
OPTIMIZATION OF ELECTRIC FIELD FREQUENCY ON
DIELECTROPHORETIC IMPEDANCE MEASUREMENT
METHOD FOR ORAL BACTERIA DETECTION
Ryo Hamada
R & D Center, Panasonic Healthcare Co., Ltd., 2131-1, Minanikata, Toon, Ehime, 791-0395, Japan
Department of Electrical and Electronic Systems Engineering, Graduate School of Information Science and Electrical
Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
Junya Suehiro
Department of Electrical and Electronic Systems Engineering, Graduate School of Information Science and Electrical
Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
Keywords: Dielectrophoresis, Impedance, DEPIM, Bacteria, Oral hygiene, Influenza, Pneumonia.
Abstract: A simple and rapid bacteria detection device for on-site evaluation of oral hygiene in hospitals and clinics
was demonstrated. The developed device utilizes dielectrophoretic impedance measurement (DEPIM)
method. We integrated a micro electrode chip on which bacteria were captured by dielectrophoresis (DEP),
an AC voltage source to induce DEP force, and an impedance measurement circuit to a portable instrument
that enables rapid and automated oral bacterial inspection in hospitals and clinics. Special considerations
have been made on effects of high electrical conductivity of oral samples on DEP force and DEPIM results.
It was shown experimentally and theoretically that using a higher electric field frequency for the DEP
bacteria trap and the impedance measurement could realize DEPIM application to bacteria inspection from
oral samples with higher conductivity. Based on these investigations, we optimized the frequency condition
of the DEPIM suitable for inspecting an oral sample along with the design and development of a portable
DEPIM apparatus for on-site inspection of oral bacteria.
1 INTRODUCTION
Microbiological infectious disease of the oral cavity
is one of the matters for greatest concern since the
relationship between influenza, pneumonia and oral
bacteria, so that accurate evaluation of the amount of
oral bacteria as a level of oral hygiene is required in
order to prevent influenza (Abe et al., 2006a) and
aspiration pneumonia (Abe et al., 2006b). In this
study, a simple and rapid bacteria detection device
for on-site evaluation of oral hygiene in hospitals
and clinics was demonstrated. The developed device
utilizes dielectrophoretic impedance measurement
(DEPIM) method (Suehiro et al., 1999). Bacteria
suspended in a solution is trapped at the gap of
interdigitated microelectrode by positive
dielectrophoresis (DEP), simultaneously, temporal
change of capacitance of the electrode is measured.
Bacteria concentration is calculated based on a
tangent slope of capacitance change. Effect of high
conductivity of oral samples on DEP force and
DEPIM results was experimentally and theoretically
validated.
2 MATERIAL AND METHODS
2.1 Electrodes
Two different electrode configurations were used. A
smooth interdigitated electrode system was
employed in all the DEPIM experiments because
this type of electrode configuration is suitable for
accurate impedance measurement (Suehiro et al.,
1999). The smooth interdigitated electrode arrays of
gold were patterned on a polycarbonate substrate by
125
Hamada R. and Suehiro J..
OPTIMIZATION OF ELECTRIC FIELD FREQUENCY ON DIELECTROPHORETIC IMPEDANCE MEASUREMENT METHOD FOR ORAL BACTERIA
DETECTION.
DOI: 10.5220/0003128901250129
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 125-129
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
a laser ablation technique. Each microelectrode strip
had a 5 μm gap in which cells were trapped and
formed into pearl-chains by positive DEP. On the
other hand, a castellated electrode configuration
(Wang et al., 1993) was employed for the visual
observation of the cell collection process using
positive DEP. The castellated electrode arrays of
chrome were patterned on a glass substrate by
photolithography technique, and the microelectrode
was surrounded by a silicon rubber spacer to form a
chamber in which 22 μl of bacterial suspension
liquid was stored.
2.2 DEP Observation Equipment
The cell suspension liquid was stored in a reservoir
tank and circularly fed to the test chamber using a
peristaltic pump (Suehiro et al., 1999). Sinusoidal
AC voltage was generated by a function generator
(WF 1945, NF Corporation, Japan) and applied to
the electrode system. Visual observation of DEP was
conducted using an inverted microscope (BX-51,
OLYMPUS, Japan) and a CCD digital camera (C-
5060Z, OLYMPUS, Japan). The flow rate of the cell
suspension liquid fed by the peristaltic pump was 2.1
ml/min, and the amplitude of the applied voltage
was 10.0 V peak–peak respectively, which were
found to be appropriate conditions for the
observation of positive DEP in the preliminary tests.
2.3 DEPIM Equipment
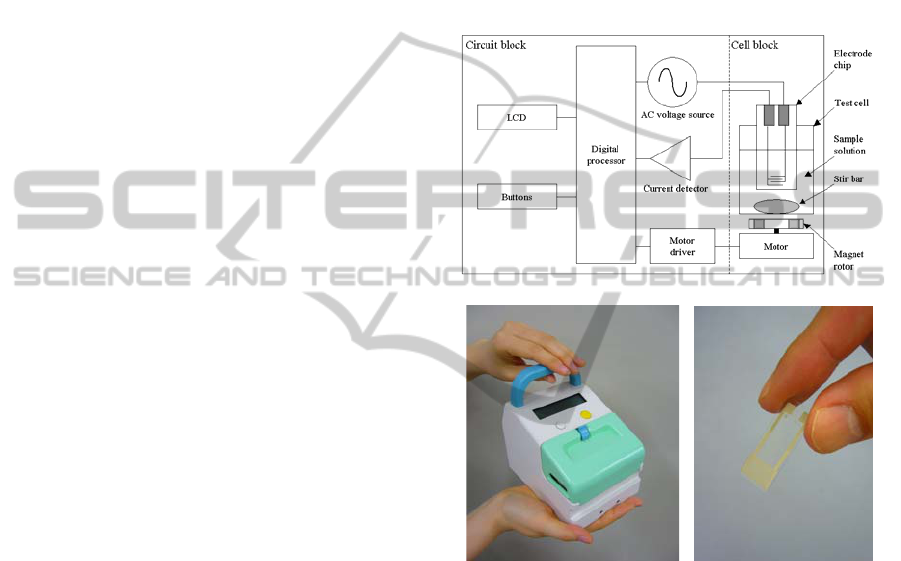
Fig. 1 shows a block diagram and a photographs of
the newly designed and developed DEPIM apparatus
and electrode chip. To enable rapid and automated
bacterial inspection in hospitals and clinics, the
apparatus was designed as a portable instrument to
enable stand-alone measurement without any other
instruments or cables.
The AC voltage source generates AC voltage,
which energizes the interdigitated electrode to
generate positive DEP force. Amplitude of the
applied voltage was 5.0 V peak–peak. AC current
flowing through the electrode is measured by the
current detector. The processor calculates the
electrode capacitance from the amplitudes of the
applied AC voltage and detected current, and the
phase difference between the two components. The
sequential measurement is carried out for 20 s, and
temporal variation of the electrode capacitance is
stored, then a tangent slope of capacitance change is
calculated in order to estimate bacteria
concentration, which has a linear relationship with
the slope.
In the test cell, 5 ml of bacterial suspension is
stored, in which the smooth interdigitated electrode
is immersed. The electrode chip is connected to the
AC voltage source and current detector. A magnetic
stirrer continuously generates a circular flow in the
test cell to enhance the DEP trapping of bacteria.
Impedance values measured by the DEPIM
apparatus were calibrated using a dummy load (a
parallel connection of resistance and capacitance
with known values), as well as a buffer with known
conductivity.
(a)
(b) (c)
Figure 1: The block diagram (a) and photographs of a
newly designed portable DEPIM apparatus (b) and an
electrode chip (c).
2.4 Bacteria Samples
For observation of the DEP trapping process and
optimization of DEPIM conditions, Escherichia coli
(E. coli) strain K-12 (NBRC3301), which have a
high growth rate and have been successfully
employed in previous works (Suehiro et al., 1999),
were employed as a dummy of oral bacteria in order
to improve efficiency of experiments. E. coli were
incubated on agar plates for 24 hours. Before each
measurement, cells were harvested from the agar
and suspended in a 0.1 M mannitol solution. After
several washings by centrifugation, they were finally
resuspended in a 0.1 M mannitol solution (1 μS/cm)
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
126
at various diluted concentrations as determined by a
colony counting method.
Conductivity of the mannitor solution was
adjusted range up to 50 μS/cm by dissolving sodium
chloride to simulate mixing of human saliva. This
value corresponds to be roughly 150 times diluted
human saliva by deionized water (Neyraud et al.,
2009), and bacteria concentration of human saliva
(Abe et al., 2008) at the dilution strength will be
detected by DEPIM method (Suehiro et al., 1999).
3 RESULTS
3.1 Observation of DEP Trapping
Process of Bacteria
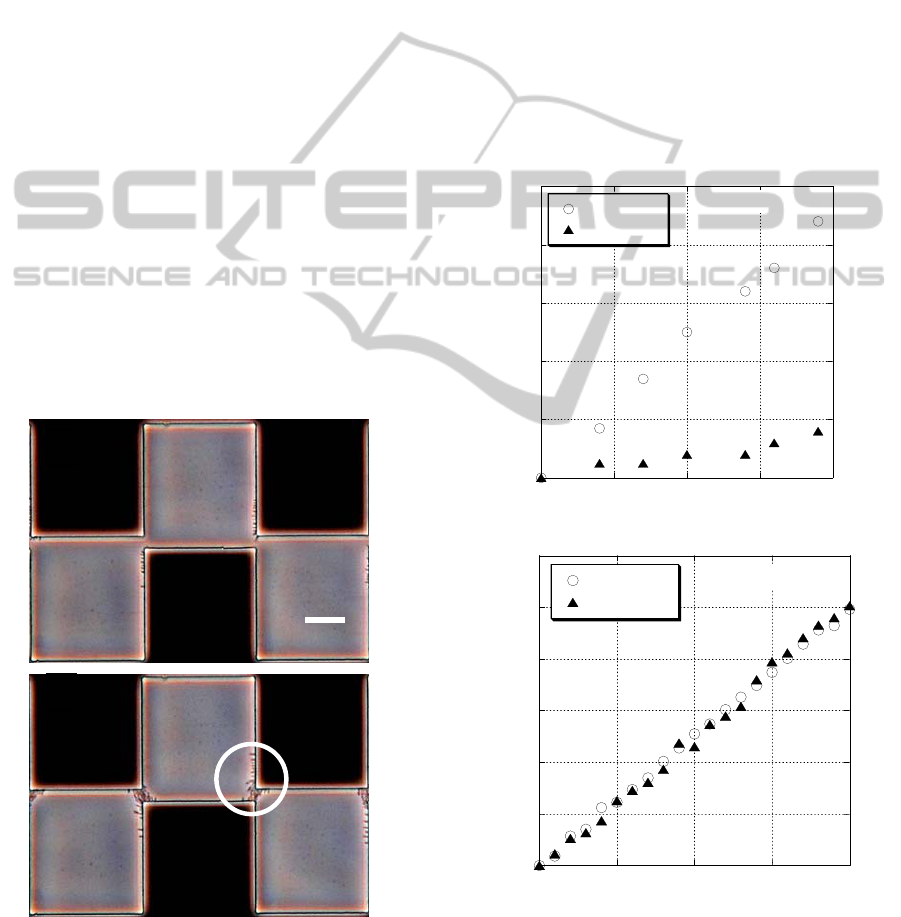
Photographs of the DEP collection of E. coli are
shown in Fig. 2. The DEP collection observations
were made at two different electric field frequencies
of 100 kHz (Fig. 2a) and 800 kHz (Fig. 2b), and
conductivity of the suspending medium of 50 μS/cm.
Bacteria were not trapped at 100 kHz, while some
bacteria were captured at 800 kHz. These
observation results suggest that positive DEP force
exerted on the bacteria becomes weak with increased
conductivity at the 100 kHz frequency.
Figure 2: DEP collection process of E. coli at medium
conductivity of 50 μS/cm and at frequency of 100 kHz (a)
and 800 kHz (b).
3.2 DEPIM Measurement using E. coli
Samples
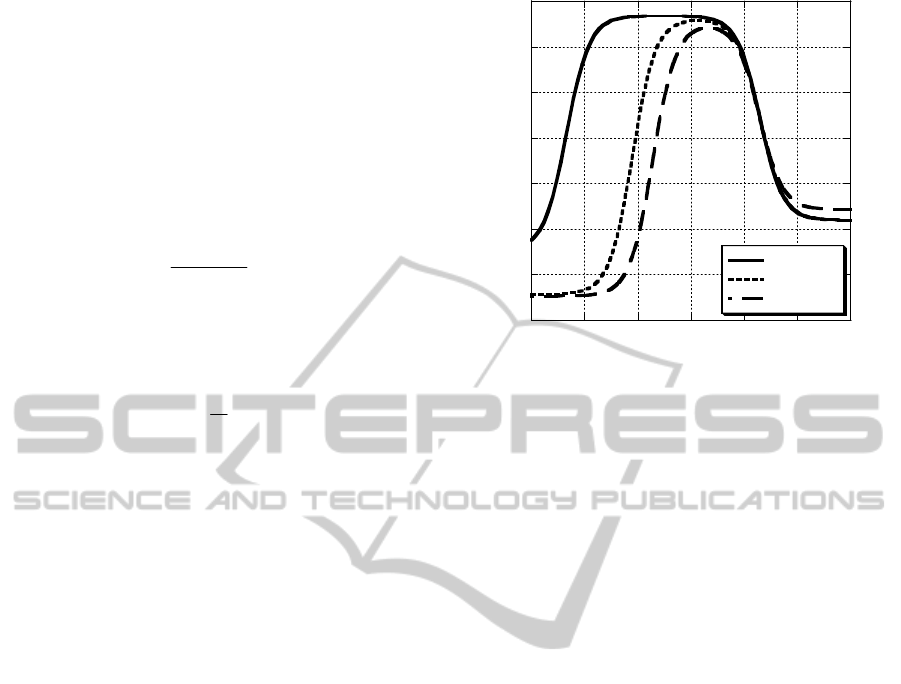
DEPIM experiments were conducted in this range of
conditions for 1 μS/cm and 50 μS/cm. Fig. 3 shows
temporal variation of the electrode capacitance
increment measured with E. coli at 5x10
6
CFU/ml
(at 100 kHz) and 2x10
7
CFU/ml (at 800 kHz).
Capacitance increase is due to the presence of
bacteria that are trapped and enriched in the
electrode gap. At a frequency of 100 kHz, the
capacitance increase rate in the case of 25 μS/cm
was obviously lowered in comparison with 1 μS/cm
(Fig. 3a). However, at a higher frequency of 800
kHz, the temporal change of capacitance was almost
the same for both the conductivities of 1 μS/cm and
50 μS/cm (Fig. 3b).
0
2
4
6
8
10
0 5 10 15 20
5 μS cm
-1
25 μS cm
-1
Capacitance change, C
T
(pF)
Time, t (sec)
ρ = 5 × 10
6
cm
-3
(a) 100 kHz
0
0.1
0.2
0.3
0.4
0.5
0.6
0 5 10 15 20
5 μS cm
-1
50 μS cm
-1
Capacitance change, C
T
(pF)
Time, t (sec)
ρ = 2 × 10
7
cm
-3
(b) 800 kHz
Figure 3: DEPIM results measured with E. coli. at
frequency of 100 kHz (a), and 800 kHz (b).
(b)
Trapped
E
.coli
(a)
20 μm
OPTIMIZATION OF ELECTRIC FIELD FREQUENCY ON DIELECTROPHORETIC IMPEDANCE MEASUREMENT
METHOD FOR ORAL BACTERIA DETECTION
127
4 DISCUSSION
The DEP force acting on a spherical particle of
radius r suspended in a medium of permittivity s is
given by (Jones, 1995)
23
)](Re[2 EKrF
sDEP
∇=
ωεπ
(1)
where E is the magnitude (RMS) of the applied field
and Re[K(ω)] is the real component of the Clausius–
Mossotti factor given by
**
**
2
)(
sp
sp
K
εε
εε
ω
+
−
=
(2)
where ε
p
*
and ε
s
*
are the complex permittivity of the
particle and surrounding medium, respectively. For a
real dielectric, the complex permittivity is defined as
ω
σ
εε
j−=
*
(3)
where ε is the permittivity and s is the conductivity
of the dielectric and ω is the angular frequency of
the applied field.
An example of a theoretical prediction of the
suspension medium conductivity dependency of
parameter Re[K(ω)] is shown in Fig. 4. One E. coli
cell is modeled as a dielectric sphere covered by
shells. The shells represent the cytoplasmic
membrane and the sphere covered by the shells
represents the cytoplasm (Huang et al., 1992).
Parameter values of E. coli are determined referring
to the referenced literature (Llamas et al., 1998). Fig.
4 indicates that Re[K(ω)] or the DEP force decreases
with increases in the medium conductivity σs at a
lower field frequency. When the medium
conductivity increases from the initial value of 1 to
50 μS/cm, DEP changes from positive-DEP to
negative-DEP at the field frequency of 100 kHz.
This suggests that E.coli cells are not captured at the
electrode gap by DEP under the condition of 50
μS/cm. On the other hand, the DEP force is hardly
dependent on σ
s
at 800 kHz. The theoretical
calculations agree well with the experimental results
shown in Fig. 2 where DEP collection of E.coli is
observed only for low medium conductivity (1
μS/cm) at 100 kHz but no clear differences are
observed with a rise in medium conductivity until 50
μS/cm at 800 kHz.
These results indicate that frequency of 800 kHz
is more appropriate than 100 kHz for DEPIM
measurement of sample with high medium electrical
conductivity, σ
s
.
-0.6
-0.4
-0.2
0
0.2
0.4
0.6
0.8
10
3
10
4
10
5
10
6
10
7
10
8
10
9
1 μS cm
-1
20 μS cm
-1
50 μS cm
-1
Re [K]
Frequency (Hz)
Figure 4: Theoretical prediction of the external medium
conductivity σ
s
dependency of Re[K(ω)] spectra.
5 CONCLUSIONS
In this study, we have described the optimization of
AC electric field frequency in the DEPIM method to
enhance the measurable range of conductivity of the
sample solution to adapt the DEPIM method for the
inspection of bacteria obtained from the human oral
cavity. Observation and theoretical calculation of
DEP, and DEPIM measurement was carried out.
From these results, it was shown that higher field
frequency is more suitable condition for bacterial
sample that has higher electrical conductivity of
solution. Consequently, it was demonstrated that the
developed portable DEPIM apparatus is useful in the
on-site evaluation of the bacterial contamination of
clinical samples from the oral cavity for quantitative
evaluation of oral hygiene to prevent influenza and
aspiration pneumonia. In addition, the developed
apparatus will be applied to other fields in which the
investigation of the sample including ionic
substances is necessary, for example, any clinical
samples besides those taken from the oral cavity, as
well aw fields relating to the environment and the
food industry.
REFERENCES
Abe S., Ishihara K., Adachi M., Sasaki H., Tanaka K.,
Okuda K., 2006a. Professional oral care reduces
influenza infection in elderly. Archives of Gerontology
and Geriatrics 43: 157–164.
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
128

Abe S., Ishihara K., Adachi M., Okuda K., 2006b. Oral
hygiene evaluation for effective oral care in preventing
pneumonia in dentate elderly. Archives of Gerontology
and Geriatrics 43: 53-64.
Abe S., Ishihara K., Adachi M., Okuda K., 2008. Tongue-
coating as risk indicator for aspiration pneumonia in
edentate elderly. Archives of Gerontology and
Geriatrics 47 (2): 267-275.
Huang Y., Holzel R., Pethig R., Wang X-B., 1992.
Differences in the AC electrodynamics of viable and
non-viable yeast cells determined through combined
dielectrophoresis and electrorotation studies. Phys.
Med. Biol. 37 (7): 1499-1517.
Jones T. B., 1995. Electromechanics of Particles,
Cambridge University Press. New York, 1
st
edition.
Llamas M., Giner V., Sancho M., 1998. The dynamic
evolution of cell chaining in a biological suspension
induced by an electrical field. J. Phys. D: Appl. Phys.
31 (21): 3160-3167.
Neyraud E., Bult J. H. F., Dransfield E., 2009. Continuous
analysis of parotid saliva during resting and short-
duration simulated chewing. Archives of oral biology
54 (5): 449-456.
Suehiro J., Yatsunami R., Hamada R., Hara M., 1999.
Quantitative estimation of biological cell
concentration suspended in aqueous medium by using
dielectrophoretic impedance measurement method. J.
Phys. D: Appl. Phys. 32: 2814-2820.
Wang X-B., Huang Y., Burt J. P. H., Markx G. H., Pethig
R., 1993. Selective dielectrophoretic confinement of
bioparticles in potential energy wells. J. Phys. D: Appl.
Phys. 26 (8): 1278-1285.
OPTIMIZATION OF ELECTRIC FIELD FREQUENCY ON DIELECTROPHORETIC IMPEDANCE MEASUREMENT
METHOD FOR ORAL BACTERIA DETECTION
129
