
SELF-ORGANISATION OF GAIT PATTERN TRANSITION
An Efficient Approach to Implementing Animal Gaits and Gait Transitions
Zhijun Yang, Juan Huo and Alan Murray
Institute of Micro and Nano Systems, School of Engineering and Electronics
Edinburgh University, Edinburgh EH16 6XD, U.K.
Keywords:
Central pattern generator, oscillatory building block, gait transitions, Self-organisation, Hopfield network.
Abstract:
As an engine of almost all life phenomena, the motor information generated by the central nervous system
(CNS) plays a critical role in the activities of all animals. Despite the difficulty of being physically identified,
the central pattern generator (CPG), which is a concrete branch of studies on the CNS, is widely recognised
to be responsible for generating rhythmic patterns. This paper presents a novel, macroscopic and model-
independent approach to the retrieval of different patterns of coupled neural oscillations observed in biological
CPGs during the control of legged locomotion. Based on the simple graph dynamics, various types of oscil-
latory building blocks (OBB) can be reconfigured for the production of complicated rhythmic patterns. Our
quadrupedal locomotion experiments show that an OBB-based artificial CPG model alone can integrate all
gait patterns and undergo self-organised gait transition between different patterns.
1 INTRODUCTION
Animal gait analysis is an ancient science. As early
as two thousand years ago, Aristotle described the
walk of a horse in his treatise (Aristotle, 1936).
In modern biological research, it is widely believed
that animal locomotion is generated and controlled,
in part by central pattern generators (CPG), which
are networks of neurons in the central nervous sys-
tem (CNS) capable of producing the rhythmic out-
puts (Stein, 1978),(Grillner, 1985),(Pearson, 1993).
The constituents of the locomotory motor system are
traditionally modelled by nonlinear coupled oscilla-
tors, representing the activation of flexor and ex-
tensor muscles by, respectively, two neurophysio-
logically simplified motor neurons (Linkens et al.,
1976),(Tsutsumi and Matsumoto, 1984),(Bay and
Hemami, 1987). Despite its mathematical accuracy
and ability to mimic some basic oscillatory features,
this approach provides, however, neither a sufficiently
detailed description of the real biological mechanisms
nor a model simple enough for application purpose.
Based on the graph dynamics, in this paper we present
a structural approach to the modelling of the complex
behavioural dynamics with a new concept of oscilla-
tory building blocks (OBB) (Yang and Franca, 2003),
(Yang and Franca, 2008). For the first time we present
that the OBB model is able to self-organise its dif-
ferent gait pattern outputs under the control of a se-
lecting signal flow in the neuro-musculo-skeletal sys-
tem. Through appropriate selection and organisation
of suitably configured OBB modules, different gait
patterns and transitions between different patterns can
be achieved for producing complicated rhythmic out-
puts, retrieving realistic locomotion prototypes and
facilitating the very large scale integrated (VLSI) cir-
cuit synthesis in an efficient, uniform and systematic
framework.
2 METHOD
Out-of-phase (walking and running) and in-phase
(hopping) are the major characteristics of observed
gaits in bipeds, while in quadrupeds more gait types
were observed and enumerated (Alexander, 1984), as
walk, trot, pace, canter, gallop, bound and pronk. Un-
like bipeds and quadrupeds, hexapod locomotion can
have more complicated combinations of leg move-
ments. Despite the variety, however, some general
symmetry rules should still be obeyed and remained
as the basic criteria for gait prediction and construc-
tion. For instance, it is a generally accepted view that
multi-legged (usually more than six legs) locomotion
often display a travelling wave sweeping along the
chain of oscillators (Collins and Stewart, 1993),(Gol-
75
Yang Z., Huo J. and Murray A. (2008).
SELF-ORGANISATION OF GAIT PATTERN TRANSITION - An Efficient Approach to Implementing Animal Gaits and Gait Transitions.
In Proceedings of the Fifth International Conference on Informatics in Control, Automation and Robotics - ICSO, pages 75-79
DOI: 10.5220/0001476400750079
Copyright
c
SciTePress
ubitsky et al., 1998). In order to construct a novel,
uniform OBB-based CPG architecture to integrate all
gaits as well as the self-organised gait transitions of
a specific legged animal, we need a case study of the
simple graph dynamics as a start.
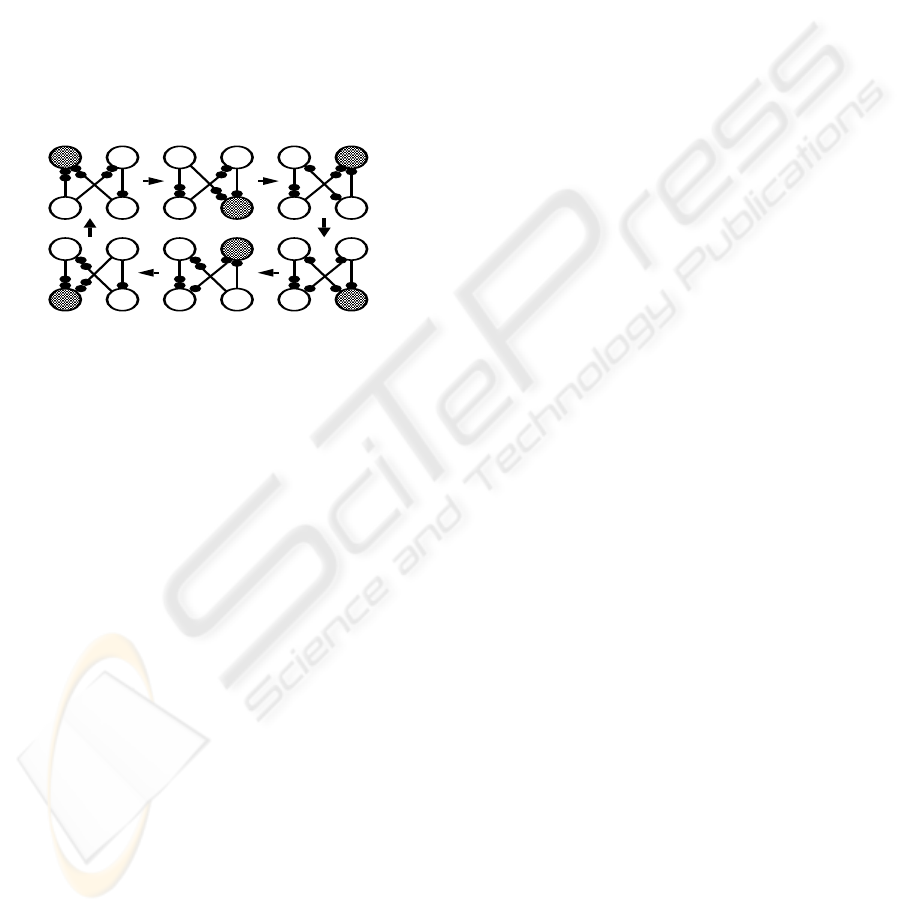
2.1 A Case of Graph Dynamics
Consider a system consisting of a set of processes and
a set of atomic shared resources represented by a con-
nected graph G = (N,E), where N is the set of pro-
cesses and E the set of edges defining the interconnec-
tion topology. An edge exists between any two nodes
if and only if two corresponding processes share at
least one atomic resource (Fig. 1).
i
j k
p
Figure 1: A simple graph dynamics representing an opera-
tion cycle, r
i
= r
j
= 2, r
k
= r
p
= 1, dark-filled circle repre-
sents a node is operating.
Between any two nodes i and j, i, j ∈ N, there can
exist e
ij
unidirected edges, e
ij
≥ 0. The reversabil-
ity of node i is r
i
, i.e., the number of edges that shall
be reversed by i to each of its neighbouring nodes,
indiscriminately, at the end of the operation. Node i
is an r
i
-sink if it has at least r
i
edges directed to it-
self from each of its neighbours. Only r
i
-sink node
can operate and reverse r
i
edges towards each of its
neighbours, the new set of r
i
-sinks will operate, and
so on. In order to avoid operation deadlock, the shared
resources between any two nodes, e
ij
, must satisfy
e
ij
= r
i
+ r
j
− gcd(r
i
,r
j
), and max{r
i
,r
j
} ≤ e
ij
≤
r
i
+r
j
−1 (Barbosa, 1996), where gcd for the greatest
common divisor. This simple graph dynamics can be
used to construct the artificial CPGs by implementing
OBB modules as asymmetric Hopfield-like networks,
where operating sinks can be regarded as firing neu-
rons in purely inhibitory neuronal networks.
2.2 Dynamics of an OBB Module
An OBB module is defined to have a pair of r
i
-sink
and r
j
-sink nodes, n
i
and n
j
, sharing the number of
e
ij
resources. The postsynaptic membrane potential
of neuron i at t instant, M
i
(t), depends on three fac-
tors, i.e., the potential at the last instant M
i
(t − 1), the
impact of its coupled neuron output v
j
(t − 1), and the
negative feedback of neuron i itself v
i
(t − 1), with-
out considering the external impulse. The selection
of system parameters, such as the neuron thresholds
and synapse weight, are crucial for modelling. In
our model, let r = max(r
i
,r
j
) and r
′
= h(r), where
h is a function of highest integer level and multi-
plying it by 10, e.g., if r
i
= 81 and r
j
= 341 then
r
′
= h(r) = h(max(81,341)) = h(341) = 10
3
. We can
further design neuron i and j’s thresholds θ
i
, θ
j
and
their synapse weights w
ij
, w
ji
as follows,
θ
i
= max(r
i
,r
j
)/(r
i
+ r
j
− gcd(r
i
,r
j
))
w
ij
= max(r
i
,r
j
)/r
′
θ
j
= (min(r
i
,r
j
) − 1)/(r
i
+ r
j
− gcd(r
i
,r
j
))
w
ji
= min(r
i
,r
j
)/r
′
(1)
The difference equation in the discrete time domain
of this system can be formulated as follows: each
neuron’s self-feedback strength is w
ii
= −w
ij
, w
j j
=
−w
ji
. The activation function is a sigmoidal Heavi-
side type. It is worth noticing that k is a local clock
pulse of each neuron, a global clock is not required.
Thus we have,
M
i
(t + 1) = M
i
(t) + w
ji
v
j
(t) + w
ii
v
i
(t)
M
j
(t + 1) = M
j
(t) + w
ij
v
i
(t) + w
j j
v
j
(t)
(2)
where,
v
i
(t) = max(0,sgn(M
i
(t) − θ
i
))
v
j
(t) = max(0,sgn(M
j
(t) − θ
j
))
(3)
We consider the designed circuit as a conservative dy-
namical system in an ideal case. The total energy is
constant, no energy loss or complement is allowed.
The sum of two neurons’ postsynaptic potential at any
given time instant is normalised to one. It is clear that
this system has the capability of self-organised oscil-
lation with the firing rate of each neuron arbitrarily
adjustable.
If a neuron has more than one connections, then
its firing state depends on the interactions of all the
connections,
V
i
(t) =
∏
n
j=1
v
j
i
(t) (4)
For instance, neuron i in Fig. 1 has connections with
both neuron j and k, the output of i is expressed as
V
i
(t) = v
j
i
(t) × v
k
i
(t), here v
j
i
(t) and v
k
i
(t) are obtained
from the above dynamical equations.
3 QUADRUPEDAL GAIT MODEL
Generally seven types of primary gait patterns are
identified for a quadruped in literature (Alexander,
ICINCO 2008 - International Conference on Informatics in Control, Automation and Robotics
76
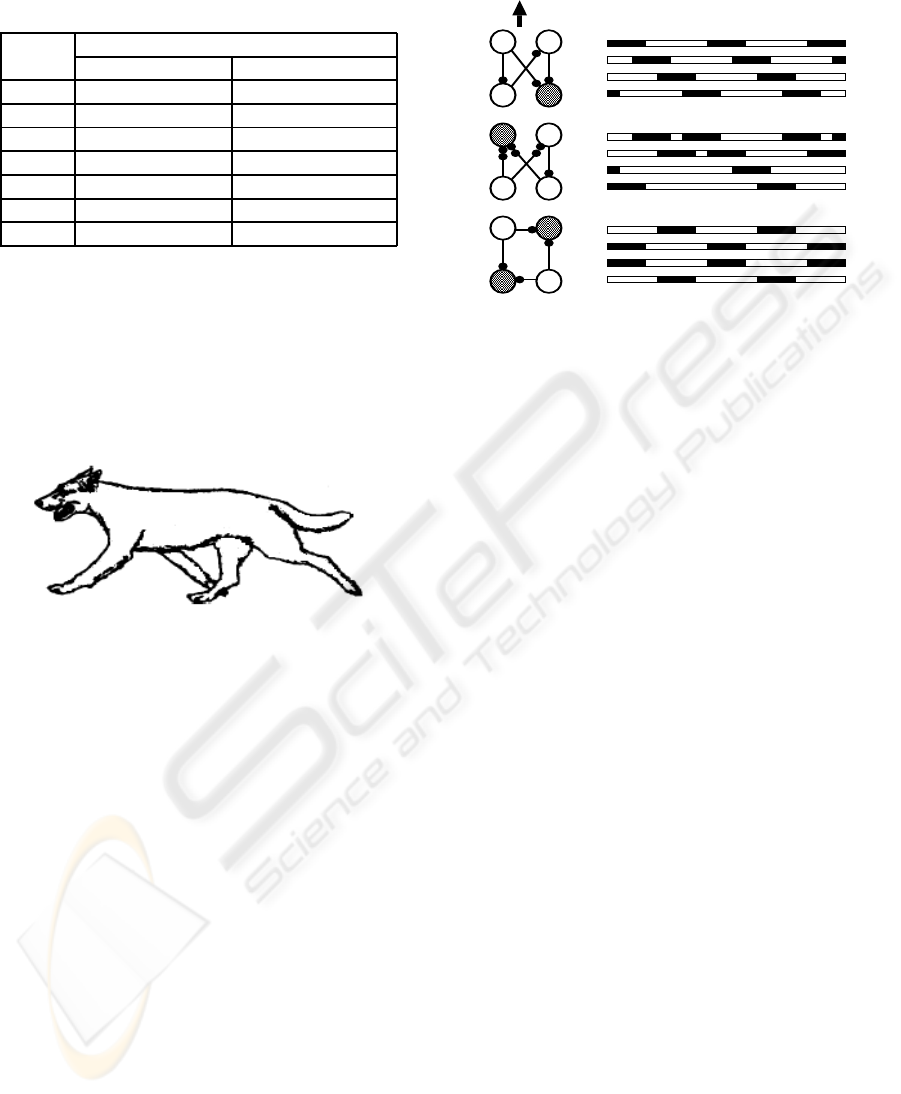
1984). These can be summarised in Table 1.
Table 1: Quadrupedal primary gaits and description.
Pattern description
Gaits Ipsilateral legs Contralateral legs
walk quarter cycle out half cycle out
trot half cycle out half cycle out
pace in phase half cycle out
gallop half cycle out quarter cycle out
bound half cycle out in phase
pronk in phase in phase
jump quarter cycle out in phase
The proposed CPG model, with adaptable, different
model parameters, is able to generate all the gait pat-
terns shown in Table 1. The signals selecting gaits
and controlling transitions in the CPG, usually in the
spinal cord, come from the higher neural level in the
prefrontal, premotor and motor cortices following the
dog’s interaction with its environment.
Figure 2: Trot gait of a dog.
We choose a dog’s risk-avoiding behaviour to build a
case study of an CPG model which is made of the
OBB modules. Suppose a dog is initially wander-
ing around in a walk gait. Suddenly it is frightened
by something and makes an abrupt left turn and es-
capes with a faster trot gait (Fig 2). In this scene the
dog’s trajectory includes three different stages in se-
quence: walk, left turn (a special gait) and trot. These
locomotion behaviours are controlled by the different
motor driving outputs from its CPG. The activity of a
dog’s leg is simplified and generated by the activity of
a flexor and an extensor motor neuron, respectively.
The firing of a neuron in the OBB drives the flexor
which lifts the leg from the ground in a swing stage.
The idleness of a neuron activates the extensor which
lands the leg on the ground in a stance stage.
The OBB network topologies for the rhythmic
pattern generation, and the phase relationship for
aforementioned three gait patterns are shown in Fig 3,
where the firing cell (gray colour) denotes its corre-
sponding leg is lifting from the ground. In the right,
black-white bar, black bars specify the firing of flex-
ors while white ones for the firing of extensors. It is
clear that the special, left turn gait has a gait pattern
circulation as shown in Fig. 1.
Head
LF RF
LH RH
RH
RF
LH
LF
RH
RF
LH
LF
RH
RF
LH
LF
(a)
(b)
(c)
Figure 3: The schematic diagram of three gaits and their
phase relations. The little black circles denote the shared
resources, gray cells are firing and white ones are idle. The
left side topology is only one configuration of a circulation
period of a pattern. RF - right front, RH - right hind, LF -
left front, LH - left hind. (a) walk, (b) left turn, (c) trot. .
From the biological knowledge we know that locomo-
tion speed is decided by both the coordinated phase
relation among legs and the duty factor, which is the
proportion of an extensor’s firing duration in one pe-
riod. When an animal’s locomotion speed increases,
the extensor firing time (corresponding to stance) will
decrease drastically while the flexor firing time (to
swing) keeps basically constant (Pearson, 1976). This
results in a lower duty factor, a relatively longer swing
stage and a faster locomotion speed. As the same with
adjusting coordinated phase relationship, the duty fac-
tor can also be modified by changing the reversabili-
ties of two coupled cells.
4 SIMULATION RESULTS
A schematic circuit diagram of OBB-based asymmet-
ric Hopfield-like neural network is shown in Fig. 4.
A computer simulated example of quadruped risk-
avoiding behaviour is conducted using this network
and the pre-defined cell reversabilities. There are to-
tally six possible connections between any two cells
in this CPG architecture (as shown in Fig. 3), among
which four connections are selected for a given gait
pattern. The selecting signals, labelled from a
1
to
a
6
, control the selection of an individual connection,
respectively, and come from the higher cortex. The
change of these signals denotes a change of connec-
tion topology. This change, together with the change
of reversabilities of the cells, decides the transition of
gait patterns.
SELF-ORGANISATION OF GAIT PATTERN TRANSITION - An Efficient Approach to Implementing Animal Gaits and
Gait Transitions
77
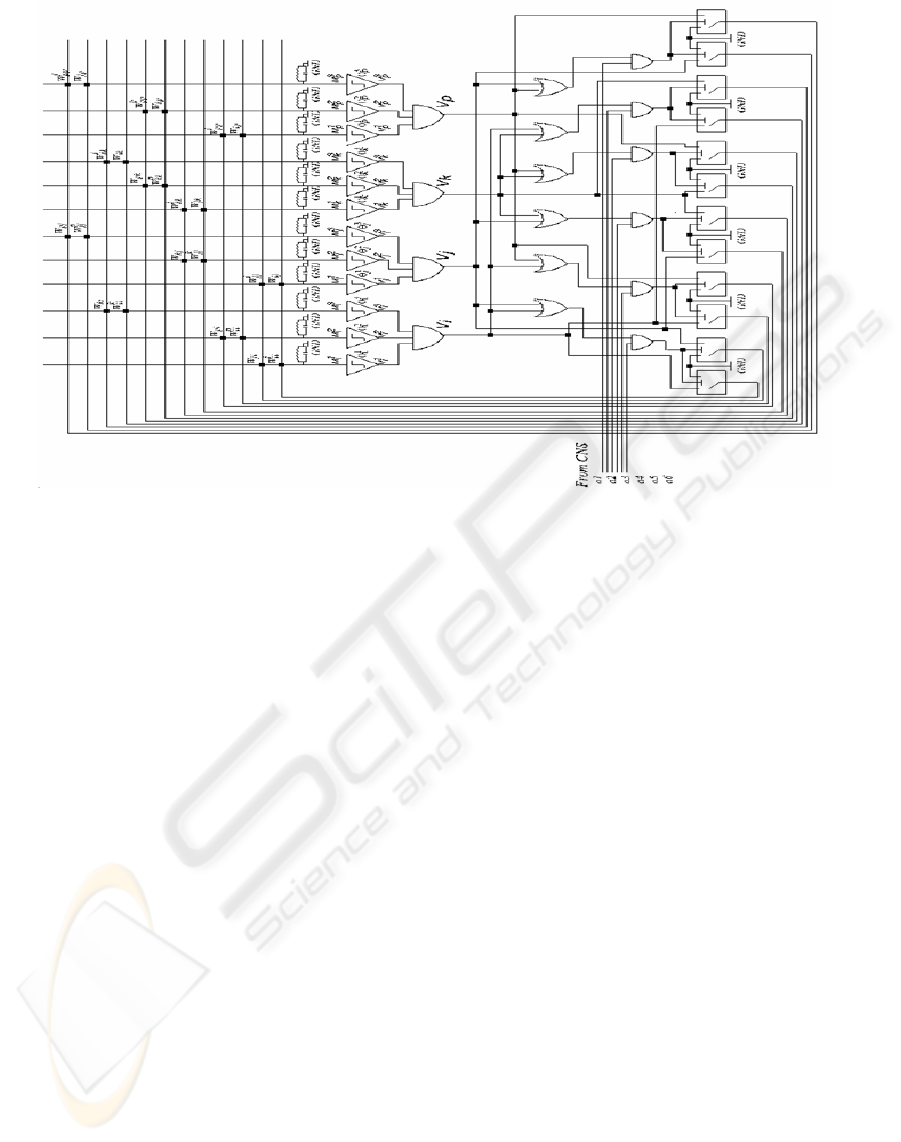
Figure 4: Quadruped CPG architecture. The cell output V
i
, V
j
, V
k
, V
p
correspond to LF, LH, RH, RF respectively. The pre-
defined cell reversabilities are r
i
= r
j
= r
k
= r
p
= 1 for the walk and trot gaits, r
i
= r
j
= 2 and r
k
= r
p
= 1 for the left turn
gait. The initial membrane potentials are M
j
i
(0) = M
k
i
(0) = M
p
j
(0) = M
k
p
(0) = M
j
k
(0) = 0.45, M
i
j
(0) = M
i
k
(0) = M
p
k
(0) =
M
j
p
(0) = M
k
j
(0) = 1.05, M
p
i
(0) = 0.55, M
i
p
(0) = 0.95. The gait select signals are [a1,a2,a3,a4,a5,a6] = [111001] for walk
and trot, [001111] for left turn. The cell thresholds and weights follow equation 1.
An array of twelve comparators, three for a cell, is
used to compare the sub-cells’ membrane potentials
with thresholds. If a connection is absent then the
relative comparator is disabled. The enabled connec-
tions of a cell converge to an AND gate, to implement
equation 4 for the cell output. If a cell has an output,
this output will make three OR gates, corresponding
to its connections with all rest cells, have an output.
These OR gate outputs are selected by the control sig-
nals from CNS at an array of AND gates, whose val-
ues are fed to an array of swithes, and form a loop to
the input weight matrix for the comparator array.
According to equation 4, The output of a cell is
the multiplication of two terminal outputs connecting
with the cell’s two neighbours. A cell will fire if and
only if its sub-cells are firing simultaneously. An ad-
ditional control (not shown) is needed to ensure that
all shared resources on an edge will not reverse un-
less the output of a cell is firing. The system param-
eters are given in the legend of Fig. 4. The choice
of initial membrane potential for every sub-cells is to
make all cells work, i.e., being firing or idle, in an
appropriate configuration of a starting gait’s circula-
tion period. After the choice of the membrane poten-
tials the system will run adaptively in its gait transi-
tion which is controlled by the CNS signals. There
is a self-organisation period observable in Fig. 5 from
walk to left turn gait. Another self-organisation be-
tween left turn to trot should exist whose effect may
be minimised if the system parameters are occasion-
ally appropriate for the next locomotion pattern at the
transition time instant.
5 CONCLUSIONS
We have presented a novel central pattern generator
model capable of generating a whole range of legged
locomotion gait patterns. This CPG architecture is
generalisable to mimic any gait patterns of any legged
animals from biped to centipede. Self-oganisation in
gait transition is observed in control of a limited num-
ber of signal flow bits from the high level cortex. This
modular system is simple and highly compatible to
circuit implementation.
ACKNOWLEDGEMENTS
This work is supported by a British EPSRC grant
EP/E063322/1, a Chinese NSF grant 60673102 and
ICINCO 2008 - International Conference on Informatics in Control, Automation and Robotics
78
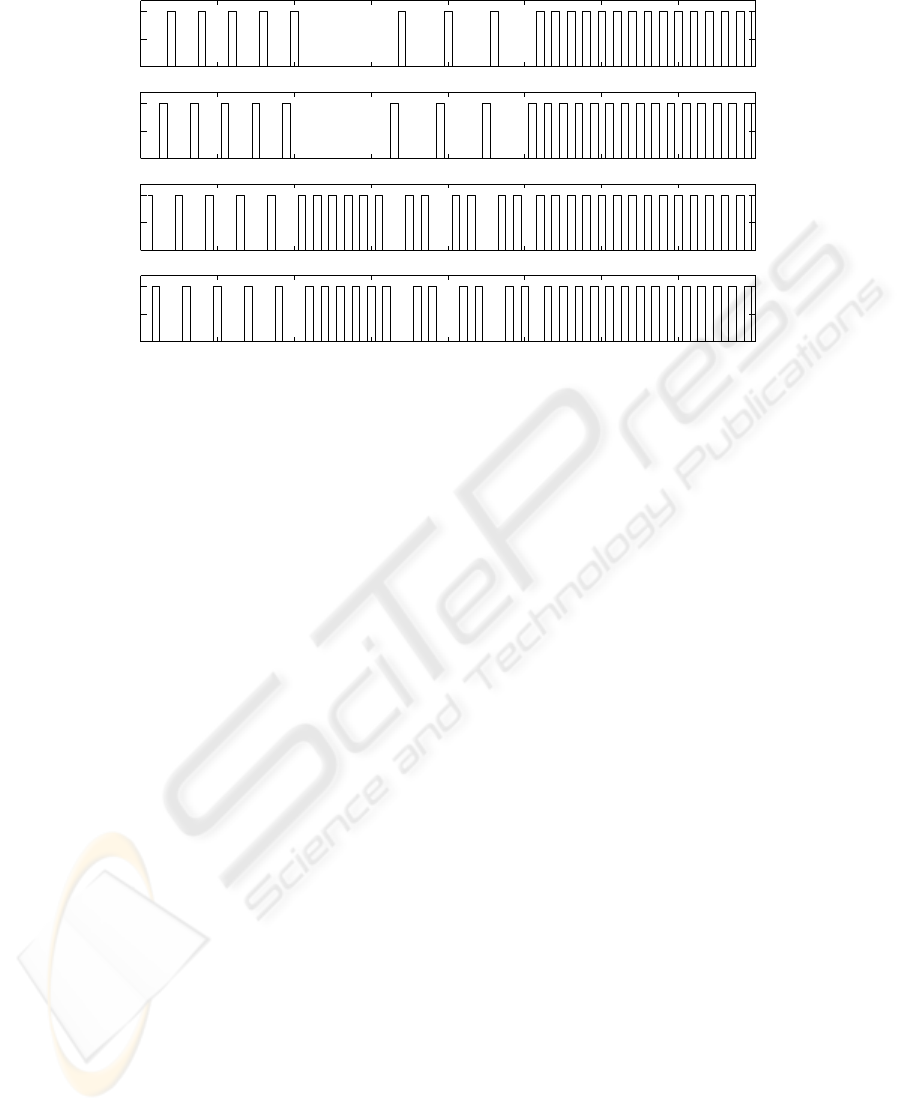
0 1 2 3 4 5 6 7 8
0
0.5
1
LF cell
0 1 2 3 4 5 6 7 8
0
0.5
1
LH cell
0 1 2 3 4 5 6 7 8
0
0.5
1
RH cell
0 1 2 3 4 5 6 7 8
0
0.5
1
RF cell
Time (second)
Figure 5: Cell output. Cell activity is equivalent to flexor activity and contrary to extensor activity in phase. The virtual dog
starts with walk for 2 seconds. After that the left turn gait lasts for 3 seconds, which include a one-second self-organisation
period. From the fifth second on it uses the faster trot gait to avoid the risk.
a Jiangsu Province grant BK2006218. We thank the
useful discussions with Dr. Felipe M.G. Franca and
Mr. Rodrigo Rodovalho.
REFERENCES
Alexander, R. (1984). The gait of bipedal and quadrupedal
animals. Intl J. Robotics Research, 3:49–59.
Aristotle (Translated version of 1936). On Parts of Ani-
mals, Movement of Animals, Progression of Animals.
By A.S. Peek and E.S. Forster (translators). Cam-
bridge, MA: Harvard University Press.
Barbosa, V. (1996). An introduction to distributed algo-
rithms. Cambridge, MA: The MIT Press.
Bay, J. and Hemami, H. (1987). Modeling of a neural
pattern generator with coupled nonlinear oscillators.
IEEE Trans. Biomedical Engr., 34:297–306.
Collins, J. and Stewart, I. (1993). Coupled nonlinear oscil-
lators and the symmetries of animal gaits. Journal of
Nonlinear Science, 3:349–392.
Golubitsky, M., Stewart, I., Buono, P., and Collins, J.
(1998). A modular network for legged locomotion.
Physica D, 115:56–72.
Grillner, S. (1985). Neurobiological bases of rhythmic mo-
tor acts in vertebrates. Science, 228:143–149.
Linkens, D., Taylor, Y., and Duthie, H. (1976). Mathemat-
ical modeling of the colorectal myoelectrical activity
in humans. IEEE Transactions on Biomedical Engi-
neering, 23:101–110.
Pearson, K. (1976). The control of walking. Scientific
American, 235:72–86.
Pearson, K. (1993). Common principles of motor control in
vertebrates and invertebrates. Annual Review of Neu-
roscience, 16:265–297.
Stein, P. (1978). Motor systems with specific reference to
the control of locomotion. Annual Review of Neuro-
science, 1:61–81.
Tsutsumi, K. and Matsumoto, H. (1984). A synaptic mod-
ification algorithm in consideration of the generation
of rhythmic oscillation in a ring neural network. Bio-
logical Cybernetics, 50:419–430.
Yang, Z. and Franca, F. (2003). A generalized locomotion
cpg architecture based on oscillatory building blocks.
Biological Cybernetics, 89:34–42.
Yang, Z. and Franca, F. (2008). A general rhythmic pattern
generation architecture for legged locomotion, In Ad-
vancing artificial intelligence through biological pro-
cess applications (A. Porto, A. Pazos, W. Buno, edi-
tors). Hershey, PA: Idea Group Inc.
SELF-ORGANISATION OF GAIT PATTERN TRANSITION - An Efficient Approach to Implementing Animal Gaits and
Gait Transitions
79
