
POLYISOPRENE – NANOSTRUCTURED CARBON COMPOSITE
(PNCC) MATERIAL FOR VOLATILE ORGANIC
COMPOUND DETECTION
Gita Sakale, Maris Knite, Valdis Teteris
Institute of Technical Physics, Riga Technical University, Azenes iela 14/24, Riga, Latvia
Velta Tupureina
Institute of Polymer Materials, Riga Technical University, Riga, Latvia
Keywords: Polymer-high structured carbon black composite, Volatile organic compound sensors.
Abstract: Our scientific group has chosen the elaboration of conductive composite material, which could be useful for
volatile organic compound detection, as one of research areas. It was found out that the most sensitive
composite material consists of polyisoprene and 10 mass parts of nanostructured carbon black. The electric
resistance changes of the composite in presence of 10 different saturated organic solvents vapour were
measured. Results obtained form our mass-sorption experiments indicated that electrical resistance of the
composite increases because of volatile organic compound (VOC) molecule absorption in the composite
matrix material. We also evaluated VOC compatibility with PNCC matrix material and estimated how the
PNCC resistance change velocity (
R
v ) versus organic solvent vapour molecule diameter varies.
1 INTRODUCTION
Available statistical data evidence about people
exposed to organic solvent daily at their workplaces,
but there are no monitoring devices used to control
VOC concentration in the room. There is also a
necessity to protect environment and equipment
from exposure to VOC. Above mentioned denotes
that there is an urgent need for VOC sensor
materials.
Devices (sorbent polymer films, metal oxide
semiconductors, quartz microbalance (quartz
resonator), laser gas sensors ect.) in the market can
not still be compared with mammal’s olfactory
system. Scientists are trying to design a prototype of
sensor which in sensing capability of different gases
could be close to mammals olfactory system and
even could be improved for practical applications.
We think that the desirable result of VOC
detection can be achieved by using polymer –
carbon black composites as gas sensor materials
because polymer matrix can be selected for direct
volatile compound detection and identifying.
In our opinion a candidate sensor material for gas
sensing should fulfil the following criteria: not
expensive constituent materials; simple production;
fast and reversible response; in-situ control of VOC;
small dimensions of sensing element and ability to
function for a long period of time.
The mechanism how polymer – carbon black
composite detects VOC is as fallows: i) the sample
of the composite material is exposed to VOC,
molecules of VOC adsorbe on the surface of
composite and diffuse into the matrix material; ii)
intermolecular chains in the polymer matrix weaken
and form intermolecular chains between VOC
molecules and macromolecules of matrix material;
iii) the matrix material swells; iv) electroconducting
pathways break down because distance between
carbon black aggregates increases; v) at the same
time tunnelling currents between carbon aggregates
in thin layers of matrix decreases and the electrical
resistance of the composite increases.
2 EXPERIMENTAL
Polyisoprene – nanostructured carbon composite
material was made by rolling highly structured nano-
size carbon black PRINTEX XE2 (specific surface
117
Sakale G., Knite M., Teteris V. and Tupureina V. (2009).
POLYISOPRENE – NANOSTRUCTURED CARBON COMPOSITE (PNCC) MATERIAL FOR VOLATILE ORGANIC COMPOUND DETECTION.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 117-122
DOI: 10.5220/0001557301170122
Copyright
c
SciTePress
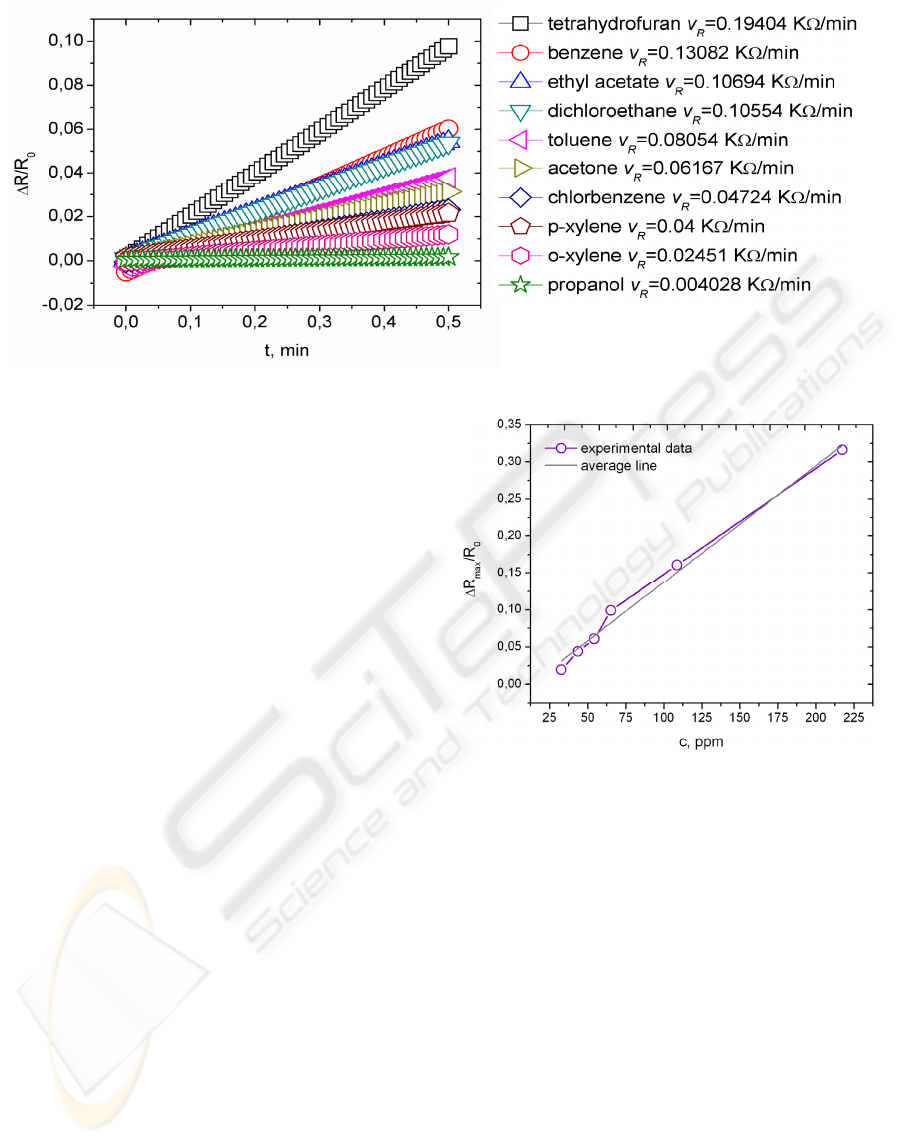
Figure 1: The change of relative resistance vs. time for the sample, held in saturated vapours of different solvents.
950 m
2
/g, mean diameter of primary particles 25 nm,
DBP absorption 380 ml/100g) and necessary
additional ingredients – sulphur and zinc oxide –
into a Thick Pale Crepe No 9 Extra polyisoprene
matrix using cold rolls. Then follows vulcanization
process (under 30BAR pressure at 150˚C for 15
minutes) when not only sulphur crossbonds form but
also possibly chemical bonds between carbon black
nanoparticles and matrix macromolecules form. As
better carbon black particle electrocodutive grid is
connected with polyisoprene macromolecules as
better sensing element to any kind of deformation is
achieved.
Preparation of samples and schematic illustration
of the experimental set-up is described in (Knite,
2007).
2.1 The Change of PNCC Electrical
Resistance Due to VOC Presence
Samples were exposed to vapour for 30 seconds and
then held in open air for relaxation processes to let
go on until the composite material reaches its initial
resistance R
0
. Then the measurement was repeated.
Composite material response to different saturated
organic solvents vapour can be seen in Fig. 1. In the
figure
R
v
denotes electrical resistance change
velocity (KΩ/min). It is obvious that the largest
resistance change velocity is obtained when PNCC
is exposed to tetrahydrofuran vapour followed by
benzene, ethylacetate and dichloroethane ect. In the
case of propanol vapour PNCC resistance did not
change at all. The composite can not sense the
presence of propanol vapour. In these experiments
PNCC samples with dimensions 50 x 5 x 1 mm
(length x width x thickness) were used.
Figure 2: Resistance change of PNCC vs. toluene vapour
concentration.
We carried out experiments with PNCC samples
with dimensions 50 x 5 x 0,25 mm also. The
composite capability to sense different toluene
vapour concentrations were tested (Fig. 2.).
Resistance of PNCC increases proportionally to
toluene vapour concentration. Here we would like to
mention that PNCC can sense concentrations which
are equal to or lower then TWA limits (TWA
indicates a time-weighted average concentration for
up to a 8-hour workday during a 40-hour workweek)
(http://www.cdc.gov). In the case of toluene vapour
TWA limit is 200 ppm.
2.2 The Relaxation Process of PNCC
Electrical Resistance
After samples were subjected to saturated organic
solvents vapour electrical resistance relaxation
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
118
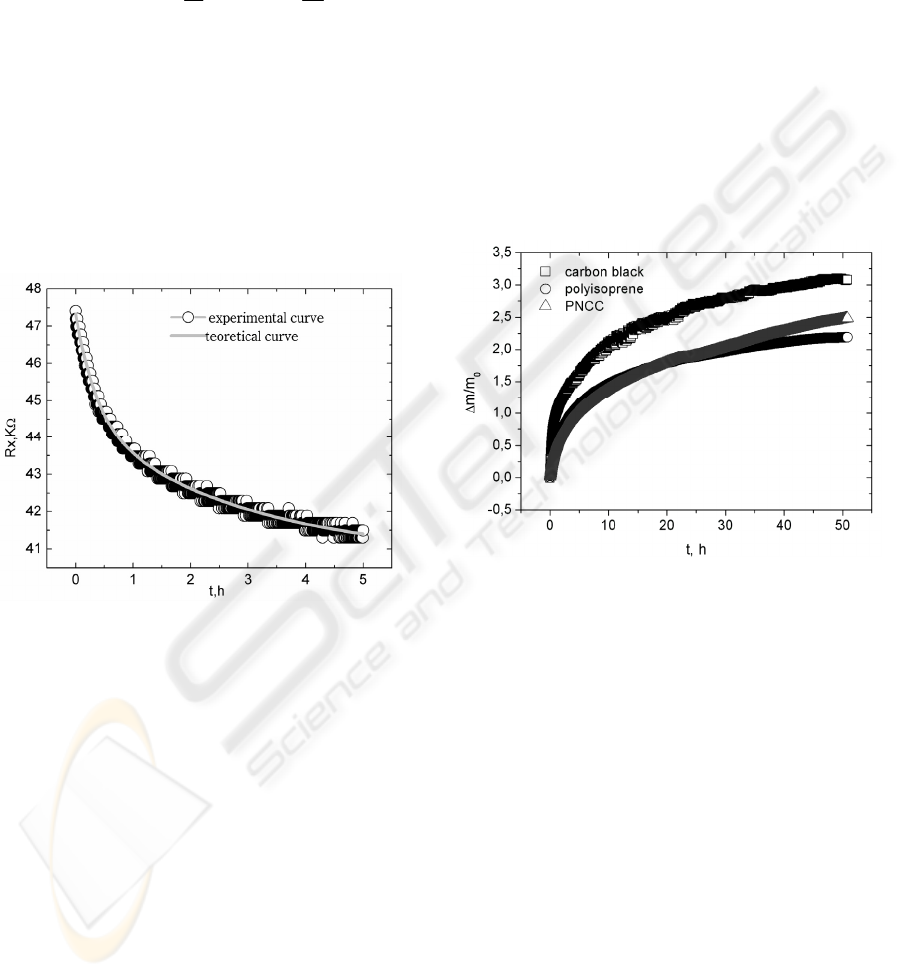
process in air were carried out. Typical PNCC
sample with thickness 1mm relaxation process is
shown in Fig.3.
Experimentally obtained data were fitted with
theoretical curve which can be characterised by
equation:
01 2
12
exp( ) exp( )
tt
RR A A
τ
τ
=+ − + −
(1)
, where
R
0
– initial electrical resistance
R – transient electrical resistance
A
1
, A
2
– constants
τ
1
, τ
2
– relaxation time (h).
From equation (1) we calculated relaxation time τ
1
and τ
2
. For all tested organic solvent vapour the
relaxation process can be divided into fast and slow
processes.
Figure 3: Electrical resistance relaxation of specimen in
open air. The sample was exibited to tetrahydrofuran for
30s.
In the case of 250μm thick samples electrical
resistance relaxation process is about one hour if the
sample has been exposed to 108,7 ppm toluene
vapour concentration.
2.3 Mass – sorption Experiments
Experiments of the change of the sample mass as a
function of time the sample is kept in organic
solvent vapour were used to find out the mechanism
of the change of resistance. For example, specimens
of pure polyisoprene, PNCC, and a pellet of
compressed high-structured carbon black powder
were held in toluene vapour for ~ 48 hours and a
mass as a function of time (sorption curve) was
recorded (Fig. 4.). Sorption of vapour in carbon filler
in the initial period (first 15 min) is approximately
three times (approximately 1,5 times after up to 104
min) as big as that of pure polyisoprene rubber. Yet
the sorption of vapour in PNCC material in the
initial period is around 1,3 times shorter if compared
with pure polyisoprene rubber, although it seems
that carbon filler should increase the vapour sorption
in the PNCC material. That can be explained, firstly,
by the fact that in the PNCC composition there are
only 10 mass parts of carbon and, secondly, in
processing (mixing and vulcanizing) the PNCC
compounds insulating polyisoprene layers are
formed between the carbon nano-particles. So, even
near the percolation threshold (10 mass parts of
carbon), when electro-conductive channels are
formed, very thin polyisoprene intermediate layers
between the nano-particles still exist and tunnelling
currents may emerge between the channels.
Figure 4: The change of mass for three materials
(polyisoprene rubber, HSCB and its composite) as
functions of the time of exposure to toluene vapour.
Only after approximately 28 h the sorption of vapour
by the PNCC material noticeably exceeds the
sorption by polyisoprene rubber, which indicates
that only after this period of time the sorption in the
filler begins to play substantial role. Consequently,
1) the obtained result indirectly proves the existence
of quantum effect – tunnelling currents in the PNCC
material, 2) sorption of vapour in the polyisoprene
matrix plays absolutely uppermost role in effect of
gas sensing (due to sorption of vapour molecules
and swelling of the matrix the distance between
carbon nano-particles increases and tunnelling
currents rapidly decrease) (Knite, Shakale 2007).
POLYISOPRENE – NANOSTRUCTURED CARBON COMPOSITE (PNCC) MATERIAL FOR VOLATILE ORGANIC
COMPOUND DETECTION
119
3 DISCUSSION
3.1 Electrical Resistance as a Function
of VOC Molecule Diameter
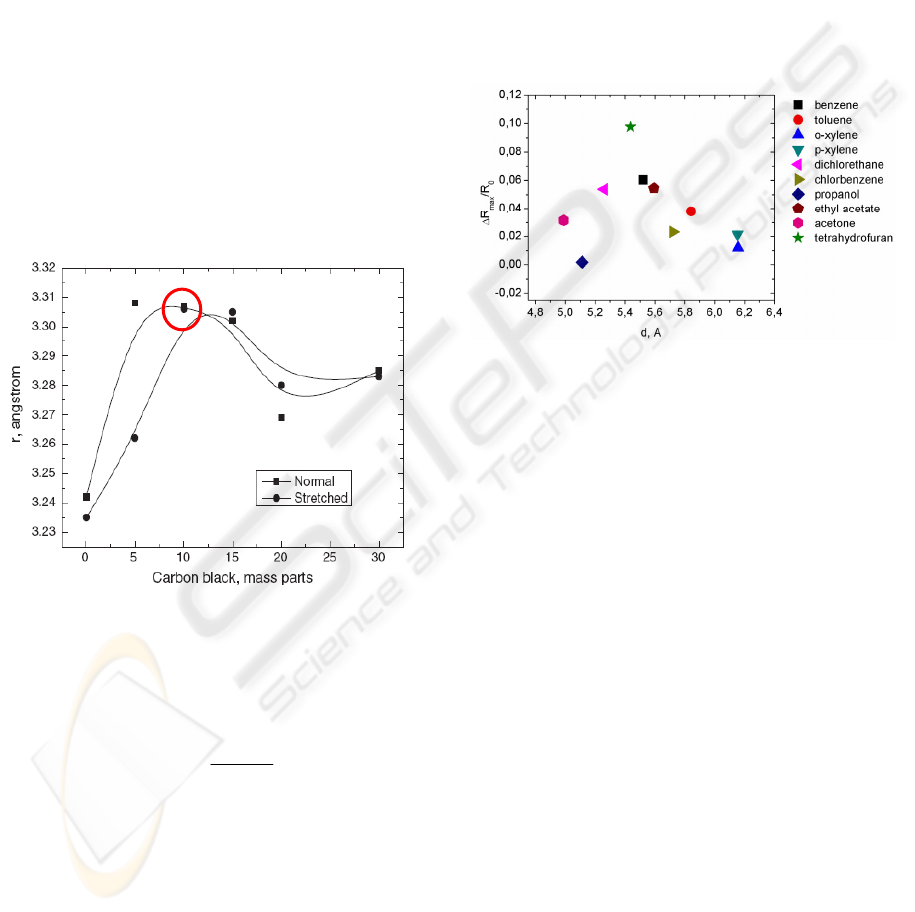
Previously we have made positron annihilation
lifetime spectroscopy measurements for PNCC in
collaboration with scientists from Monash
University in Australia (Knite, 2006). The purpose
of these experiments was to evaluate free volume
cavities dimensions in the composite material when
it is stretched and in normal (unloaded) state. As it
can be seen from Fig. 5 free volume mean radius for
PNCC containing 10 mass parts of carbon black in
normal state is 3,305Å. Then diameter of free
volume cavities is 6,61Å. When molecule diameters
of VOC are smaller than the diameter of free volume
cavities in the composite material then there is no
need for extra activation energy for molecule
diffusion into the matrix material.
Figure 5: Mean radius of free volume cavities in PNCC as
a function of carbon black content in normal and stretched
(Δl =15 mm) states measured by PALS.
Molecule volume of VOC was calculated using
equation (Askadcli, 1983):
ρ
⋅
⋅
=Δ
∑
A
i
i
N
Mk
V
(2)
, where
k – Atoms packing density
N
A
– Avogadro number, mol
-1
∑
Δ
i
i
V - Van der Valse volume of VOC molecule,
which consists of discrete atom volume sum, cm
3
M – Molar mass of VOC, g/mol
ρ
– Density of VOC, g/cm
3
.
After calculation of VOC molecule volume we
accepted approximation that all molecules take the
form of sphere. Finally we calculated diameters of
VOC molecules. We suppose with increasing
molecule diameter of organic solvent vapour the
maximum PNCC electrical resistance change
ΔRmax/R0 should be decreased.
In Fig. 6 we can see that above mentioned
realizes only partly. ΔRmax/R0 increases with
increasing molecule diameter starting from acetone
vapour until tetrahydrofuran vapour. In our opinion
this means that ΔR
max
/R
0
is not dependent only from
organic solvent vapour molecule dimensions.
Figure 6: Electrical resistance change of PNCC vs. organic
solvents vapour molecule diameter.
ΔR
max
/R
0
decreases with increasing molecule
diameter of organic solvents vapour in Fig. 6 from
tetrahydrofuran vapour until o- and p-xylene vapour.
In the case of toluene and chlorbenzene an exception
has to be made. Toluene vapour causes greater
ΔR
max
/R
0
change than chlorbenzene vapour, while
molecule diameter of toluene molecule is lager than
that of chlorbenzene.
3.2 VOC Compatibility with Polymer
Matrix Material
The law “like dissolves the like” is already well
enough known. Polar solvents dissolve in polar
solvents and analogous non-polar dissolve in non-
polar solvents. This also can be attributed to organic
solvents and polymer materials.
To evaluate organic solvents compatibility with
polyisoprene matrix material we compared solvents
dielectric permeability (ε) values to polyisoprene ε
value. As ε value of organic solvents is closer to
polyisoprene ε as the matrix material better absorbs
solvent molecules and then greater
R
v
change is
observed.
From literature data (Brandrup, 1989) we know
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
120
that polyisoprene dielectric permeability value is
2,68. Organic solvent vapour dielectric permeability
values are summarized in Table 1.
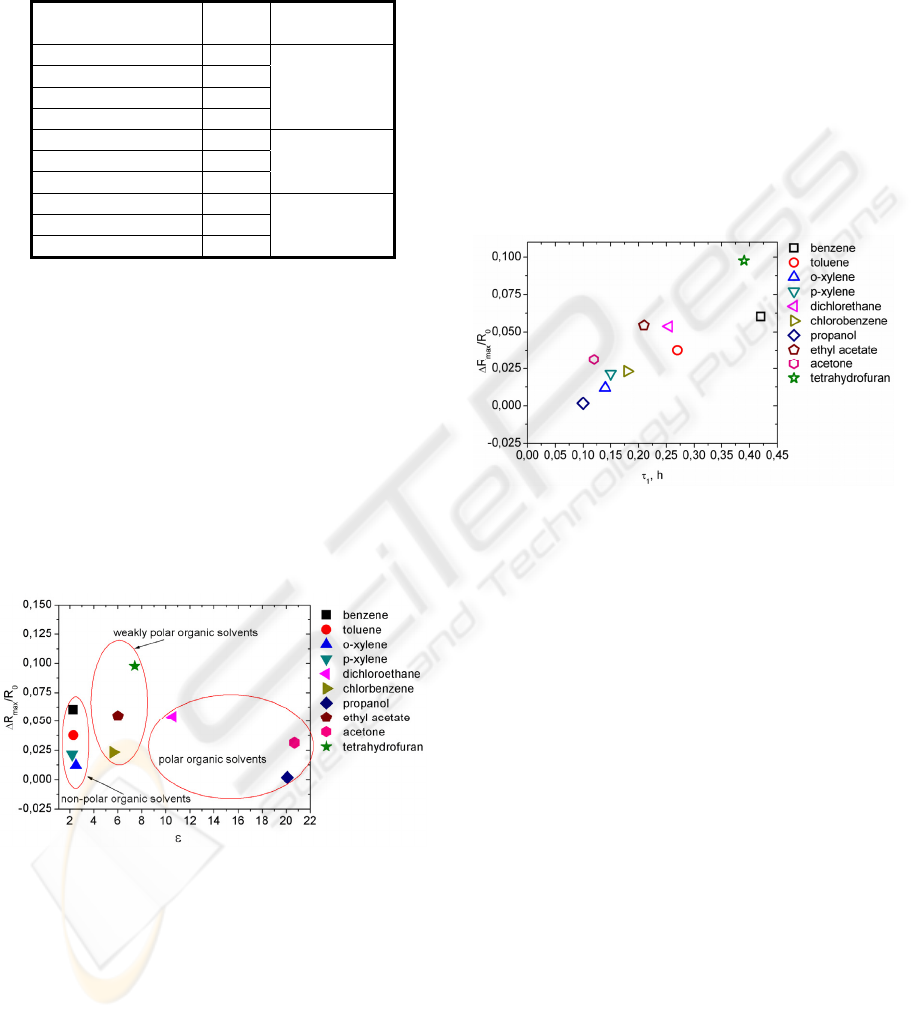
Table 1: VOC dielectric permeability values.
Substnace ε
Type of
solvent
P-xylene 2,2
Non-polar
Benzene 2,3
Toluene 2,3
O-xylene 2,5
Chlorobenzene 5,6
Weakly polar Ethyl acetate 6
Tetrahydrofurna 7,4
Dichloroethane 10,6
Polar Propanol 20,1
Acetone 20,7
From Fig. 7 we can see that PNCC resistance
response to organic solvent vapour is concentrated
into three groups. First let us begin with polar
organic solvents group. As we can see in this group
small electric resistance change is observed while
molecule dimensions of these solvents are the
smallest from all tested organic solvents. It can be
explained by acetone, propanol and dichlorethane
vapour non-compatibility with the composite matrix
material. Thus, we can conclude that resistance
change in this group is more dependent of organic
solvents vapour compatibility with the composite
matrix material.
Figure 7: PNCC resistance change vs. organic solvents
dielectric permeability values.
We will continue discussion with weakly polar
and non-polar solvents group. From Fig. 6 and Fig. 7
we can see that in these groups molecule dimensions
and dielectric permeability compensate each other
and both affect the composites response to organic
solvents vapour. For example, tetrahydrofuran
vapour molecule diameter is the smallest from these
two groups and the largest ΔR
max
/R
0
value is
obtained, while ε value is 7,4.
Above we compared toluene and chlorbenzene
vapour caused electrical resistance change versus
vapour molecule dimensions. If we look at Fig. 7
then we can see that chlorbenzene belongs to weakly
polar solvents group. Chlorbenzene vapour is less
compatible with the composite matrix material. For
that reason PNCC resistance change in chlorbenzene
vapour is smaller then in toluene vapour. If we
compare mass of molecule for toluene and
chlorbenzene then for toluene it is 15,30·10
-23
g and
for chlorbenzene it is 18,69
-23
g. From kinetic-
molecular theory we know that for molecules with
smaller mass the motion velocity is higher. This also
explains why resistance change of PNCC is larger in
the case of toluene vapour.
Figure 8: Relaxation time τ
1
vs. resistance change in
organic solvents vapour.
Composites organic solvent vapour sensing
mechanism is based on matrix swelling, which
causes increased distance between carbon black
aggregates and resistance change of the composite.
As larger is value ΔR
max
/R
0
as larger is amount of
organic solvent vapour molecules the composite has
absorbed and as longer should be relaxation process.
According to afore said relaxation time τ
1
and τ
2
should be proportional to ΔR
max
/R
0
change.
Concerning Fig. 8 we can say that τ
1
are nearly
proportional to ΔR
max
/R
0
, but there are also some
exceptions which are related to previously described
molecule dimensions and organic solvents
compatibility with polymer matrix material. But
from Fig. 9 we can not find any mathematical
relationship between ΔR
max
/R
0
change and τ
2
values.
We conclude that fast relaxation processes (τ
1
)
are determined by vapour molecule diffusion from
swelled matrix interior layers to the surface and
crosslinked macromolecule relaxation. Slow
relaxation processes (τ
2
) are related to
electroconductive grid relaxation made of carbon
black particles.
POLYISOPRENE – NANOSTRUCTURED CARBON COMPOSITE (PNCC) MATERIAL FOR VOLATILE ORGANIC
COMPOUND DETECTION
121
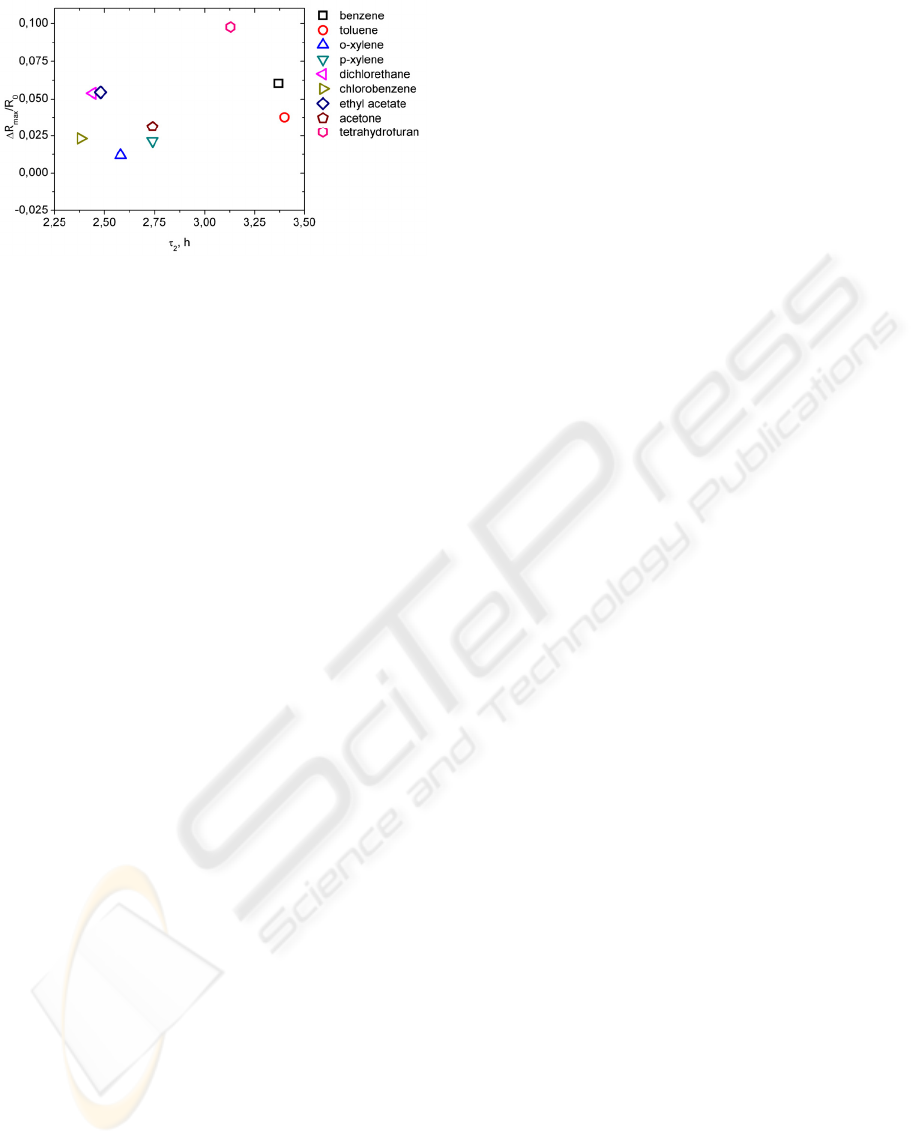
Figure 9: Relaxation time τ
2
vs. resistance change in
vapour of organic solvents.
4 CONCLUSIONS
In conclusion we can say that for the production of
PNCC we used not expensive and available
materials. The production of the composite is rather
simple if the production procedure is strictly
performed. When PNCC is exposed to VOC the
electrical resistance of the composite increases
rapidly and the effect is reversible. Electric
resistance change velocity is dependent of both VOC
molecule diameters and VOC compatibility with
PNCC matrix material.
Better sensitivity PNCC exhibits when it is
exposed to non-polar or weakly polar solvent
vapour. So, we can declare that PNCC sensing of
VOC is selective.
Further, in our work, we are going to try to
decrease relaxation time of electrical resistance to
couple of minutes or even seconds. We suppose that
PNCC can be used for VOC detection with some
improvements, for example, reducing dimensions of
the composite to optimal size. Else, functioning of
PNCC needs to be evaluated in long time period.
REFERENCES
Knite, M., Klemenok, I., Shakale., G., Teteris, V., and
Zicans, J., 2007. J. Alloys Comp.
Knite, M., Shakale, G., Klemenoks, I., Ozols, K., Teteris,
V., 2007. Journal of Physics: Conference Series 93,
012031.
Askadcli, A. A., Matveev, J. I., 1983. Chemical structure
and physical characteristics of polymers, Kimija:
Moscow.
Knite, K., Hill, A.J., Pas, S.J., Teteris, V., Zavickis, J.,
2006. Materials Science & Engineering C, V 26.
Brandrup, J., Immergut, E.H., 1989. Polymer handbook,
John Wiley&Sons: New York, Chichester, Brisbane,
Toronto, Singapore, 3
rd
edition.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
122
