
CONTROL OF CELL ADHESION AND FUNCTIONS
USING SELF-ORGANIZED HONEY COMB-PATTERNED
POLYMER FILMS
Masaru Tanaka
Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1Katahira, Aoba-ku, Sendai, Japan
Akinori Tsuruma, Sadaaki Yamamoto
Sadaaki Yamamoto Creative Research Initiative “Sousei” (CRIS), Hokkaido University, N20W10 Kita-ku, Sapporo, Japan
Masatsugu Shimomura
Institute of Multidisciplinary Research for Advanced Materials, and World Premier International Research Center
Tohoku University, 2-1-1Katahira, Aoba-ku, Sendai, Japan
Keywords: Self-organization, Honeycomb, Scaffold, Tissue engineering, Cell adhesion, Stem cell.
Abstract: The design of nano- and microstructures based on self-organization is a key area of research in the search
for new biomaterials and biodevices, and such structures have a variety of potential applications in tissue
engineering scaffolds and medical implants. 3D scaffolds of appropriate pore size and porosities and with
interconnected pores are required to facilitate cell adhesion, proliferation, differentiation, and eventual tissue
regeneration in a natural manner. We have reported the honeycomb-patterned polymer film with highly
regular pores that is formed by self-organization. The honeycomb films exerted a strong influence on cell
morphology, proliferation, cytoskeleton, focal adhesion, and ECM production profiles. Our studies
demonstrated that the neural stem / progenitor cells morphology, proliferation, and differentiation are
controlled by the pore size of the honeycomb film. It is expected that the honeycomb films will have great
potentials as biomaterials for tissue regeneration in a growth factor free proliferation process of stem cells.
1 INTRODUCTION
It is well established that surface topography
influences implant integration. Many in vitro studies
have extended these observations to cells in culture,
demonstrating that scaffold architecture and surface
chemistry considerably influence cell behavior.
Therefore, cell adhesion, proliferation, and
differentiation can be regulated by controlling
surface topography.
We have reported a honeycomb-patterned
polymer film (honeycomb film) with regular pores,
which is formed by self-organization. The
honeycomb films strongly affected cell morphology,
proliferation, cytoskeleton, focal adhesion, and
extracellular matrix (ECM) production profiles
(Tanaka, 2006., Tanaka, 2007., Yamamoto, 2007.,
Mcmillan, 2007., Tanaka, 2008., Arai, 2008.,
Tsuruma, 2008.). These studies were performed on
cells cultured in the absence of growth factors.
In neural tissue engineering, the preparation of
neural stem/progenitor cells (NSCs) is required for
the treatment of diseases of the nervous system
(Steinman, 2003). NSCs are self-renewing,
immature, undifferentiated, and multipotent cells.
They can differentiate into cells constituting the
central neural system, such as neurons, astrocytes,
and oligodendrocytes (Roberti, 2000., Wurmser,
2004., Wang, 2006.). The use of NSCs is a potential
therapy for diseases of the nervous system. In order
to increase the feasibility of this technique, viable
method, the preparation, culture, and seeding of
NSCs are steps that have to be carefully controlled.
The main problem associated with the use of stem
cells is that when these cells are extracted from an
390
Tanaka M., Tsuruma A., Yamamoto S. and Shimomura M. (2009).
CONTROL OF CELL ADHESION AND FUNCTIONS USING SELF-ORGANIZED HONEY COMB-PATTERNED POLYMER FILMS.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 390-393
DOI: 10.5220/0001545403900393
Copyright
c
SciTePress
individual, they start differentiating (specializing
into a specific cell type); thus, they lose their stem
cell characteristics. Thus, the reintroduction of these
cells into patients is problematic. In order to
overcome this problem, a culture environment
wherein the stem cells can be maintained in the
undifferentiated state is required. The proliferation
of self-renewing NSCs is required for cell therapy.
We studied the effects of the pore size of the
honeycomb films on the proliferation and
differentiation of NSCs.
2 MATERIALS AND METHODS
Honeycomb films were fabricated using
biodegradable polymers poly(ε-caprolactone) (PCL)
and a copolymer of dodecylacrylamide and ω-
carboxyhexylacrylamide. The honeycomb film was
prepared on a glass substrate by employing a
previously described method (Sato, 2002., Tanaka,
2004., Tanaka, 2007). The flat film was prepared by
a spin coater in dry condition. NSCs were derived
from the cerebral cortex of embryonic 14 day mice.
The NSCs were seeded on the films at a density of 2
× 10
4
cells/cm
2
. NSCs were cultured in serum
medium (Opti-MEM, 10 % Fetal Bovine Serum, 55
μM 2-mercaptoethanol) for 24 hr. After that, NSCs
were cultured in serum-free medium. The
morphologies of neurons were examined by a
scanning electron microscope (SEM) and a confocal
laser scanning microscope. NSCs were
immunostained for Nestin and BrdU. Cell number
was estimated by measuring of DNA concentration
from the extracted samples.
3 RESULTS AND DISCUSSION
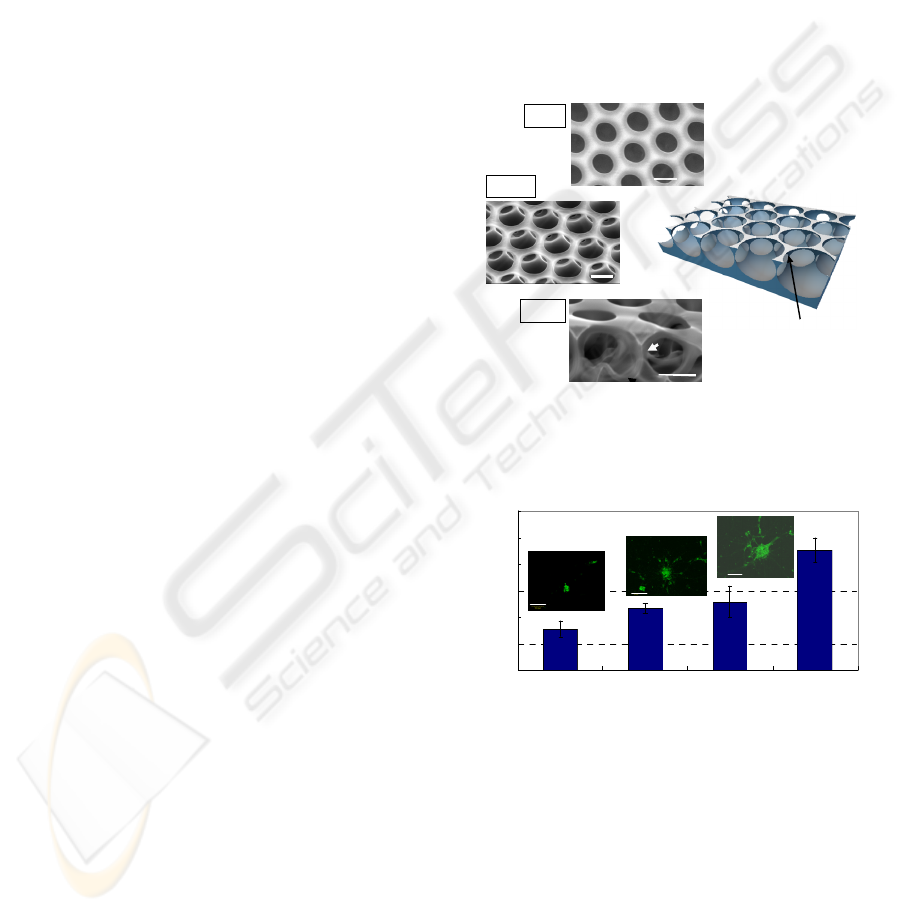
Scanning electron microscopy (SEM) revealed a
highly regular hexagonal arrangement of pores
(honeycomb pattern), and a well-interconnected,
uniform pore structure (Fig. 1a–d). NSCs cultured
on flat films differentiated into round neurons with
neurites that extended randomly on the film. The
morphology of cells on the honeycomb films
depended on the pore size of the films. On films
with a pore size of 1.5 μm, the cell bodies were flat.
Their neurites extended randomly and jumped over
the pore of the film. On films with a pore size of 10
μm, the cell bodies were round and the cells adhered
to the rims of the films. Their neurites extended
along the rims forming a simple network. The
honeycomb film provided positive cues to support
neurite extension. The cells on films with pore sizes
of 5 and 8 μm exhibited a morphology similar to that
of cells on films with a pore size of 10 μm.
Interestingly, on honeycomb films with a pore size
of 3 μm, several large spheroids were observed. The
neurites gathered to form large bundles, which
radiated out from the periphery of the spheroids.
NSCs on honeycomb films with a pore size of 3 μm
formed spheroids of diameter 30~90 μm (Fig. 2);
such spheroid formation was not observed for NSCs
either on other honeycomb films (with pore sizes of
1.5, 5, 8, and 10 μm) or on flat films.
Top
layer
Bottom
layer
Pillar
d
1
0 μm
10
μ
m
Tilted
5
μ
m
μm
5
m
Closs
Top
Pillar
Bottom
10 μ m10
μ
m
Top
(a)
(b)
(c)
(d)
Top
layer
Bottom
layer
Pillar
d
1
0 μm
10
μ
m
10
μ
m
μ
m
Tilted
5
μ
m
μm
5
μm
5
m
Closs
Top
Pillar
Bottom
10 μ m10
μ
m
Top
(a)
(b)
(c)
(d)
Top
layer
Bottom
layer
Pillar
d
1
0 μm
10
μ
m
Tilted
5
μ
m
μm
5
m
Closs
Top
Pillar
Bottom
10 μ m10
μ
m
Top
(a)
(b)
(c)
(d)
Top
layer
Bottom
layer
Pillar
d
1
0 μm
10
μ
m
10
μ
m
μ
m
Tilted
5
μ
m
μm
5
μm
5
m
Closs
Top
Pillar
Bottom
10 μ m10
μ
m
Top
(a)
(b)
(c)
(d)
Figure 1: SEM images of the honeycomb film in different
views. (a) Surface, (b) tilted, and (c) cross-section. (d)
Schematic representation of the 3D structure of the
honeycomb film.
Culture time (days)
Diamet er (μm)
0
20
40
60
80
100
120
35 710
3 days
7 days
10 days
Culture time (days)
Diamet er (μm)
0
20
40
60
80
100
120
35 710
3 days
7 days
10 days
Figure 2: Spheroids diameter on the honeycomb film (pore
size:3 µm). Fluorescent images indicate spheroids stained
for Nestin. Bar: 50 µm.
In order to characterize the cells on the flat and
honeycomb films, the cells were immunostained for
nestin and microtubule-associated protein 2 (MAP2)
and were labeled with bromodeoxyuridine (BrdU).
Nestin and MAP2 are selective markers for NSCs
and neurons, respectively. Nestin expression
decreases when NSCs differentiate and mature into
neurons. BrdU is selectively incorporated into the
nuclei of proliferating cells, and thus indicates cell
CONTROL OF CELL ADHESION AND FUNCTIONS USING SELF-ORGANIZED HONEY COMB-PATTERNED
POLYMER FILMS
391

growth. Immunostaining for nestin and labeling with
BrdU revealed that the spheroids were aggregates of
self-renewed NSCs. The diameter of the spheroids
increased with the culture time (Fig. 2).
Figure 3: The removability and phenotype of the spheroids
on the honeycomb film (pore size: 3 μm).
Figure 4: Comparison of our method for proliferation of
NSCs using honeycomb films with the conventional
neurosphere method.
These results implied that honeycomb films with
pore size less than the cell size promoted the
proliferation of NSCs, but prevented their
differentiation. We found that the number of total
neural cells increased after 3 days owing to the
maintenance of the undifferentiated state and to the
proliferation of NSCs.
In order to determine the removability of the
spheroids from honeycomb films and to ascertain the
phenotypes of the cells, cells obtained from the
spheroids were cultured in a standard culture dish
(Fig. 3). All cells of the spheroids adhered to the
culture dish. The cells extended neurites after 2 d
and were positive for MAP 2, suggesting their
differentiation into neurons. This result implied that
the cells in the spheroids could differentiate into
neurons and confirmed the finding of the
immunostaining experiment that the spheroids were
aggregates of self-renewed NSCs.
The conventional neurosphere culture method is
widely used for the proliferation of NSCs (Cattaneo,
1990., Louis, 2004). This technique involves the use
of serum-free culture medium supplemented with
growth factors (fibroblast growth factor-2: FGF-2
and/or epidermal growth factor: EGF) (Fig. 4). The
NSCs obtained by this method are expected to be
supplied to lost and dysfunctional nervous systems
in order to regenerate neural tissue. In this
technique, NSCs are cultured without the attachment
to a surface (floating culture) because the NSCs
immediately differentiate into neurons when they are
attached to the substrate surface. Improvements in
the neurosphere culture technique, which include the
use of U-bottomed wells, have recently been
reported (Mori, 2006). However, our technique
required neither growth factors nor the floating
culture system wherein the cells are not attached to a
surface.
In our technique, some NSCs were observed to
be encapsulated by the 3 μm pores immediately after
cell seeding. At this adhesion arraignment, the NSC
contacts to the pore at around the cell body. Such
circular adhesion may result in a small adhesion
area; thus, the NSCs are in an environment similar to
that in the neurosphere method, that is, they are
suspended to prevent contact with surfaces. Thus,
cell encapsulation, in contrast to cell adhesion that is
observed on flat surface, is probably the reason for
the control of NSC proliferation by surface
topography; such encapsulation is characteristic to
the honeycomb film with a pore size of 3 μm.
4 CONCLUSIONS
Our study revealed that the morphology,
proliferation, and differentiation of NSCs are
controlled by the pore size of the honeycomb film.
This is a novel approach to NSC culture in
regenerative medicine, wherein the proliferation and
differentiation of the NSCs are controlled by the
surface topography of scaffolds. Honeycomb films
are potentially useful biomaterials for neural tissue
regeneration, which can help in the proliferation of
NSCs in the absence of growth factors.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
392

ACKNOWLEDGEMENTS
This work is supported by Grants-in-Aid and
CREST from Japan Science and Technology
Corporation (JST), and Special Coordination Funds
for Promoting Science and Technology of Ministry
of Education, Culture, Sports, Science and
Technology, Japan.
REFERENCES
Arai, K., Tanaka, M., Yamamoto, S., Shimomura, M.,
2008. Colloids Surf A, 313-314, 530.
Cattaneo, E., Mckay, R., 1990. Nature 347, 762.
Louis, S. A., Reynolds, B. A., 2004. Meth. Mole. Biol.
290, 265.
Mcmillan, J. R., Akiyama, M., Tanaka, M., Yamamoto, S.,
Goto, M., Abe, R., Sawamura, D., Shimomura, M,
Shimizu, H., 2007. Tissue Engineering, 13, 789.
Mori, H., Ninomiya, K., Kino-oka, M., Shofuda, T. 2006.
J. Neurosci. Res,. 84, 1682.
Roberti, Y.L., Ronald, D.G., 2000. J. Neurosci. 20, 3725.
Sato, K., Hasebe, K., Tanaka, M., Takebayashi, Nishikawa,
K., Kawai.T., Matsushita, M., Shimomura, M., and S,
Todo., 2002. Inter. J. Nanosci. 1, 689.
Steinman, L., 2003. Nature, 422, 671.
Tanaka, M. Takebayashi, M., Miyama, M., Nishida, J.,
Shimomura, M., 2004. Biomed. Mater. Eng., 14, 439.
Tanaka, M., Nishikawa, K., Okubo, H., Kamachi, H.,
Kawai, T., Matsushita, M., Todo, S., and Shimomura,
M., 2006. Colloids Surf A, 284–285, 464.
Tanaka, M., Takayama, A., Ito, E., Sunami, H.,
Yamamoto, S., Shimomura, M., 2007. J. Nanosci.
Nanotech, 7, 763.
Tanaka, M., Yoshizawa, K., Tsuruma, A., Sunami, H.,
Yamamoto, S., Shimomura, M., 2008. Colloids Surf A,
313-314, 515.
Tsuruma, A., Tanaka, M., Yamamoto, S., Shimomura, M.,
2008. Colloids Surf A, 313-314, 536.
Wang, J. H., Hung, C. H., Young, T. H., 2006.
Biomaterials, 27, 3441.
Wurmser, A. E., Palmer, T. D., Gage, F. H., 2004.
Science, 304, 1253.
Yamamoto, S., Tanaka, M., Sunami, H., Ito, E., Yamashita,
S., Morita, Y., Shimomura, M., 2007. Langmuir, 23,
8114.
CONTROL OF CELL ADHESION AND FUNCTIONS USING SELF-ORGANIZED HONEY COMB-PATTERNED
POLYMER FILMS
393
