
NITRITE BIOSENSING WITH DISPOSABLE
ELECTRODE STRIPS
A Preliminary Study
Marcelo Rodrigues
1
, Cátia Correia
1
, Célia M. Silveira
1
, José. J. G. Moura
1
, Estibaliz Ochoteco
2
,
Elena Jubete
2
and M. Gabriela Almeida
1,3
1
REQUIMTE — Dept. de Química, Faculdade de Ciências e Tecnologia,
Universidade Nova de Lisboa, 2829-516 Monte de Caparica, Caparica, Portugal
2
CIDETEC-IK4 — Sensors and Photonics Unit, Parque Tecnológico de San Sebastián, Pº Miramon, 196,
20009 Donostia — San Sebastián, Spain
3
Escola Superior de Saúde Egas Moniz, Monte de Caparica, 2829-511 Caparica, Portugal
Keywords: Nitrite, Cytochrome c nitrite reductase, Electrochemical biosensors, Screen printed electrodes.
Abstract: This paper presents the results of a preliminary study on the construction of miniaturized biosensing devices
for nitrite analysis in clinical samples. Following our previous works regarding the development of
amperometric nitrite biosensors using the nitrite reducing enzyme (ccNiR) from Desulfovibrio desulfuricans
ATCC 27774, now we aim to reduce the size of the experimental set-up according to the specific needs of
biomedical applications. For this, thick-film strip electrodes made of carbon conductive inks deposited on
plastic supports were modified with ccNiR previously mixed with the conductive graphite ink, in the
presence of propanone or methylethylketone. Then, the enzyme electrodes were dried at 40°C or 60°C, to
simulate the curing procedure typically done after screen-printing. In this way, the biocompatibility of
ccNiR with these organic solvents and thermal treatments was evaluated and the composition of the mix
enzyme/conductive ink was optimized. The analytical performance of these electrodes was satisfactory,
with a sensitivity of 52 A.μM
-1
.cm
-2
within a linear range of 0.001 - 1 mM.
1 INTRODUCTION
The detection of nitrites in physiological fluids such
as plasma and urine is commonly used for clinical
diagnosis and has gained an increasing importance
in biomedical research. In fact, the nitrate-nitrite-NO
pathway is emerging as an important mediator of
blood flow regulation, cell signaling, energetics and
tissue responses to hypoxia (Bryan, 2005; Lundberg,
2009; Hord, 2009). Most of the strategies used for
analytical determination of NO
3
-
and NO converge
to the quantification of NO
2
-
. However, the classical
protocols for nitrite assessment lack the sensitivity
and selectivity needed for the analysis of
physiological samples (Almeida, 2010). For
example, urine test strips are routinely used for
screening nitrites in patients with infection, but
results are just qualitative as they are obtained by
visual comparison to a color chart. Plasma analysis
is much less frequent, owing to limitations of the
analytical methods, including blood sampling and
processing (Ellis, 1998). As a consequence, there is
a growing demand for improved analytical tools,
increasingly sensitive, reliable and, preferentially,
easy-to-use and inexpensive.
An alternative approach relies on the
construction of biosensing devices using stable
enzymes with high catalytic activity and specificity
for nitrite. Due to its high selectivity, turnover and
stability, the multihemic cytochrome c nitrite
reductase (ccNiR) from the sulphate reducing
bacterium Desulfovibrio desulfuricans ATCC
27774, which performs the six electron reduction of
nitrite to ammonia (Almeida, 2003), has proven to
be a promising candidate for the development of an
electrochemical nitrite biosensor (Almeida, 2007;
Chen, 2007; da Silva, 2004; Silveira 2010a,b;
Zhang, 2009).
Miniaturization is critical for both health care
and physiological studies. The screen-printing
technology has been widely used for the large-scale
32
Rodrigues M., Correia C., M. Silveira C., J. G. Moura J., Ochoteco E., Jubete E. and Gabriela Almeida M..
NITRITE BIOSENSING WITH DISPOSABLE ELECTRODE STRIPS - A Preliminary Study.
DOI: 10.5220/0003757700320036
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 32-36
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

fabrication of disposable biosensors. Besides the
portable dimensions, screen-printed electrodes
(SPEs) are low-cost and versatile in terms of formats
and materials (Jubete, 2009).
In this work, thick-film strip electrodes were
fabricated using carbon based conductive pastes
printed on plastic supports. The working electrodes
were later modified with an extra layer of the carbon
ink, previously diluted with either propanone or
methyl ethyl ketone (MEK) and mixed in different
proportions with ccNiR. The activity of ccNiR
towards nitrite after immobilization in this harsh
environment (solvents exposure and heat dry) was
evaluated by cyclic voltammetry, and has proved to
be highly satisfactory.
2 MATERIALS AND METHODS
2.1 Reagents
Acetone (propanone; 99%; b.p. 56°C) and
propanone (methylethylketone, MEK; 99%, b.p.
79°C) were from Pronalab. The remaining chemicals
were analytical grade and were used without further
purification. Solutions were prepared with deionized
(DI) water (18 MΩcm) from a Millipore MilliQ
purification system.
The graphite conductive ink was obtained from
Acheson. Alumina slurries (0.05 and 1.0 µm) were
from Buehler.
ccNiR was purified from Desulfovibrio
desulfuricans ATCC 27774 cells grown in nitrate, as
previously described by Almeida and co-workers
(Almeida, 2003).
2.2 Electrochemical Measurements
For the optimization studies, a conventional three-
electrode electrochemical cell was used, with an
Ag/AgCl reference electrode, a Pt counter electrode
(both from Radiometer) and a home-made working
electrode made of pyrolytic graphite disks (4 mm
diameter) and modified with the enzyme/ink layer.
The characterization of the optimized electrode
was performed after replacing the previous system
by carbon paste screen-printed electrodes (CPSPEs)
with a three electrode configuration (Figure 1),
including an Ag/AgCl pseudo-reference, a graphite
paste counter electrode and a graphite paste working
electrode (3.1 mm diameter). The CPSPEs were
fabricated at CIDETEC facilities, as described by
Ochoteco and co-workers (Ochoteco, 2009).
Figure 1: A screen-printed three-electrode system. (1)
working electrode; (2) reference electrode; (3)
counter-electrode.
The one-compartment electrochemical cell
containing 0.1 M KCl in 0.05 M Tris-HCl buffer, pH
7.6 as supporting electrolyte, was thoroughly purged
with Argon before each experiment. Measurements
were performed with a potentiostat Autolab PSTAT
12 (Eco-Chemie) monitored by the control and data
acquisition software GPES 4.9. The cyclic
voltammograms (CV) were plotted at room
temperature (22 ± 2°C), with a scan rate of 20 mV/s,
in the potential window [0.0; -0.8] V (vs reference
system). To evaluate the biosensors response to the
analyte (0.001 - 7 mM), the cell was successively
spiked with standard solutions of nitrite. After each
addition, the electrochemical cell was deoxygenated
and the CV was registered. The catalytic currents
(I
cat
) were measured at the inversion potential; all
values were subtracted from the non-catalytic
current recorded in the absence of nitrite (I
c
). Each
experiment was replicated two times.
2.3 Bioelectrodes Preparation
Prior to coating, the pyrolytic graphite electrodes
(PGEs) were polished with alumina slurry in cloth
pads. Then, the electrodes were thoroughly washed
with DI water and ethanol and ultrasonicated in
water for 5 min. The electrodes’ surface was further
washed with DI water and dried with compressed
air.
CPSPEs were used as produced, with no pre-
activation.
The conductive carbon inks were previously
diluted with an organic solvent (acetone or MEK) in
a 1:1 ratio and homogenised with the help of an
ultrasound bath. The inks’ suspensions were then
mixed with ccNiR in different proportions (4:1, 2:1,
1:1 and 1:2 ink/enzyme). Finally, a 5 μL drop was
placed on the surface of the working electrodes
which were cured for 20 min. inside an oven set at
40°C or 60°C. Control experiments were carried out
with no curing treatment and/or no carbon ink; in
such cases, the ccNiR layer was dried at room
1
2
3
NITRITE BIOSENSING WITH DISPOSABLE ELECTRODE STRIPS - A Preliminary Study
33
temperature.
3 RESULTS
3.1 Response to Nitrite
Regardless of the composition of the electrode
material and enzyme modifying layer, the CVs
displayed a sigmoidal shape in the presence of nitrite
(not shown), as previously observed in bare PGEs
and carbon nanotubes modified electrodes (Silveira
2010a,b). This reflects the electrocatalytic reduction
of nitrite into ammonium as a consequence of the
direct electron transfer between the electrode and
ccNiR.
In general, the plots catalytic current (I
cat
) vs
nitrite concentration could be fitted to a hyperbolic
equation, denoting a Michaelis-Menten profile. The
assessment of the analytical performance of each
bioelectrode was based on the measurement of the
following parameters: catalytic efficiency (I
max
/I
c
),
sensitivity of detection (slope of the linear range),
correlation coefficient (r
2
) and quantification range.
3.2 Temperature and Solvent Effects
Preliminary experiments were carried out in order to
check if the chemicals (organic solvents) and the
thermal treatment (curing) required for printing the
working electrode component in CPSPEs were
compatible with nitrite reductase activity. In this
regard, three different PG electrodes were modified
with i) ccNiR only, ii) ccNiR mixed with carbon ink
diluted in acetone and iii) the same as (ii) but with
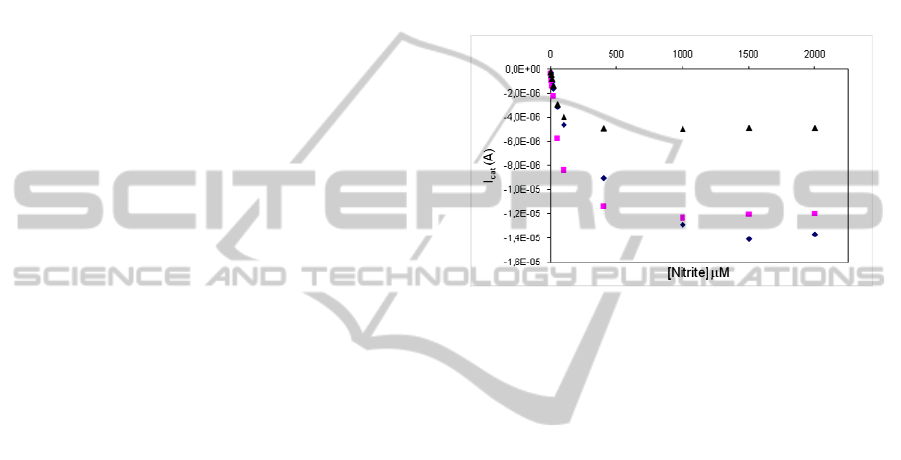
an extra curing step at 60°C. As seen in Figure 2, the
maximum catalytic current (I
max
) has increased about
three times in the presence of the carbon ink. This
should be related with the resultant enlargement of
the electroactive area. Apparently, the carbon
ink/acetone composite had no critical effect on
catalytic activity.
When comparing the response profiles obtained
with or without electrode curing (both in the
presence of conductive ink), one can see that the
thermal treatment does not have a strong influence
on I
max
. On the other hand, the sensitivity of the
sensor, as given by the slope of the linear range of
the plot, decreased about 55%. This indicates that
partial protein denaturation has occurred.
3.3 Electrode Optimization
3.3.1 Enzyme/Carbon Ink Ratio
Different proportions of enzyme and carbon ink
suspended in acetone were early tested in order to
choose the best composition. The one using the
highest amount of protein (1:2 ratio, corresponding
to 3.3 μg of ccNiR) displayed the best results (not
shown) without relevant loss of ink, and was
selected for further studies.
Figure 2: Electrocatalytic response to nitrite of ccNiR
(3.3 μg) modified PG electrodes: (S) without carbon
conductive ink and thermal treatment (sensitivity:
32x10
-8
A.μM
-1
.cm
-1
); () pre-mixed with the carbon
conductive ink diluted in acetone (sensitivity:
73x10
-8
A.μM
-1
.cm
-1
); (¡) pre-mixed with the carbon
conductive ink diluted in acetone and cured at 60°C
(sensitivity: 33x10
-8
A.μM
-1
.cm
-1
).
3.3.2 Selection of Organic Solvent and
Curing Temperature
Normally, inks for screen printing contain organic
solvents that are later evaporated by heating. If other
ingredients like ccNiR need to be included, it is
highly recommended to lower the viscosity of the
paint in order to facilitate the mixing process. For
this reason, prior to enzyme incorporation, the
carbon ink used in this work was diluted with two
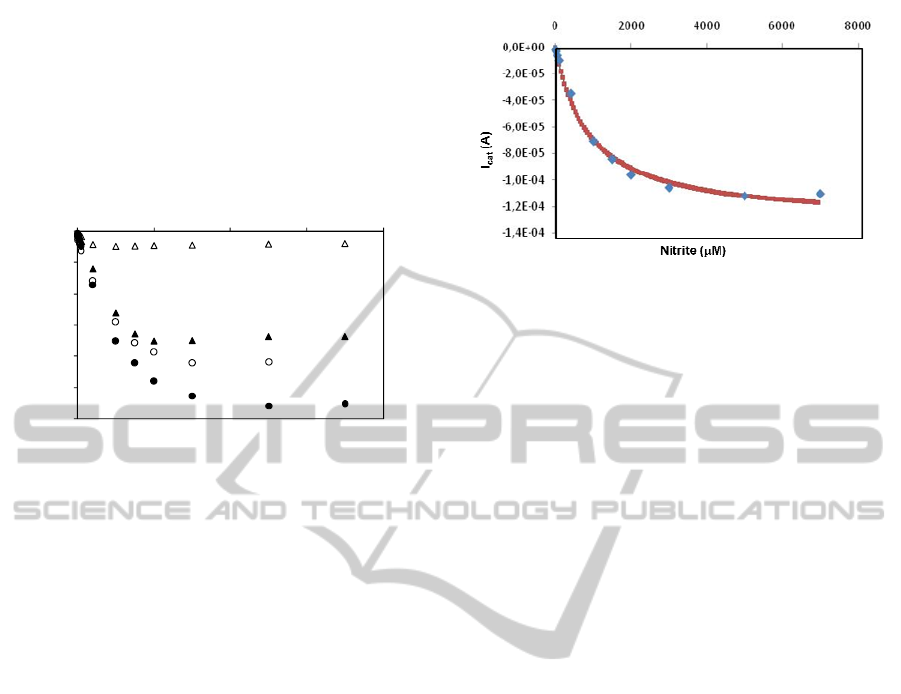
different organic solvents - MEK or acetone. It is
worth noting that acetone is less commonly used for
inks dilution than MEK, although it has a lower
boiling point that could permit the use of lower
curing temperatures. Actually, the response to nitrite
was much higher when this solvent was used instead
of MEK (Figure 3) and, accordingly, the linear range
was also wider. Therefore, acetone has proved to be
less harmful to the protein.
In order to evaporate residual organic solvents,
most CPSPEs have to be dried thermally. Although a
temperature of 60°C is normally selected for the
curing process of those used in the present work, due
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
34
to the presence of the biocatalyst, we have also
tested the lowest permitted heating temperature, i.e.,
40°C. Interestingly, the differences on nitrite
reducing activity were generally small, except when
MEK was used for ink dilution, which generated
much lower catalytic currents. Most likely, this
solvent did not evaporate completely at 40°C,
enhancing its detrimental effect on enzyme activity.
Figure 3: Electrocatalytic response to nitrite of ccNiR
(3.3 μg) modified PG electrodes, pre-mixed with the
carbon conductive ink, prepared and treated in the
following ways: (U) diluted in MEK and cured at 40°C;
(S) diluted in MEK and cured at 60°C; ({) diluted in
acetone and cured at 60°C. (z) diluted in acetone and
cured at 40°C.
In accordance to the results obtained in this
combined study, we have selected acetone for ink
solubilization and a curing temperature of 40°C.
3.4 Application of Carbon Paste Screen
Printed Electrodes (CPSPEs)
Following the optimization of the ccNiR containing
conductive paints, the enzyme/carbon inks were
deposited on CPSPEs. The CVs displayed higher
background currents (not shown), which is most
likely related to the roughness of the SPEs surfaces,
generating higher capacitive currents. Nevertheless,
the analytical parameters (sensitivity: 52 A.μM
-1
.cm
-2
;
linear range: 0.001 - 1 mM) remained similar (Figure
4). The data were fitted to the Michaelis-Menten
kinetic model using the software GraphPad Prism
4.0. Accordingly, the K
m
app
is 0.9 ± 0.1 mM, which
is about 250 times higher than the value previously
determined by protein film voltammetry (Silveira,
2010c). This means that the diffusion of nitrite
within the carbon ink is a very slow process and
should be responsible for the wide linear range of
the calibration curves.
Figure 4: Nitrite response of CPSPEs modified with ccNiR
in accordance with the optimized procedure
(3.3 μg enzyme in a 1:1 mix of acetone:graphite ink;
curing temperature = 40°C). Sensitivity: 52 A.μM
-1
.cm
-2
;
linear range: 0.001 - 1 mM. The calibration curve was
fitted to a hyperbolic equation according to the Michaelis-
Menten using the GraphPad Prism program
(K
m
app
= 0.9 ± 0.1 mM; I
max
= 1.32 x 10
-4
± 0.04 x 10
-4
A;
r
2
= 9.94 x 10
-1
).
4 CONCLUSIONS
This R&D project was designed to address a critical
and growing need for real-time monitoring of nitrites
and to provide better analytical tools for its clinical
diagnosis. Our previous results have demonstrated
the feasibility of using ccNiR in the construction of
bioelectrodes for a selective nitrite analysis
(Almeida, 2007; Chen, 2007; da Silva, 2004;
Silveira 2010 a,b; Zhang, 2009). Herein we have
shown the biocompatibility of the painting materials
and the electrode curing procedure with ccNiR
activity. The success of this preliminary work opens
up the possibility of including the enzyme directly in
the printing paste used for the fabrication of thick-
film electrodes, facilitating the mass production of
easy-to-use nitrite biosensors. If coupled to a
portable potentiostat, these enzyme containing
disposable electrode strips will turn a long and
elaborated laboratory protocol into a simple task,
quickly executed onsite.
ACKNOWLEDGEMENTS
The authors thank the financial support from
Associated Laboratory REQUIMTE.
REFERENCES
Almeida, M. G., Serra, A. S., Silveira, C., Moura, J. J. G.,
2010. “Nitrite Biosensing Via Selective Enzymes – A
‐1,2E‐04
‐1,0E‐04
‐8,0E‐05
‐6,0E‐05
‐4,0E‐05
‐2,0E‐05
0,0E+00
0 2000 4000 6000 8000
I
cat
(A)
[Nitrite]μM
NITRITE BIOSENSING WITH DISPOSABLE ELECTRODE STRIPS - A Preliminary Study
35

Long But Promising Route” Sensors, 10(12) 11530-
11555; doi:10.3390/s101211530.
Almeida, M. G., Macieira, S., Gonçalves, L. L., Huber, R.,
Cunha, C. A., Romão, M. J., Costa, C., Lampreia, J.,
Moura, J. J. G., Moura I., 2003. “The Isolation and
characterization of Cytochrome c Nitrite Reductase
Subunits (NrfA and NrfH) from Desulfovibrio
desulfuricans ATCC 27774. Re-evaluation of the
spectroscopic data and redox properties”, Eur. J.
Biochem. 270, 3904-3915.
Almeida, M. G., Silveira, C. M., Moura, J. J. G., 2007.
“Biosensing Nitrite Using the System Nitrite
Redutase/Nafion/Methyl Viologen - A Voltammetric
Study” Biosens. Bioelectron. 22, 2485-2492.
Bryan, N. S., Fernandez, B. O., Bauer, S. M., Garcia-
Saura, M. F., Milsom, A. B., Rassaf, T., Maloney, R.
E., Bharti, A., Rodriguez, J., Feelisch, M., 2005.
“Nitrite is a signaling molecule and regulator of gene
expression in mammalian tissues” Nat. Chem. Biol. 1,
290-297.
Chen, H., Mousty, C., Cosnier, S., Silveira, C. Moura, J. J.
G., Almeida, M. G., 2007. “Highly Sensitive ccNiR
Biosensor Electrical-Wired by [ZnCrAQS
2
] for Nitrite
Determination” Electrochem. Comm. 9, 2240-2245.
da Silva, S., Cosnier, S., Almeida, M. G., Moura, J. J. G.,
2004. “An Efficient Poly(pyrrole)-Nitrite Reductase
Biosensor for the Mediated Detection of Nitrite”
Electrochem. Comm. 6, 404-408.
Ellis, G., Adatia, I., Yazdanpanah, M., Makela, S. K,
1998. “Nitrite and nitrate analyses: A clinical bioche-
mistry perspective”. Clin. Biochem. 31, 195-220.
Hord, N. G., Tang, Y., Bryan N. S., 2009. “Food sources
of nitrates and nitrites: The physiologic context for
potential health benefits” Am. J. Clin. Nutr. 90, 1-10.
Jubete, E., Loaiza, O. A., Ochoteco, E., Pomposo, J. A.,
Grande, H., Rodrıguez, J., 2009. “Nanotechnology: A
Tool for Improved Performance on Electrochemical
Screen-Printed (Bio)Sensors” J. Sensors, Article ID
842575, 13 pages, doi:10.1155/2009/842575.
Lundberg, J. O. et al., 2009. “Nitrate and nitrite in biology,
nutrition and therapeutics” Nature, 12, 865-869.
Ochoteco, E., Jubete, E., Pomposo, J. A., Grande, H. et al.
2009 Patent Application Number E200930539.
Silveira, C. M., Gomes, S. P., Araújo, A. N., Couto, C. M.
C. M., Montenegro, M. C. B. S. M., Silva, R., Viana,
A. S., Todorovic, S., Moura, J. J. G., Almeida, M. G.,
2010a. “An Efficient Mediatorless Biosensor for
Nitrite Determination” Biosens. Bioelectron. 25, 2026-
2032.
Silveira, C., Baur, J., Cosnier, S., Moura, J. J. G.,
Holzinger, M., Cosnier, S., Almeida, M. G., 2010b.
"Enhanced direct electron transfer of a multihemic
nitrite reductase on SWCNT modified electrodes"
Electroanalysis, 22, 2973–2978.
Silveira, C. M., Besson, S., Moura, I., Moura, J. J. G.,
Almeida, M. G., 2010c. ”Measuring the Cytochrome c
Nitrite Reductase Activity – Practical considerations
on the enzyme assays”, Bioinorg. Chem. Applic.,
Volume 2010, Article ID 634597, 8 pages,
doi:10.1155/2010/634597
Zhang, Z., Xia, S., Leonard, D., Jaffrezic-Renault, N.,
Zhang, J., Bessueille, F., Goepfert, Y. S, Wang, X.,
Chen, L., Zhu, Z., Zhao, J., Almeida, M. G., Silveira,
C. M., 2009. “A novel nitrite biosensor based on
conductometric electrode modified with cytochrome c
nitrite reductase composite membrane” Biosens.
Bioelectron. 24, 1574–1579.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
36
