
WIENER-HAMMERSTEIN PARAMETER ESTIMATION USING
DIFFERENTIAL EVOLUTION
Application to Limb Reflex Dynamics
Oliver P. Dewhirst, David M. Simpson, Natalia Angarita, Robert Allen
Institute of Sound and Vibration Research, University of Southampton, Southampton, U.K.
Philip L. Newland
School of Biological Sciences, University of Southampton, Southampton, U.K.
Keywords:
Wiener-Hammerstein, Differential evolution, Locust, Limb reflex dynamics, Neuromuscular modelling.
Abstract:
The nonlinear Wiener-Hammerstein model, which consists of a static (no memory) non-linearity sandwiched
between two dynamic (with memory) linear elements, provides a parsimonious and accurate model for
representing a number of biological systems. In this study we compare the performance of two Wiener-
Hammerstein parameter estimation methods; a commonly used nonlinear local optimisation method and the
global optimisation method Differential Evolution. The accuracy and convergence properties of the two meth-
ods is tested using experimental data collected from the locusts hind limb reflex control system and using
computer simulations.
1 INTRODUCTION
A greater understanding of neuromuscular control of
limb movement is vital for optimising the treatment
of patients with neuromuscular dysfunction. System
identification methods provide a valuable tool for in-
vestigating the complex mechanical and neural com-
ponents of such systems (Bai et al., 2009; Dempsey
and Westwick, 2004). Our work uses the locust as
a model system because it provides the opportunity
to develop new system identification techniques and
gain physiological insight into a related but simpler
and more accessible system.
Nonlinear Wiener or Volterra seriesmodels, a gen-
eralisation of the convolution integral for a linear sys-
tem, have often been used to model the nonlinear
responses generated by reflex limb control systems
(Newland and Kondoh, 1997) and other biological
systems (Song et al., 2009). These series are often
truncated to second order because it is difficult to vi-
sualise, interpret and calculate, in terms of compu-
tational cost and length of data required, the higher
order kernels.
Cascade models, a restricted subset of the Volterra
series, have been shown to provide a parsimonious
and easier to interpret alternative for representing
such systems (Dewhirst et al., 2009; Dempsey and
Westwick, 2004). Cascade models consist of linear
dynamic (with memory) elements and static (no mem-
ory) nonlinear elements. Common configurations in-
clude the Wiener (linear - nonlinear), Hammerstein
(nonlinear - linear) and the Wiener-Hammerstein (lin-
ear - nonlinear - linear) model shown in Figure 1.
Methods to estimate the parameters of the Wiener-
Hammerstein model are currently receiving much at-
tention for control applications (Schoukens et al.,
2009) but not for application to biological systems.
A key feature of the locust’s hind leg control sys-
tem is a reflex control loop. This system uses a me-
chanical loop structure attached to the tibia by an
apodeme to move a stretch sensor called the Femoro-
tibial Chordotonal Organ (FeCO). Sensory neurons in
the FeCO convert mechanical stimuli into electrical
signals which are transmitted to motor neurons which
activate muscle contraction (Newland and Kondoh,
1997). Previous work (Dewhirst et al., 2009) found
that the Wiener-Hammerstein model was the opti-
mum structure for representing the dynamic reflex re-
sponse of the Fast Extensor Tibia (FETi) motor neu-
ron in the locusts hind leg control system, the system
studied in the current work.
As the cascade models are nonlinear in their pa-
271
P. Dewhirst O., M. Simpson D., Angarita N., Allen R. and L. Newland P. (2010).
WIENER-HAMMERSTEIN PARAMETER ESTIMATION USING DIFFERENTIAL EVOLUTION - Application to Limb Reflex Dynamics.
In Proceedings of the Third International Conference on Bio-inspired Systems and Signal Processing, pages 271-276
DOI: 10.5220/0002741902710276
Copyright
c
SciTePress

Figure 1: The Wiener-Hammerstein model.
rameters and have a differentiable cost function their
parameters can be estimated using a nonlinear local
optimisation method. One of the most widely used
local optimisation methods for estimating the parame-
ters of the Wiener-Hammerstein models for biological
applications was developed by Korenberg and Hunter
(Korenberg and Hunter, 1986) (the KH method). As
the cost function of the model is multi-modal, such
methods require careful parameter initialisation to en-
sure that they do not convergeto a local rather than the
global minimum (Schoukens et al., 2008; Dempsey
and Westwick, 2004).
An alternative approach is to use a nonlinear
global optimisation method such as simulated anneal-
ing which has been developed to avoid the problem
of local minima. As these methods can suffer from
slow convergence and poor local search characteris-
tics their application has often been restricted to find-
ing initial parameter estimates for the local methods
(Juusola et al., 2003). One type of evolutionary algo-
rithm, Differential Evolution (DE), however, has been
shown to give fast robust convergence and good lo-
cal search performance. It also requires few control
parameters and is easy to use and implement (Storn
and Price, 1997). DE has been applied to estimate the
parameters of Hammerstein and Wiener models (Al-
Duwaish, 2000) but we do not believe to the Wiener-
Hammerstein model.
In this study we compare the performance of the
DE and KH methods for estimating the parameters
of the Wiener-Hammerstein model from experimental
data obtained from the locusts leg control system and
computer simulations.
2 METHODS
The linear elements of the Wiener-Hammerstein
model (Figure 1) have a Finite Impulse Response
(FIR) structure. A polynomial function was chosen to
represent the static nonlinear element. The noise free
output of the Wiener-Hammerstein model is given by
y(t) =
T
∑
σ=1
g(σ)
Q
∑
q=0
c
(q)
T
∑
τ=1
h(τ)u(t − σ − τ)
!
q
(1)
Where T is the length of the FIR filter and Q is the
polynomial order.
2.1 Differential Evolution
The parameters of the Wiener-Hammerstein model
are coded in a vector containing D elements
x =
h(1), . . . , h(T), c
0
, . . . , c
Q
, g(1), . . . , g(T)
(2)
Differential Evolution (DE) works with a population,
P, of NP D-dimensional parameter vectors (Storn and
Price, 1997)
P
(G)
=
x
(G)
1,1
x
(G)
1,2
··· x
(G)
1,D
x
(G)
2,1
x
(G)
2,2
··· x
(G)
2,D
.
.
.
.
.
.
.
.
.
.
.
.
x
(G)
NP,1
x
(G)
NP,2
··· x
(G)
NP,D
(3)
where x
(G)
1,1
x
(G)
1,2
···x
(G)
1,D
are elements of x. Each param-
eter vector
x
(G)
i
=
x
(G)
i,1
, x
(G)
i,2
, . . . ,x
(G)
i,D
i = 1, 2, . . . , NP (4)
represents a possible solution to the optimisation
problem. The population is initialised randomly and
evolves in generations (iterations), G. With each gen-
eration a mutant vector, v
i
, is formed from each of the
NP parent vectors, x
i
, following
v
(G+1)
i
= x
(G)
r
1
+ F
x
(G)
r
2
− x
(G)
r
3
(5)
with the multiplication factor F set by the user (F >
0) and random, mutually different indexes r
1
, r
2
, r
3
∈
{1, 2, . . . ,NP} which are also different from i. The
trial vector
u
(G+1)
i
=
u
(G+1)
i,1
, u
(G+1)
i,2
, . . . , u
(G+1)
i,D
(6)
is generated using the crossover operator
u
(G+1)
i, j
=
(
v
(G+1)
i, j
if (rnd( j) ≤ CR)
x
(G)
i, j
if (rnd( j) > CR)
(7)
where j = 1, 2, . . . , D and rnd( j) is the j
th
output from
a random number generator with a uniform distribu-
tion. The cross over constant CR ∈ [0, 1] is set by
the user; rn(k) ∈ {1, 2, . . . , D} is a randomly chosen
index used to ensure that at least one of the parame-
ters from v
i, j
is transferred to u
i, j
. The widely used
Minimum Mean Square Error (MMSE) cost function
is used to compare the performance of the trial and
parent vectors.
J =
1
N
N−1
∑
t=0
(z(t) − ˆy(t))
2
(8)
where N is the signal length in samples. A greedy
selection procedure (does not reconsider past genera-
tions) is used to determine if the parent vector should
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
272
be retained for the next generation, or replaced by
the trial vector. These steps are repeated either for a
specific number of generations or until a certain cost
function value is reached. The DE method was set
up with F = 0.5, CR = 0.9, NP = 10 × D (Storn and
Price, 1997) and was run for 900 iterations.
2.2 The Korenberg Hunter Method
Korenberg and Hunter (Korenberg and Hunter, 1986)
use an iterative two step approach to estimate the pa-
rameters of the Wiener-Hammerstein model. Their
method is summarised in Algorithms 1 and 2.
Algorithm 1. KH parameter estimation.
Require: Initial estimate of
ˆ
h(τ) = (
1
fs
, 0, 0, . . . ,0)
1: Filter u(t) by
ˆ
h(τ) to obtain ˆx(t)
2: Fit the Hammerstein system between ˆx(t) and z(t)
using Algorithm 2
3: If first iteration or significant improvement in
model accuracy continue, else exit with current
parameters
4: Obtain a new estimate of
ˆ
h(τ) using the
Levenberg-Marquardt (LM) (Marquardt, 1963)
gradient based method to minimise J (Equation
8) given the current estimate of the Hammerstein
system. Return to step 2
Algorithm 2. Hammerstein parameter estimation.
1: Fit a linear system between z(t) and ˆx(t) to obtain
an initial estimate of the inverse of ˆg(τ), ˆg(τ)
−1
2: Filter z(t) by ˆg(τ)
−1
to obtain ˆw(t)
3: Fit a polynomial ˆm(·) between ˆx(t) and ˆw(t)
4: Obtain a new estimate of ˆw(t) given ˆm( ˆx(t))
5: Re-estimate ˆg(τ) given ˆw(t) and z(t)
6: Calculate the %MSE (Equation 9) difference be-
tween model output ˆy(t) and measured outputz(t)
7: If the %MSE reduction is small compared to last
iteration, exit with current parameters
8: Else, compute a new estimate of ˆg(τ)
−1
using the
current ˆw(t) and z(t). Return to step 2
2.3 Experimental Data
The performance of the two parameter estimation
methods was measured using experimental data col-
lected from the locust (Schistocerca gregaria). A
brief summary of the experimental methods used by
Newland and Kondoh (Newland and Kondoh, 1997)
follows. The locusts were mounted in modelling
clay; the apodeme of the FeCO was exposed by dis-
section and was attached by forceps to an electro-
mechanical shaker (LDS, type 101). The FeCO was
stimulated by applying a 27Hz low pass filtered Gaus-
sian White Noise (GWN) signal to the shaker to move
the apodeme (Figure 2 A). The synaptic inputs to
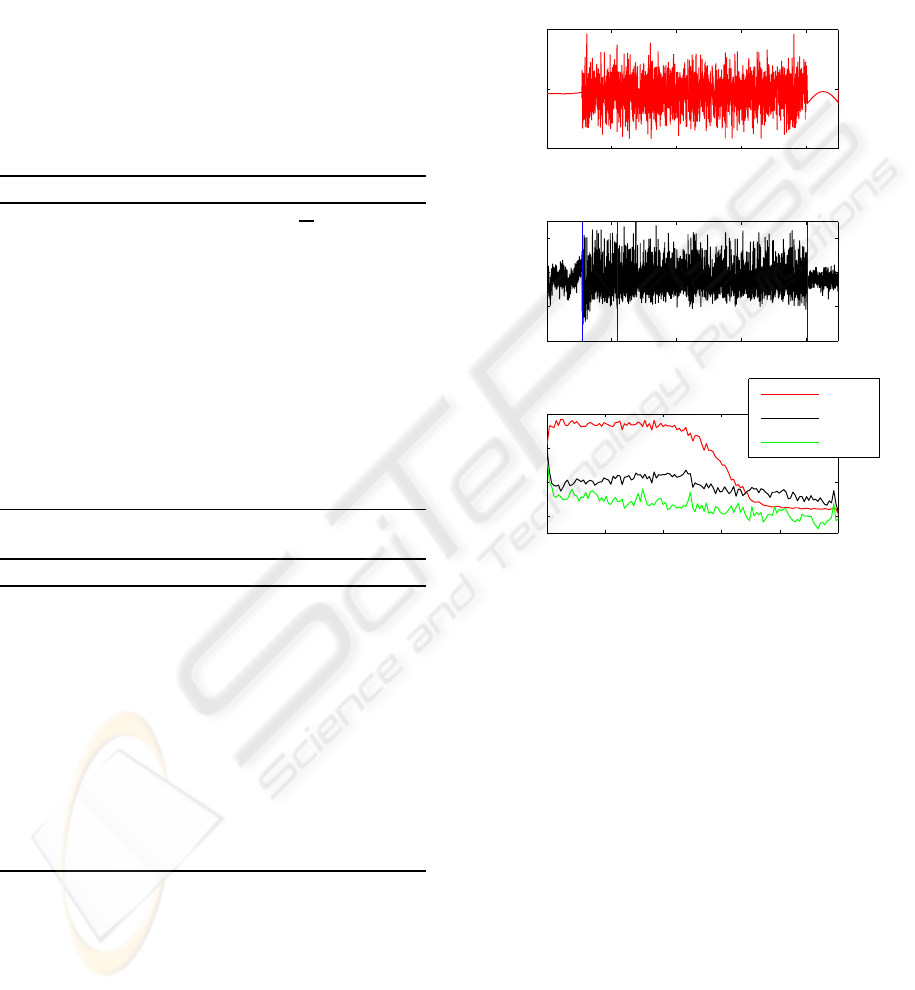
0 10 20 30 40
0
60
120
Time (s)
Joint angle (degs.)
A)
0 10 20 30 40
−0.4
−0.2
0
0.2
Time (s)
Amplitude (mV)
B)
s1 s2 s3
0 10 20 30 40 50
−60
−40
−20
0
Frequency (Hz)
Power (dB)
C)
Input
Output
Noise
Figure 2: The band limited (0-27Hz) GWN input signal
applied to move the apodeme of the FeCO (A). A typical
output signal recorded from FETi (B). Noise, the transient
adapting response of FETi and its steady state adapted re-
sponse are marked as s1, s2 and s3 respectively. The spec-
trum of the input signal, adapted FETi response (s3) and
noise signal (s1) are plotted in C.
the FETi motor neuron, caused by stimulation of the
apodeme of the FeCO, were measured by inserting a
glass microelectrode into its soma. A typical record-
ing from the soma of the FETi motor neuron is shown
in Figure 2 B. The high level of measurement and
background neural noise, initial transient response
and steady state adapted response of FETi can be
seen in Figure 2 B sections s1, s2 and s3 respectively.
Models were fitted between the first 600 samples (6s
with fs=100Hz) of the steady state adapted response
of FETi (Figure 2 B s3) and the corresponding sam-
ples of input signal. The performance of the parame-
ter estimation methods was determined by calculating
the %MSE difference between the predicted output of
the model and the measured output of the neuron us-
WIENER-HAMMERSTEIN PARAMETER ESTIMATION USING DIFFERENTIAL EVOLUTION - Application to
Limb Reflex Dynamics
273
ing 200 samples of validation data.
MSE = 100×
1
N
∑
N
t=1
(y(t)− ˆy(t))
2
1
N
∑
N
t=1
y(t)
2
(9)
Before the performance of the two parameter estima-
tion methods was compared, the number of model
parameters was optimised. As six sets of data were
available, six models were estimated using the KH
method. The %MSE performance of each of these
models with a varying number of parameters was cal-
culated, using validation data. The optimum num-
ber of parameters was determined by calculating the
mean of the minimum %MSE value. As the gain of
the locust system can be arbitrarily assigned to the
linear and nonlinear elements of the model these ele-
ments have been normalised to allow comparison.
2.4 Simulations
Computer simulations were used to investigate the
robustness of the two parameter estimation methods
to experimental conditions. The simulated system
was based on one of the Wiener Hammerstein mod-
els identified from experimental data using the KH
method. Measurement noise coupled with the limited
bandwidth of the input signal (Figure 2 C) introduces
random estimation errors into the linear elements of
the model. As the estimation error occurs where there
is little input power (> 27Hz) and system dynamics it
was removed using a low pass filter (zero phase, 3
rd
order Butterworth, f
c
= 27Hz).
“Ideal” and “low pass with measurement noise”
data sets were generated using the simulated system.
To create the “ideal” data set, a GWN signal was gen-
erated and applied to the simulated system. The in-
put signal in the “low pass with measurement noise”
data set represents the input signal used in the locust
experiments. It was created by filtering the GWN in-
put signal with a low pass filter (zero phase, 5
th
order
Butterworth, f
c
= 27Hz). The effect of measurement
noise on the parameter estimation methods was inves-
tigated using a Monte Carlo simulation (100 trials).
In each trial measurement noise with a Gaussian dis-
tribution was added to the output from the simulated
system generated using the same low pass input sig-
nal. The noise was added to give a Signal to Noise
Ratio of 5dB, a level similar to that found in the lo-
cust recordings.
3 RESULTS AND DISCUSSION
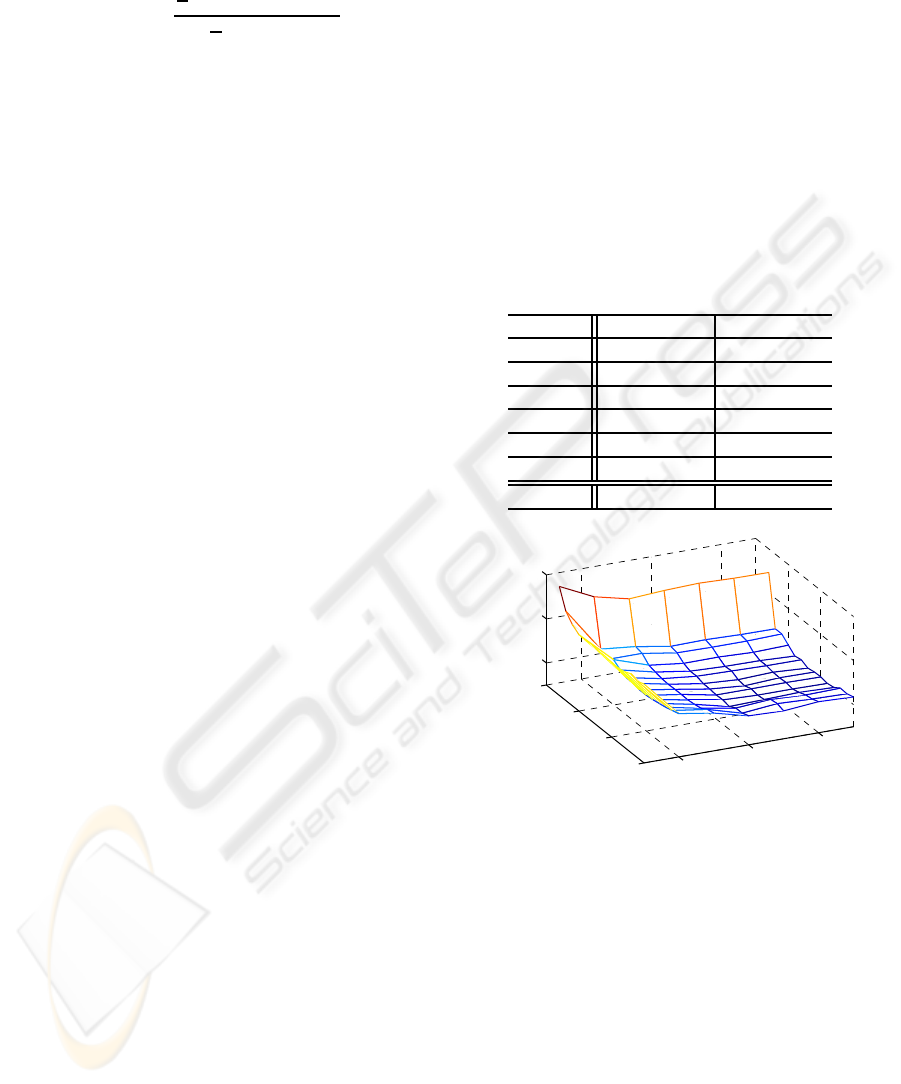
The variation in mean %MSE performance of the
Wiener-Hammerstein model (parameters estimated
using the KH method) with the number of parameters
it contains is plotted in Figure 3. This result shows
that optimum performance is obtained with 9 parame-
ters in each linear element and a 5
th
order polynomial,
though the minimum is in an almost flat area of the pa-
rameter space. The mean %MSE performance of the
models, containing the optimum number of parame-
ters, with their parametersestimated using the KH and
DE methods is very similar (30.0% and 29.0% respec-
tively, Table 1). Both methods also produce similar
mean parameter estimates (Figure 4).
Table 1: %MSE performance of the Wiener-Hammerstein
models, with their parameters estimated using the KH and
DE methods, on validation data. Models contain the opti-
mum number of parameters.
Animal KH method DE method
1 31.0 29.2
2 29.4 32.4
3 17.6 19.6
4 36.0 33.3
5 32.1 28.0
6 34.1 31.3
Mean 30.0 29.0
0
10
20
30
2
4
6
30
40
50
↓
Polynomial order (Q)
Total no. of linear
parameters (2T)
%MSE
Figure 3: Mean (n=6) %MSE performance variation for val-
idation data with the total number of linear parameters in the
model and polynomial order. The minimum mean MSE is
30.0% and occurs with a total of 18 linear parameters and a
5
th
order polynomial (marked with an arrow).
The non white spectrum of the input signal (Fig-
ure 2 C) coupled with the high levels of measure-
ment noise (Figure 2 B s1) results in the parameter
estimates containing high frequency estimation error
(Figure 4). The rather high %MSE performance of
the models, suggests that they provide a poor fit to
the system. The recordings prior to the start of stim-
ulation, when the input is constant, show consider-
able measurement noise (Figure 2 B s1). Analysis
has shown that the spectrum of the residual signal (the
difference between the model and the measured out-
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
274
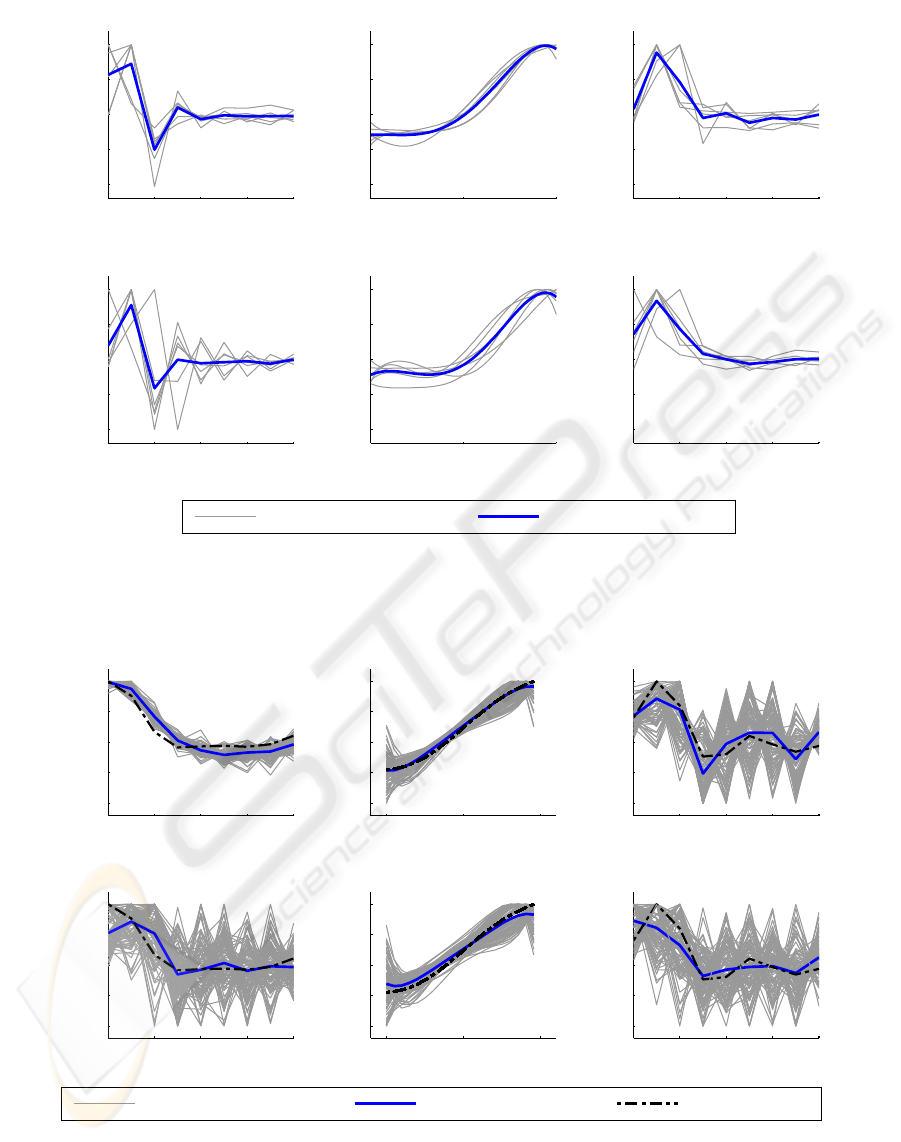
0 20 40 60 80
−1
−0.5
0
0.5
1
Lag (ms)
Amplitude
A)
−1 0 1
−1
−0.5
0
0.5
1
Input
Output
B)
0 20 40 60 80
−1
−0.5
0
0.5
1
Lag (ms)
Amplitude
C)
Model estimates (6 animals)
Mean of model estimates
0 20 40 60 80
−1
−0.5
0
0.5
1
Lag (ms)
Amplitude
D)
−1 0 1
−1
−0.5
0
0.5
1
Input
Output
E)
0 20 40 60 80
−1
−0.5
0
0.5
1
Lag (ms)
Amplitude
F)
Figure 4: The parameters of the Wiener-Hammerstein models of the FETi system estimated using the KH method (A-C) and
the DE method (D-F). The first linear element h(τ) is plotted in A and D, the second linear element g(τ) in C and F. The
polynomial function is plotted in B and E. Note that the parameters have been normalised to allow comparison.
0 20 40 60 80
−1
−0.5
0
0.5
1
Lag (ms)
Amplitude
A)
−1 0 1
−1
−0.5
0
0.5
1
Input
Output
B)
0 20 40 60 80
−1
−0.5
0
0.5
1
Lag (ms)
Amplitude
C)
Model estimates (100 trials)
Mean of model estimates
Simulated system
0 20 40 60 80
−1
−0.5
0
0.5
1
Lag (ms)
Amplitude
D)
−1 0 1
−1
−0.5
0
0.5
1
Input
Output
E)
0 20 40 60 80
−1
−0.5
0
0.5
1
Lag (ms)
Amplitude
F)
Figure 5: Results of Monte Carlo simulations comparing the performance of the KH (A to C) and the DE (D to F) pa-
rameter estimation methods using the “low pass with measurement noise” data set. In each trial measurement noise with
a Gaussian distribution was added to the output of the simulated system generated using the same low pass input sig-
nal.
WIENER-HAMMERSTEIN PARAMETER ESTIMATION USING DIFFERENTIAL EVOLUTION - Application to
Limb Reflex Dynamics
275

put signal) is very similar to the spectrum of the noise
signal. This suggests that model fit is good, as one
cannot expect the model to be able to predict the ran-
dom measurement noise.
Computer simulations using the “ideal” data set
showed that both parameter estimation methods could
accurately identify the simulated system. The nor-
malised parameter bias error for both methods was
less than 0.1%. The results of the Monte Carlo sim-
ulation using the “low pass with measurement noise”
data set are shown in Figure 5. Under these simu-
lated experimental conditions, neither method could
retrieve the parameters of the simulated system. It is
interesting to note that the DE estimates of the pa-
rameters of h(τ) (Figures 4 and 5 D) contain more
high frequencyestimation error than the KH estimates
(Figures 4 and 5 A) for both experimental and simu-
lated data. This is probably because the LM method
(Westwick and Kearney, 2003) used to estimate h(τ)
in the KH algorithm (Algorithm 1 step 4) approxi-
mates the Hessian
ˆ
H as
ˆ
H =
1
N
J
T
J+ µ
k
I
M
(10)
and the µ
k
I
M
term improves the condition of
ˆ
H.
4 CONCLUSIONS
The accuracy of two Wiener-Hammerstein parame-
ter estimation methods has been compared using ex-
perimental data and computer simulations. Our work
has shown that DE gives fast robust convergence and
good local search performance, providing similar ac-
curacy as the local optimisation method developed by
Korenberg and Hunter (Korenberg and Hunter, 1986).
The DE method offers the advantageof simplicity and
flexibility, for example making it easy to use different
cost functions. Also, as it is a global method, it should
be less sensitive to parameter initialisation, but addi-
tional work is require to verify this feature. Further
investigation into the convergence properties of both
methods and ways to reduce parameter bias and vari-
ance under experimental conditions is required.
ACKNOWLEDGEMENTS
The authors would like to thank the Gerald Kerkut
Trust, the LSI Forum, the ISVR Rayleigh Scholarship
and the BBSRC for their support.
REFERENCES
Al-Duwaish, H. (2000). A genetic approach to the identi-
fication of linear dynamical systems with static non-
linearities. International Journal of Systems Science,
31(3):307–313.
Bai, E., Cai, Z., Dudley-Javorosk, S., and Shields,
R. K. (2009). Identification of a modified wiener-
hammerstein system and its application in electrically
stimulated paralyzed skeletal muscle modeling. Auto-
matica, 45(3):736 – 743.
Dempsey, E. and Westwick, D. (2004). Identification
of hammerstein models with cubic spline nonlinear-
ities. IEEE Transactions on Biomedical Engineering,
51(2):237–245.
Dewhirst, O., Simpson, D., Allen, R., and Newland, P.
(2009). Neuromuscular reflex control of limb move-
ment - validating models of the locust’s hind leg con-
trol system using physiological input signals. In IEEE
EMBS 09, 4th International Conference on Neural En-
gineering. IEEE EMBS.
Juusola, M., Niven, J., and French, A. (2003). Shaker
k+ channels contribute early nonlinear amplification
to the light response in drosophila photoreceptors. J
Neurophysiol, 90(3):2014–2021.
Korenberg, M. and Hunter, I. (1986). The identification of
nonlinear biological systems: lnl cascade models. Bi-
ological Cybernetics, 50(2):125–134.
Marquardt, D. W. (1963). An algorithm for least-squares
estimation of nonlinear parameters. SIAM Journal on
Applied Mathematics, 11(2):431–441.
Newland, P. and Kondoh, Y. (1997). Dynamics of neurons
controlling movements of a locust hind leg iii: Exten-
sor tibiae motor neurons. The Journal of Neurophysi-
ology, 77(5):3297–3310.
Schoukens, J., Pintelon, R., and Enqvist, M. (2008). Study
of the lti relations between the outputs of two coupled
wiener systems and its application to the generation
of initial estimates for wiener-hammerstein systems.
Automatica, 44(7):1654 – 1665.
Schoukens, J., Suykens, J., and Ljung, L. (2009). Wiener
hammerstein benchmark. In Proc. 15 IFAC Sympo-
sium on System Identification, St Malo, France.
Song, D., Marmarelis, V., and Berger, T. (2009). Paramet-
ric and non-parametric modeling of short-term synap-
tic plasticity. part i: Computational study. J Comput
Neurosci, 26(1):1 – 19.
Storn, R. and Price, K. (1997). Differential evolution a sim-
ple and efficient heuristic for global optimization over
continuous spaces. Journal of Global Optimization,
11:341–359.
Westwick, D. and Kearney, R. (2003). Identification of Non-
linear Physiological Systems. Wiley-IEEE Computer
Society Pr, Piscataway, New Jersey, 1st edition.
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
276
