
RFID-based Semantic-enhanced Ubiquitous Decision
Support System for Healthcare
Michele Ruta, Floriano Scioscia, Tommaso Di Noia and Eugenio Di Sciascio
Politecnico di Bari
via Re David 200, I-70125
Bari, ITALY
Abstract. We present an innovative Decision Support System for healthcare ap-
plications, based on a semantic enhancement of RFID standard protocol. Seman-
tically annotated descriptions of both drugs and patient’s case history stored in
proper RFID tags are used to help doctors in providing the correct therapy. The
proposed system allows to discover possible inconsistencies in a therapy suggest-
ing alternative treatments.
1 Introduction
RFID is an automatic identification technology, relying on storing and remotely re-
trieving information located on a tag exploiting proper interrogators, namely readers.
The miniaturization of electronic components and circuits nowadays allows an RFID
transponder to be applied-to or incorporated-into objects, animals, or persons for iden-
tification and tracking purposes. Some tags can be interrogated at distance and also
by-passing possible physical barriers and obstacles. They usually contain a unique code
which is read by the interrogator and can be used to identify the associated object via a
networked database on a server. Nevertheless, transponders with larger internal memory
open new interesting possibilities and enable further applications. Notice that current
Radio Frequency (RF) identification methods only enable elementary recognition ap-
plications which exploit queries over a database for retrieving object features and prop-
erties. If tagged objects, animals or persons expose to a reader not simply a numeric
identifier but a compressed semantic annotation, they may describe themselves without
referring to a centralized database, also allowing the possibility to update those de-
scriptions in real time and when needed considering information not completely known
when the tag has been attached/implanted. This is particularly useful in case: (i) a de-
pendable or networked link toward the fixed information server is unavailable; (ii) the
information related to the object/subject has to be always and straightaway available;
(iii) an advanced description of object/subject characteristics and capabilities is needed
in order to enable complex inference procedures over data stored within the tag.
All the above features have an undoubted interest in the healthcare sector. E-health-
care information systems include applications for tele-medicine, tele-health, and tele-
home care services. RFID technology now has significant impact on healthcare systems,
with specific reference to tracking and management of patients and drugs within hospi-
tals. Benefits of RF identification in those cases include error prevention in identifying
Ruta M., Scioscia F., Di Noia T. and Di Sciascio E. (2009).
RFID-based Semantic-enhanced Ubiquitous Decision Support System for Healthcare.
In Proceedings of the 3rd International Workshop on RFID Technology - Concepts, Applications, Challenges , pages 79-88
DOI: 10.5220/0002203500790088
Copyright
c
SciTePress

staff and regulation of accesses in various divisions for patients and doctors. Although
these applications are noteworthy, a more advanced exploitation of RFID could further
enhance the impact of this technology in e-healthcare. In this paper we present a novel
Decision Support System (DSS) for innovative medical applications based on a seman-
tic enhancement of RFID standard protocol. Thanks to semantic annotation of both
drugs to be administered and patient’s case history, the proposed system helps physi-
cians in confirming and then choosing the best therapy based on the medical record of
the patient.
By referring to semantic metadata stored within RFID tags attached to drugs pack-
aging and patient’s RFID wristband, a matchmaking can be performed to discover
possible inconsistencies in a therapy, also suggesting further treatment options to the
physician. We borrowed ideas and technologies devised for the Semantic Web initia-
tive. We set our stage in an e-healthcare context, where RFID tags are dipped into an
enhanced Bluetooth framework. In a previous work both the RFID EPCglobal data ex-
change protocol
1
and the Bluetooth Service Discovery Protocol
2
have been modified to
enable support for advanced inference services, while maintaining legacy applications
[1]. Here we introduce novel semantic-based value-added services for decision support
in healthcare.
The remaining of the paper is structured as follows. In the next section relevant
related work referred to the exploitation of RFID technology in the medical field is
surveyed. Section 3 outlines the framework, explaining the discovery and matchmaking
algorithms devised for the purposes outlined above. Section 4 illustrates the system
architecture and the approach in an example scenario; finally conclusion and future
work close the paper.
2 RFID for Healthcare
Hospital activities are characterized by complex workflows requiring the interaction
of several different actors and the coordination of multiple facilities [2]. Supply chain
management has suggested that the identification capability of RFID can be leveraged
to improve healthcare services. Patients, equipment and staff can be tagged with RFID
transponders and tracked within a hospital by a network of RFID readers deployed in
key locations. Actions recognized via RFID can be logged automatically, thus avoid-
ing lengthy and error-prone manual data input by personnel. Research studies and pilot
projects have evaluated the impact of such infrastructures in ordinary hospital activities
[3,4] as well as in emergency conditions due to disasters [5] or epidemics [6]. The inte-
gration of RFID into a Hospital Information System (HIS) allows to: automate checks
for security (authorization enforcement) and safety (prevention of human error) during
critical processes such as patient admission, checkout and drug administration; reduce
response times in emergency situations; improve efficiency of resource allocation. Ul-
timately, these benefits lead to higher confidence and satisfaction of both patients and
personnel [2].
1
EPCglobal. http://www.epcglobalinc.org
2
Bluetooth. http://www.bluetooth.com
80

The integration of RFID with other pervasive computing technologies –such as
wireless protocols and sensor networks– is leading to further innovative applications
in the tele-medicine area, particularly for ubiquitous persistent monitoring of elderly
or disabled people as well as for patient follow-up during rehabilitation phase [7,8].
Context-awareness is the key aspect of such approaches to improve quality of health-
care services. Challenges and benefits were clearly evidenced in a prototype of RFID-
enabled smart hospital bed [9], whose architecture resembles our solution. That sys-
tem, however, provided only basic identification features and lacked more advanced
knowledge-based capabilities. Our proposal takes a step further in this direction, by
combining a pervasive and context-aware computing framework with decision support
features based on Knowledge Representation (KR). Decision support to clinical activity
is widely acknowledged as one of the most important benefits of medical informatics
[10]. Research has also evidenced that artificial intelligence and rule-based systems can
be effective in helping clinicians to reduce errors in both diagnosis and treatment. Nev-
ertheless, the first generation of Computerized Physician Order Entry (CPOE) systems
was mostly based on manual data entry and a fragmented collection of non-integrated
utilities. Experience taught that, in such cases, the improvements in overall quality of
patient care are not always clear, since the decrease in some kinds of errors is counter-
acted by slowdownsin operations and an increased frequencyof other types of mistakes.
Decision support is highly effective only when it is automatic and seamless [10].
Ontology-based knowledge modeling can ensure that only highly relevant informa-
tion about patient’s clinical conditions and appropriate treatments are supplied to physi-
cians. The use of lightweight wireless computing infrastructures and of widely-adopted
KR technologies can promote interoperability and integration of solutions designed for
hospital centres hosting tele-medicine applications. Finally, as pointed out in [7], ubiq-
uitous computing technologies allow the capture of health data at an unprecedented
scale: knowledge-based approaches can assist in the management, analysis and inter-
pretation of such data for research purposes and/or to improve clinical best practices.
3 Inference Services for Decision Support in Healthcare
In the approach we propose here, non-monotonic inferences presented in [11] are ex-
ploited to retrieve suitable treatments for a given disease taking into account the case
history of the patient. The system will calculate a score, based on the semantic com-
patibility between diseases affecting the patient and characteristics of available drugs,
so allowing to: (i) find possible inconsistencies in a proposed therapy; (ii) arrange best
treatment options in relevance order; (iii) explain the matchmaking outcomes in both
cases.
In what follows, the Description Logics (DLs) setting we adopt are briefly recalled
3
.
We refer to [11,13] for several examples and wider argumentation. From now on we
assume to model ontologies (Terminological Boxes T in DL-words), patient diseases
and drugs annotations in a language whose semantics can be mapped to the ALN
3
We assume the reader be familiar with basics of DLs formalisms and reasoning [12].
81

DL, for instance (a subset of) OWL-DL
4
or the more compact XML-based DIG [14]
language.
DL-based systems provide two basic reasoning services for T , namely (a) Satis-
fiability and (b) Subsumption in order to check (a) if a formula C is consistent w.r.t.
the ontology –T 6|= C ⊑ ⊥– or (b) if a formula C is more specific or equivalent to a
formula D –T |= C ⊑ D. It is possible to define at least five different match classes
based on subsumption and satisfiability: exact, subsumption (full), plug-in, intersection
(potential) and disjoint (partial) match.
Both subsumption and satisfiability can be only used to check if there exists an ex-
act correspondence between two formulas. Hence they are not completely adequate in
scenarios like the healthcare ones, where simple yes/no answers are insufficient because
exact matches are quite rare. In the proposed approach, considering a disease descrip-
tion S and a drug annotation D, solving the Concept Contraction Problem (CCP) and
the Concept Abduction Problem (CAP) [11] (see later on for further details) we are able
to provide a support in decision making for doctors determining a therapy.
Hereafter basics of algorithms to solve abduction and contraction problems are re-
ported:
– given a partial match between D and S, solving a CCP one can compute what has
to be given up G and kept K in D in order to have a potential match between
K (a contracted version of D) and S. Hence, the result of a CCP is a pair hG, Ki
representing respectively elements in D conflicting with S and the (best) contracted
D compatible with S;
– given a potential match between D and S, solving a CAP one can compute what
has to be hypothesized in S in order to have a full match with D (or its contracted
version K). Hence, the result of a CAP is a concept H representing in some way
what is underspecified in S in order to completely satisfy D. Please note that we
say underspecified instead of missing. This is because we are under an Open World
Assumption.
Of course, for both Concept Contraction and Concept Abduction we have to define
some minimality criteria on G (giveup as few things as possible) and on H (hypothesize
as few things as possible). Algorithms to solve CAPs and CCPs for ALN have been
proposed in [11] (not reported here for brevity) and they have been properly adapted
and exploited in our e-healthcare scenario based on RFID.
3.1 Matchmaking for Healthcare
In the application presented here, Concept Contraction and Concept Abduction in-
ference algorithms are used in a slightly different fashion w.r.t. current matchmaking
problems. Patient case history and drug annotations have distinct structures and are dif-
ferently described with each other
5
, so inference services outlined above have to be
properly used to reach the desired goals.
4
OWL Web Ontology Language. http://www.w3.org/TR/owl-features/
5
The illustrative example presented in Section 4 will clarify these aspects.
82

With reference to classical matchmaking –especially devised for e-commerce [13]–
where a demand is compared with a set of supplies, in the proposed approach we have
to compare the drug annotation stored within the packaging tag with the patient clinical
description in her RFID wristband. Notice that the semantic-based matchmaking is a
non-symmetric one and the final purpose of our framework is to assess if a given drug
encounters patient’s diseases. This can be performed enriching the disease semantic an-
notation with the drugs classes suitable to cure the disease itself. In this way, Concept
Abduction allows to verify if a given treatment is suitable or not. For what concerns
contraindications, in the approach we propose treatments and disease descriptions are
modeled exploiting disjoint concepts in order to refer to interested organs and bodily
systems. Hence, if a given drug may present some undesired effects for a specific pa-
tient, the abduction check will fail due to the incompatibility between semantic descrip-
tions of drug and disease. So, thanks to Concept Contraction algorithm, the physician
can “see” the incompatibilities within a therapy annotation (i.e., the adverse indications
for the patient) which will make the part of the therapy to give up. The remaining K
component will be then used for a new abduction process.
Summarizing, the steps for therapy verification are reported hereafter:
1. the system perform a Concept Abduction between therapy description D and pa-
tient’s case history S;
2. if D ⊓ S are satisfiable w.r.t. T , the proposed therapy is verified by the system;
3. if D ⊓ S are incompatible, Concept Contraction algorithm allows to extract the
contraindications of the treatment for building a new compatible request to be sub-
mitted against the drugs in the hospital Knowledge Base (KB), in order to find a
new pharmaceutical D *;
4. the system performs a new Concept Abduction between D * and S to verify the
new therapy.
Note that step 3 returns a list of further options in a relevance order. By means
of rankPartial and rankPotential algorithms [11], the system measures the seman-
tic distance (i.e., the compatibility level) between each treatment annotation and the
description of the patient case history. Results are arranged according to the semantic
correspondence with the disease.
4 Case Study
The proposed approach was tested in a case study for a hospital rheumatology unit.
A specific ontology was devised for connective tissue diseases, an important class of
autoimmune rheumatic diseases. Figure 1 shows a relevant excerpt of it, reported in
classical DL notation for the sake of readability.
The prototypical system simulates a “smart bed” tablet computer, equipped with a
touchscreen and an RFID reader (see Figure 2 for details). The device connects to the
HIS through semantic-enhanced Bluetooth Service Discovery Protocol, via a hotspot
placed in the ward within radio range of beds. Each resource (patient, staff member and
drug) is identified by means of an RFID tag with unique EPC code, unique identifier of
83

Anatomy
- Immune System ⊑ Anatomic P art
- Adverse Immune System ⊑ Anatomic P art ⊓ ¬Immune System
- Circulatory System ⊑ Anatomic P art
- Adverse Circulatory System ⊑ Anatomic P art ⊓ ¬Circulatory System
- Skeletal System ⊑ Anatomic P art
- Muscular System ⊑ Anatomic P art
- Adverse Skeletal System ⊑ Anatomic P art ⊓ ¬Skeletal System
- Adverse M uscular System ⊑ Anatomic P art ⊓ ¬Muscular System
- Bone ⊑ Skeletal System
- Adverse Bone ⊑ ¬Bone ⊓ Adverse Skeletal System
- V isual System ⊑ Anatomic P art
- Adverse V isual System ⊑ Anatomic P art ⊓ ¬V isual System
- Eye ⊑ V isual System
- Adverse Eye ⊑ Adverse V isual System ⊓ ¬Eye
Diseases
- Disease ⊑ ∃aff ects
- Musculoskeletal System Disease ⊑ Disease ⊓ ∀aff ects.(Adverse Skeletal System ⊓
Adverse M uscular System)
- Immune System Disease ⊑ Disease ⊓ ∀affects.Adverse Immune System
- Autoimmune Disease ⊑ Immune System Disease
- Connective T issue Disease ⊑ Autoimmune Disease
- Systemic Lupus Erythematosus ⊑ Connective T issue Disease⊓ ∀af f ects.(Adverse Integumentary System⊓
Adverse Hematopoietic System ⊓ Adverse Joint ⊓ Adverse Kidney ⊓ Adverse N ervous System ⊓
Adverse Lung ⊓ Adverse M uscle ⊓ Adverse Gastrointestinal T ract ⊓ Adverse Circulatory System)
- Severe SLE ⊑ Systemic Lupus Erythematosus ⊓ ∀therapy.(N SAID ⊓ P lasmapheresis ⊓ Corticosteroid ⊓
Immunomodulator Immunosuppressant)
- M ild SLE ⊑ Systemic Lupus Erythematosus ⊓ ∀therapy.(N SAID ⊓ Corticosteroid ⊓
Immunomodulator Immunosuppressant)
Treatments
- Drug ⊑ T reatment
- P hysiotherapy ⊑ T reatment
- P lasmapheresis ⊑ T reatment
- N SAID ⊑ Drug
- Corticosteroid ⊑ Drug
- Immunomodulator Immunosuppressant ⊑ Drug
- Anti T N F alpha ⊑ I mmunomodulator Immunosuppressant
Fig.1. Excerpt of the ontology engineered for the case study.
Semantic
-
enhanced RFID
INFERE
Semantic
enhanced RFID
INFERE
RFID
RFID
Reader
WNIC
Drug’s RFID tag
Patient’s RFID tag
Fig.2. System architecture.
the reference ontology (OUUID), semantic-based annotation in compressed DIG format
and data-oriented resource attributes [1].
The key capabilities of the system (control of drug submission procedures and de-
cision support to the physician for therapy management) will be better explained by
means of a small example. The smart bed hosts a patient with a mild form of Systemic
Lupus Erithematosus (SLE) and a generic disease of the muscular and skeletal system.
This is expressed w.r.t. the reference ontology with the following ALN formula:
S: Mild SLE ⊓ M usculoskeletal System Disease
84
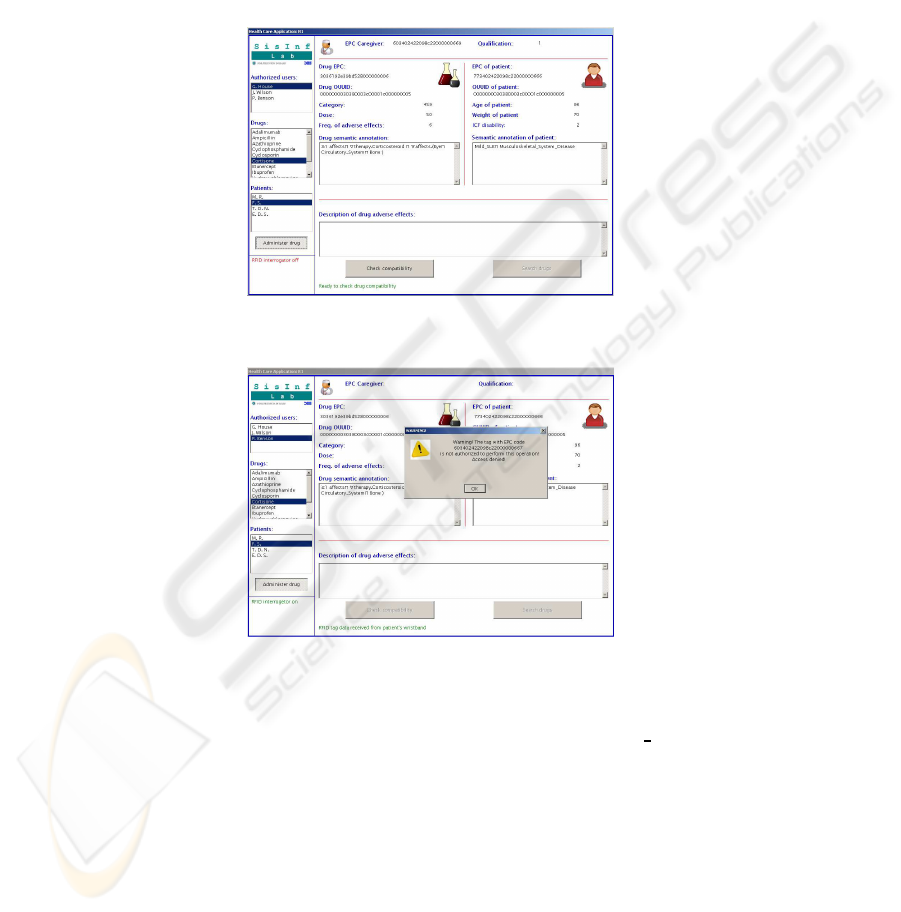
A rheumatologist approaches the bed to give cortisone to the patient. The RFID
reader detects the triple {patient, staffmember,drug} so that the display is activated:rel-
evant information extracted from tags is shown and the authorized operation is recorded
into the HIS. Figure 3 shows the output in our current prototype (the panel on the left
hand side allows to computer simulate tag reading events). If an unauthorized staff
member –e.g., a janitor or a physician from another ward– approached the bed with
a drug, then the system would provide a warning as shown in Figure 4 and record the
event into the HIS log.
Fig.3. Prototype display: access granted.
Fig.4. Unauthorized access is detected.
Cortisone is described in the KB as:
D: ∃af fects ⊓ ∀therapy.Corticosteroid⊓ ∀affects.Eye ⊓ Circulatory System⊓ Bone
so it has potential adverse effects towards eyes, bones and circulatory system. Our pa-
tient has no eye problems, but a skeletal disease, while SLE can affect the circulatory
system. Special care must be taken for this patient during treatment of SLE with corti-
sone. The system is capable to perform this inference automatically and issue a warning
to the doctor. Figure 5 shows the warning, with an alert and the description of the con-
flicting characteristics in a box in the lower part of the display. Further decisions are
then left to the judgment of the human expert. Steps 1 and 2 of the sequence in Section
85
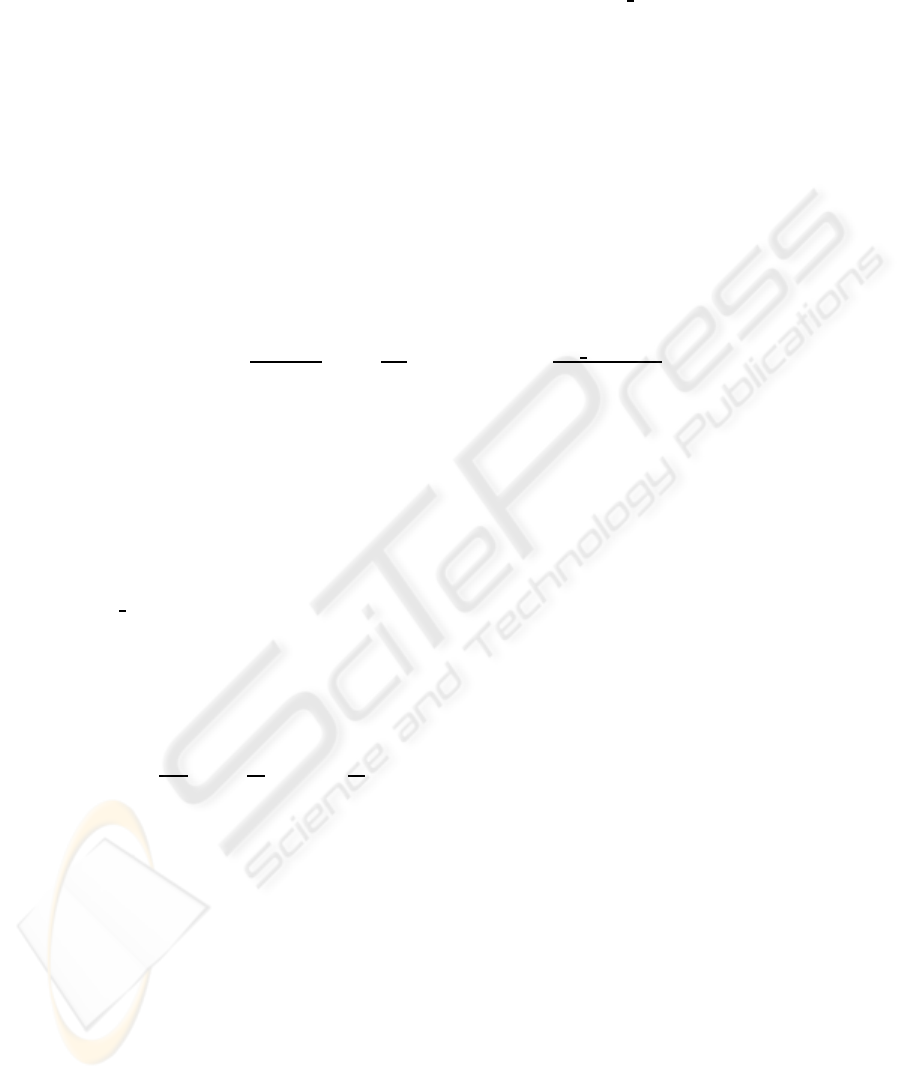
3.1 produce the following outcome: Give up: ∀affects.Circulatory System ⊓ Bone
Keep: ∀therapy.Corticosteroid ⊓ ∀af f ects.Eye
The physician can now query the system for other therapy options. The smart bed
computer sends via Bluetooth a request to the ward hotspot. For each drug in the hos-
pital KB, rankPotential is computed w.r.t. the patient’s description. If compatibility
arises, rankPartial is computed to extract and evaluate the incompatible part of the de-
mand, then rankPotential is computed again for the “Keep” part.
In order to improve flexibility of decision support, the matchmaking framework ex-
plained in Section 3 is combined with context-specific variables by means of an overall
utility function. The following parameters are taken into account: (1) age of patient;
(2) estimated frequency of drug adverse effects; (3) severity of patient’s condition, ex-
pressed in a numeric scale from 0 to 4 according to guidelines of the International
Classification of Functioning, Disability and Health framework issued by World Health
Organization [15]. The utility function has the following formula:
f
u
=
r
par
+r
pot
max
r
pot
· tanh
age
α
· sever ity · tanh
adv frequency
β
The function was modeled as a distance measure, hence a lower value means a bet-
ter overall match. The first factor allows different drugs to be ranked according to their
compatibility w.r.t. patient’s conditions: r
par
and r
pot
are the rankPartial and rankPo-
tential values between the drug and the patient, while max
r
pot
is the highest (worst)
rankPotential considering all drugs in the hospital KB (i.e., the less effective treatment).
The next components take patient’s age and severity into account: a younger patient or
with a lower impairment level will tolerate therapy better in general (the model is not
defined for pediatric patients). The last factor models drug contraindications, where
adv f requen c y is the statistical frequency of the main adverse effects, expressed in
number of occurrences per 100 patients. Empirical evaluation has suggested values for
the two tunable weights α = 50 and β = 10 respectively.
Let us suppose the patient is 96 years old and has an overall impairment degree of
2. Cortisone has a 6% frequency of adverse effects. With respect to the patient, cortisone
has a rankPartial value of 4 and its compatible part has a rankPotential of 7, whereas
the maximum rankPotential among all drugs in the KB is 9. Hence the final outcome
is: f
u
=
4+7
9
· tanh
96
50
· 3 · tanh
6
10
= 1.2575
Iteration of the procedure over each drug in the KB produces the ranking depicted in
Figure 6, which is shown to the physician. We notice that prednisone is quite similar to
cortisone (both are corticosteroids), having the same risks toward the particular patient;
however, its estimated frequency of adverse effects is lower (5% vs 6%), hence it is
preferable.
The physician selects an appropriate therapy and leaves. RFID reader detects the
event and the application screen is closed.
Even though expressiveness of the logic language is limited by the need to provide
acceptable reasoning performance, the example showed that careful modeling of the
domain can provide useful knowledge-based decision support. Our prototypical DSS
system helps the domain expert in an unobtrusive way, by automatically invoking infer-
ence procedures upon relevant fragments of knowledge extracted directly from RFIDs.
86
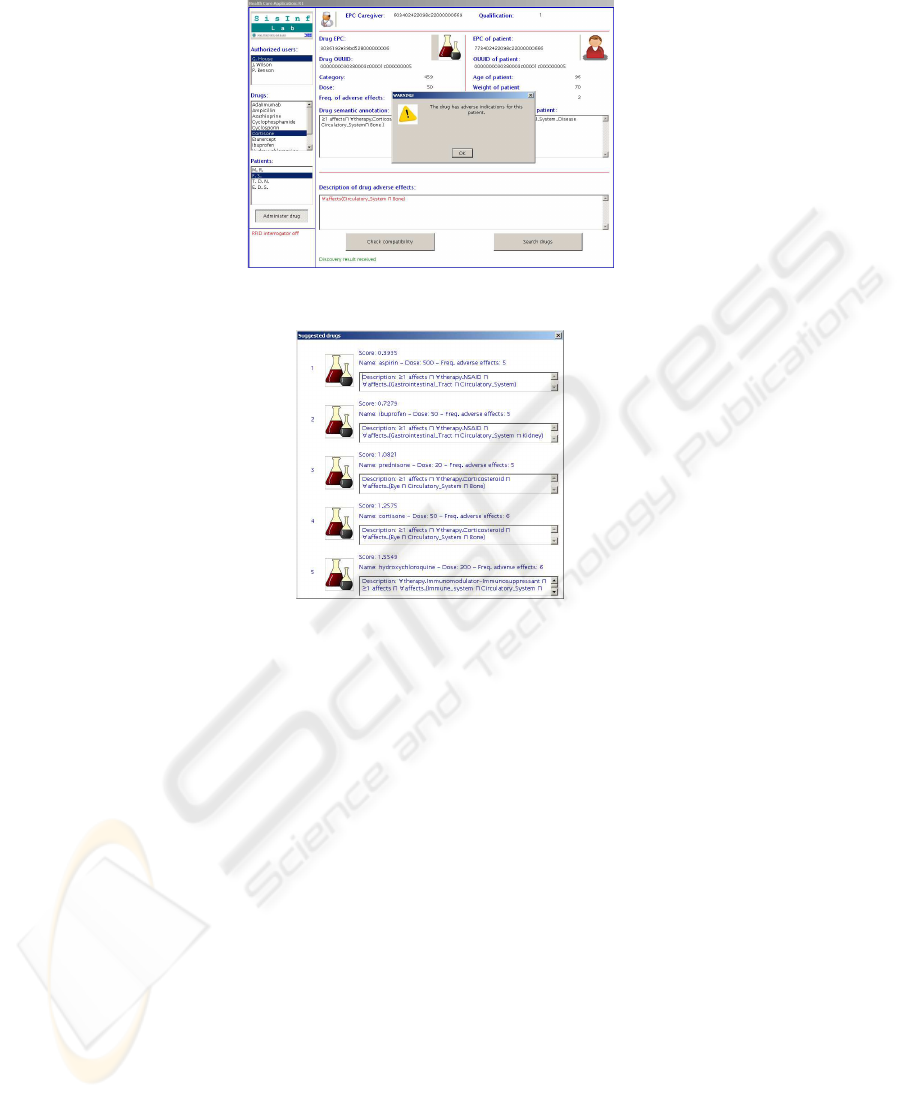
Fig.5. Physician is alerted of potential adverse effects.
Fig.6. Suggested drugs for the patient, in relevance order.
5 Conclusions and Future Work
We have presented a novel DSS for healthcare purposes based on a semantic enhance-
ment of RFID standard protocol. The proposed system exploits semantic annotations
of drugs to be administered as well as of patient’s case history, to help medical person-
nel in providing the correct therapy. Thanks to the semantic metadata accompanying
the description of both drugs (stored on RFID tags attached to packaging) and diseases
(saved on patient’s RFID wristband), it is possible to discover possible inconsistencies
in a therapy and to suggest alternative care options.
Future work includes: wider tests on the proposed methods; extension of the pro-
totype to support multiple hospital rooms and beds; improvement of the user interface,
possibly with guidance by medical personnel without specific computing knowledge.
Acknowledgements
The authors are grateful to Crescenzio Scioscia (M.D. at Rheumatology Unit, Depart-
ment of Internal Medicine and Public Health, University of Bari) for guidance in the
87

modeling of the problem domain and acknowledge partial support of Apulia Region
Strategic Project PS 121.
References
1. Di Noia, T., Di Sciascio, E., Donini, F.M., Ruta, M., Scioscia, F., Tinelli, E.: Semantic-based
bluetooth-rfid interaction for advanced resource discovery in pervasive contexts. Interna-
tional Journal on Semantic Web and Information Systems (IJSWIS) 4 (2008) 50–74
2. Sbrenni, S., Piazza, T., Farinella, E.: La tracciabilit`a del paziente in strutture ospedaliere.
Notiziario dell’Istituto Superiore di Sanit`a 20 (2007) 3–8
3. Sangwan, R., Qiu, R., Jessen, D.: Using RFID tags for tracking patients, charts and med-
ical equipment within an integrated health delivery network. In: Networking, Sensing and
Control, 2005. Proceedings. IEEE. (2005) 1070–1074
4. Holzinger, A., Schwaberger, K., Weitlaner, M.: Ubiquitous Computing for Hospital Appli-
cations: RFID-Applications to Enable Research in Real-Life Environments. In: 29th Annual
International Computer Software and Applications Conference, 2005 – COMPSAC 2005.
Volume 2. (2005)
5. Fry, E., Lenert, L.: MASCAL: RFID Tracking of Patients, Staff and Equipment to Enhance
Hospital Response to Mass Casualty Events. In: AMIA Annual Symposium Proceedings.
Volume 2005., American Medical Informatics Association (2005) 261
6. Wang, S., Chen, W., Ong, C., Liu, L., Chuang, Y.: RFID Application in Hospitals: A Case
Study on a Demonstration RFID Project in a Taiwan Hospital. In: Proceedings of the 39th
Annual Hawaii International Conference on System Sciences, 2006 – HICSS’06. Volume 8.
(2006)
7. Ho, L., Moh, M., Walker, Z., Hamada, T., Su, C.: A prototype on RFID and sensor networks
for elder healthcare: progress report. In: Proceedings of the 2005 ACM SIGCOMM work-
shop on Experimental approaches to wireless network design and analysis, ACM New York,
NY, USA (2005) 70–75
8. Jovanov, E., Milenkovic, A., Otto, C., de Groen, P.: A wireless body area network of intel-
ligent motion sensors for computer assisted physical rehabilitation. Journal of NeuroEngi-
neering and Rehabilitation 2 (2005) 1–10
9. Bardram, J.: Applications of context-aware computing in hospital work: examples and design
principles. In: Proceedings of the 2004 ACM symposium on Applied computing, ACM New
York, NY, USA (2004) 1574–1579
10. Handler, J., Feied, C., Coonan, K., Vozenilek, J., Gillam, M., Peacock, P., Sinert, R., Smith,
M.: Computerized Physician Order Entry and Online Decision Support. Academic Emer-
gency Medicine 11 (2004) 1135–1141
11. Di Noia, T., Di Sciascio, E., Donini, F.: Semantic matchmaking as non-monotonic reasoning:
A description logic approach. Journal of Artificial Intelligence Research (JAIR) 29 (2007)
269–307
12. Baader, F., Calvanese, D., Mc Guinness, D., Nardi, D., Patel-Schneider, P.: The Description
Logic Handbook. Cambridge University Press (2002)
13. Colucci, S., Di Noia, T., Pinto, A., Ragone, A., Ruta, M., Tinelli, E.: A non-monotonic
approach to semantic matchmaking and request refinement in e-marketplaces. International
Journal of Electronic Commerce 12 (2007) 127–154
14. Bechhofer, S., M¨oller, R., Crowther, P.: The DIG Description Logic Interface. In: Proceed-
ings of the 16th International Workshop on Description Logics (DL’03). Volume 81 of CEUR
Workshop Proceedings., Rome, Italy (2003)
15. Organization, W.H.: International Classification of Functioning, Disability and Health.
World Health Organization, Geneva (2001)
88
