulator.
While several papers have addressed atomistic
model acceleration on GPUs (Anderson, 2008;
Shkurti, 2010; Stone, 2010; van Meel, 2008; Rapa-
port, 2011; Bauer, 2011), the specific features of CG
models have only recently gained attention (Shkurti,
2010; Zhmurov, 2010).
In this paper, we characterize specific bottlenecks
of CG models proposing specific strategies for han-
dling them, through the implementation of an accel-
erated CG model. We identify the impact of charac-
teristics and data structures on the optimization of the
CG application. Moreover, we explore the speed-up
achieved across various architectures and quantify the
overhead of the additional structures required by the
CG nature simulation methodology to take into ac-
count interaction among heterogeneous bead types.
339
Shkurti A., Acquaviva A., Ficarra E., Orsi M. and Macii E..
CHARACTERIZATION OF COARSE GRAIN MOLECULAR DYNAMIC SIMULATION PERFORMANCE ON GRAPHIC PROCESSING UNIT ARCHI-
TECTURES.
DOI: 10.5220/0003790703390342
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2012), pages 339-342
ISBN: 978-989-8425-90-4
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

The rest of this paper is organized as follows. Sec-
tion 2 provides background on CUDA programming
and the simulated model. Section 3 covers the opti-
mization methods, while Sections 4 and 5 present and
discuss the achieved results and conclude the work.
2 BACKGROUND
2.1 CUDA Environment
The CUDA environment represents a parallel pro-
gramming model oriented on highly parallel comput-
ing, while making use of a large number of com-
putational cores. With respect of general purpose
CPUs, much more transistors are assigned to data pro-
cessing rather than to flow control or data caching.
However, memory latency is hidden with compute-
intensive calculations. The GPU is divided into a
set of Streaming Multiprocessors (SM) in which hun-
dreds of threads reside concurrently. A large num-
ber of CUDA threads, can be launched in parallel
by means of kernels, which are C functions executed
in parallel from all CUDA threads launched (CUDA,
2011).
2.2 Coarse Grain Simulator
BRAHMS (Biomembrane Reduced ApproacH
Molecular Simulator) is a CG lipid bilayer simulator
we optimized and accelerated for CUDA environ-
ment. It describes a method for CG modeling of lipid
bilayers of biomembranes and contains several origi-
nal features such as specific original representations
for water and charges (Orsi, 2010). It simulates the
displacement in time of the particles of a system,
calculating some of its macro properties such as
temperature, lateral pressure, potential and kinetic
energy (Orsi, 2010). It employs Molecular Dynamics
and in particular Newton Leapfrog Equations to move
the positions of beads, which in CG models represent
clusters of atoms, and their velocities one time step
forward (Rapaport, 2004).
3 GPU OPTIMIZATION OF
COARSE GRAIN MODELS
Code profiling of the CG simulator has been per-
formed to identify the most computation demanding
parts of the code which resulted to be: (i) Non-bonded
forces computation; (ii) Integration timestep; (iii)
Neighbor structure generation, accounting for about
95.2%, 2% and 1% of the total execution time of the
sequential simulator, respectively.
Accordingly to the bottlenecks identified, we have
implemented three kernels to be executed on the de-
vice (GPU), one for each of the most onerous parts
of code. Concerning data structures, a comprehensive
one is required containing for each bead, the informa-
tion on its position, velocity, force, torque, orienta-
tion, angular momentum in the body-fixed frame, ro-
tation matrix, bead type, mechanical type, lipid iden-
tifier and lipid unit identifier.
The integration algorithm exploits Leapfrog equa-
tions (Rapaport, 2004). This algorithm is imple-
mented by the integration kernel on the GPU, to avoid
additional data transfers between host and device.
For the calculation of the non-bonded forces, a
neighbor structure is needed to avoid considering a
contribution for each pair of beads, which would lead
to a quadratic time complexity. Therefore the system
is divided into cells of beads, where each cell has a
cubic shape and the edge size equal to the maximum
cut-off distance among cut-offs established for differ-
ent bead types. Only bead pairs with a distance value
under the cut-off are considered as neighbors. Hence,
the search for neighbors is applied to bead pairs of the
same cell or the eventual 26 adjacent cells.
In the CPU version, the cell, neighbor and inter-
action type structures cannot be efficiently mapped
on a GPU. Therefore we have created new structures
for the cells, neighbor beads and relative interaction
type storage, which provide coalesced
1
accesses for
CUDA threads.
Implementation and update of neighbor and inter-
action type structures within the GPU is needed to
obtain maximum performance from GPU by avoid-
ing data transfer overheads between CPU and GPU.
To this purpose we have implemented a kernel called
cudaneigh, in which we have used an algorithm sim-
ilar to the algorithm proposed in (Anderson, 2008)
for AL models, where the texture type used was one-
dimensional. Since the texture cache is optimized for
2D spatial locality (CUDA, 2011), we have used two
dimensional textures in our code even for the other
two kernels and employ them when the accesses in
device memory are not coalesced.
The non-bonded forces computation is imple-
mented by the kernel forces, that exploits the opti-
mized access to neighbor and interaction type struc-
tures. Compared to what has been done for AL mod-
els in (Anderson, 2008), we use an additional interac-
tion type structure with the same size and organization
1
When different threads need some particular piece of
information, they find it in the same relative address, calcu-
lated as base address+ absolute thread identifier.
BIOINFORMATICS 2012 - International Conference on Bioinformatics Models, Methods and Algorithms
340
as the neighbor structure and we use two dimensional
textures for random accesses in memory, to retrieve
the needed information.
In addition, we created separate structures for all
beads positions, all beads velocities, all beads orien-
tations and all beads types, as these data are the most
frequentlyaccessed by the GPU. In this way, we could
enable it to access the information contained in our
new structures in a coalesced way.
4 RESULTS
In this Section we present results about the speed-
up of the GPU version of BRAHMS with respect
to the CPU, considering: (i) Biological systems of
different dimensions and complexity (i.e. lipid, wa-
ter); (ii) Different versions of BRAHMS GPU code
(i.e. customized or not for water systems simulation);
(iii) Three different GPU architectures: GeForce
GTX295, GeForce GTX480 and Tesla C2050 that
have respectively 240, 448 and 480 cores and an
Intel
R
Core
TM
i7-920 Processor CPU architecture.
In the charts presented, we consider systems hav-
ing from 840 to 218904 number of beads
2
. As for
complexity, we consider systems composed by het-
erogeneous types of beads: Either water systems
uniquely containing water molecules or lipid systems
containing lipid molecules in water solution. We de-
velop the GPU water only version customized to wa-
ter systems, thus treating all the beads as a unique
type (water beads), to evaluate the impact of addi-
tional structures used for handling interactions among
the lipids. The GPU complete version handles also the
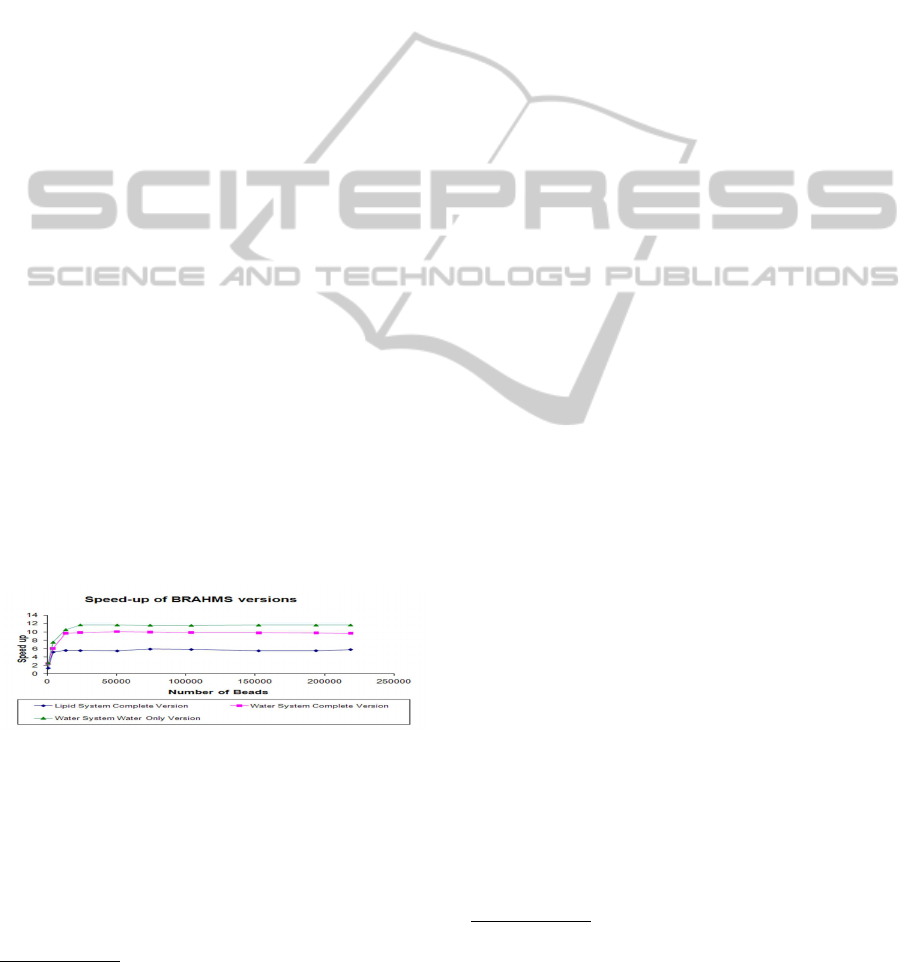
beads of lipid molecules. In Figure 2, we report
Figure 2: Comparison among the speed-up of different
BRAHMS versions with respect to the CPU achieved on
the GTX295 architecture.
the comparison among the speed-ups (related to the
CPU version) of two biological systems, lipid and wa-
ter only, characterized by different complexity simu-
lated on the GTX295 architecture. For water systems,
we distinguish between water only and complete ver-
sion. Single precision floating point arithmetic is used
2
The largest system simulated for the reported exper-
iments using the slowest version of the simulator, takes
about 200 minutes.
for these simulations. For a number of beads lower
than 23K the speed-up is lower than the maximum
achievable because the parallelism of the GPU is not
completely exploited. The water only version is the
fastest version with speed-ups up to 12x, due to the
absence of structures to handle different bead types.
On the other hand, the lower performance of the com-
plete version in the case of lipid systems with respect
to the case of water systems is due to the fact that
for lipid systems there are six different types of beads
involved instead of the single type considered in wa-
ter systems. This leads to divergent execution flows
among the threads, thus leading to a performance hit.
From Figure 2, we can also observe the effect of
lipid data structures, highlighted by the gap between
the water-only and complete version for water sys-
tems. The maximum difference in the total speed-up
between the water-only and complete version for wa-
ter systems is 36.4% when single precision arithmetic
is used for floating point operations and 15.71% when
double precision arithmetic is employed.
The larger impact of lipid structures for single pre-
cision arithmetic computation is due to the constant
overhead for memory accesses and additional control
statements associated to beads types and interactions,
which has a larger relative contribution to a shorter
execution (i.e. the single precision arithmetic simula-
tion).
In Figures 3 and 4 we report respectively for lipid sys-
tems and water systems, the speed-ups
3
achieved by
the three considered architectures for an increasing
system size. The double precision arithmetic has been
used for floating point operations.
For lipid systems we achieve speed-ups up to
6.35x, 5.82x and 2.24x for respectively GTX480,
Tesla C2050 and GTX295 architectures, while for
water systems the respective speed-ups achieved are
12, 83x, 12.05x and 4.94x.
The GTX295 architecture is the slowest architecture,
as we expected, due to its lower number of cores and
hence of parallelism. GTX480 has a higher paral-
lelism with respect to Tesla C2050, having one ad-
ditional SM (32 additional cores), which causes the
better performance of GTX480 versus Tesla C2050.
When evaluating speed-up results, we have to take
into account that: (i) The CPU used for comparison
represents a single core but high performance archi-
tecture; (ii) The characteristics of the CG model, such
as different force field for pairs potentials that in-
3
The speed-ups presented in Figures 3 and 4 are relative
only to the GPU implemented part of the application which
stands for 98% of the total execution time of the application.
We decided to present only this part, because the servers
where the GPU cards are situated have different CPUs.
CHARACTERIZATION OF COARSE GRAIN MOLECULAR DYNAMIC SIMULATION PERFORMANCE ON
GRAPHIC PROCESSING UNIT ARCHITECTURES
341

Figure 3: Speed-up of lipid simulations achieved on differ-
ent architectures with respect to the CPU execution.
Figure 4: Speed-up of water simulation achieved on differ-
ent architectures with respect to the CPU execution.
cludes particular representation for water and charges
(Orsi, 2010), which depends on the type of interac-
tion, lead to frequent divergent branches which im-
pact the overall speed-up; (iii) The complexity of the
force fields considered requires a large amount of lo-
cal physical resources (i.e. registers), which have to
be distributed among all threads on an SM impacting
on performance; (iv) Furthermore, the pair interac-
tion potentials computation causes a relatively large
amount of scattered memory accesses, leading to a
considerable texture cache miss rate. That is because
neighbors of a bead are not spatially clustered. This
issue could be addressed through additional optimiza-
tions obtained by adapting techniques developed for
AL simulations (Anderson, 2008) that cannot be ap-
plied as is for CG models. These optimizations will
be the object of future work.
Although the three architectures we evaluated are
compliant with the IEEE 754 standard, we observe
a difference in the results of CPU versus GPU, af-
ter thousands of simulation steps. Anyway, this is
expected and acceptable as long as the ensemble av-
erage, as for instance the average temperature or the
pressure, is coherent after a reasonably large number
of steps which is the case of our simulations. These
simulations on the different architectures are coherent
from an ensemble viewpoint: Mean values related to
energy, pressure and temperature, both of GPU and
CPU simulations, are the same. This is what matters
in this application due to the chaoticity of molecular
dynamics simulation systems.
5 CONCLUSIONS
In this work, we characterized the speed-ups of
coarse grain simulations, considering biological sys-
tems with different number of beads and among three
different GPU architectures. To this purpose, we im-
plemented an accelerated version of a CG lipid bilay-
ers simulator. We introduced specific optimizations
and data structures required by CG models for han-
dling differenttypes of beads and interactions and cal-
culated their overhead in the performance. We char-
acterized the impact of biological system complexity
in terms of beads type, observing that lipid systems
achieve a speed-up almost 2.5 times slower than wa-
ter systems. We finally determined the impact of GPU
architectures on the acceleration performance.
REFERENCES
Anderson, J. A. (2008). General purpose molecular dynam-
ics simulations fully implemented on graphics pro-
cessing units. In volume 227. Journal of Computa-
tional Physics.
Bauer, B. A. (2011). Molecular dynamics simulations of
aqueous ions at the liquid vapor interface accelerated
using graphics processors. In volume 32. Journal of
Computational Chemistry.
CUDA (2011). Nvidia cuda c programming guide.
In http://developer.download.nvidia.com/compute/
cuda/4 0 rc2/toolkit/docs/CUDA C Programming
Guide.pdf. NVIDIA.
MacCallum, J. L. (2006). Computer simulation of the dis-
tribution of hexane in a lipid bilayer:spatially resolved
free energy, entropy and enthalpy profiles. In volume
128. Journal of the American Chemical Society.
Marrink, S. J. (2007). The martini force field: coarse
grained model for biomolecular simulations. In vol-
ume 111. Journal of Physical Chemistry B.
Orsi, M. (2010). A quantitative coarse-grain model for lipid
bilayers. In volume 112. IEEE CS.
Rapaport, D. C. (2004). The Art of Molecular Dynamics
Simulation. Cambridge University Press, New York,
2nd edition.
Rapaport, D. C. (2011). Enhanced molecular dynamics per-
formance with a programmable graphics processor. In
volume 182. Computer Physics Communications.
Shkurti, A. (2010). Gpu acceleration of simulation tool
for lipid-bilayers. In IEEE International Conference
on Bioinformatics and Biomedicine Workshops. IEEE
CS.
Stone, J. E. (2010). Gpu-accelerated molecular modeling
coming of age. In volume 29. Journal of Molecular
Graphics and Modelling.
van Meel, J. A. (2008). Harvesting graphics power for md
simulations. In volume 34. Molecular Simulation.
Wohlert, J. (2006). Dynamics in atomistic simulations
of phospholipid membranes: Nuclear magnetic reso-
nance relaxation rates and lateral diffusion. In volume
125. Journal Of Chemical Physics.
Zhmurov, A. (2010). Sop-gpu: Accelerating biomolecular
simulations in the centisecond timescale using graph-
ics processors. In volume 78. Proteins.
BIOINFORMATICS 2012 - International Conference on Bioinformatics Models, Methods and Algorithms
342
