
TOWARDS A UNIFIED MODEL FOR THE RETINA
Static vs Dynamic Integrate and Fire Models
Pedro Tom´as, Jo˜ao Martins and Leonel Sousa
INESC-ID / IST TU Lisbon, Rua Alves Redol 9, 1000-029, Lisboa, Portugal
Keywords:
Retina Models, Stochastic Leaky Integrate and Fire, Poisson-based model, Dynamic model.
Abstract:
Many models have been proposed to describe the visual processing mechanisms in the retina. The spike
generation mechanism of the models is typically performed by a Poisson process. Alternatively, a more
realistic approach can be used by implementing an integrate and fire mechanism. In this paper we show
that the Stochastic Leaky Integrate and Fire (SLIF) model is equivalent to a non-linear Poisson-based model.
Furthermore, it proposes a dynamic model for the retina visual processing path, achieved through modulations.
For estimating this model a two-step approach is proposed: i) an initial estimation is computed by using a
spike-triggered analysis, and ii) the likelihood of the spike train is maximised by gradient ascent.
1 INTRODUCTION
Vision is a fundamental sense in one’s everyday life
that gained even more relevance in the modern soci-
ety; most of the information, art and entertainment re-
lies on it. A continuously growing number of research
groups have been dedicating their efforts to help visu-
ally impaired people by developing visual prostheses
capable of conveying some kind of vision; a endeav-
our which demands the development of accurate and
reliable retina models.
Retinal ganglion cells respond to visual stimuli by
eliciting spikes whenever the inner-membranevoltage
potential surpasses a given threshold. This response,
y(t), is characterised by the time spikes occurred; it
can be mathematically represented as a sum of Dirac
functions δ(t−t
k
) centred at the time instantst
k
, when
the inner-membrane voltage potential surpassed the
threshold: y(t) =
∑
δ(t − t
k
).
Several retina models, based mainly on general
neuron models, have been proposed. Two of the most
typical are the Poisson based model (Chichilnisky,
2001) and the Stochastic Leaky Integrate and Fire
(SLIF) (Paninski, 2006) model. However, in this pa-
per it is shown that, under some constraints, they
are equivalent. Moreover, a dynamic model is pro-
posed based on developed mathematical machinery
used to prove the equivalencebetween these two mod-
els. This dynamic model modulates its output not
only by the stimulus characteristics but also by the
recent spike firing history. To estimate this model’s
parameters several other issues are addressed, such as
the tuning and initialisation of this model, where it is
used spike-triggered analysis. At the end some exper-
imental results are provided.
This paper is organised as follows. Section 2
shows the equivalence between the SLIF model and
a Poisson based model. Section 3 presents the pro-
posed dynamic model and describes its tuning. Some
experimental results are drawn in Section 4, and sec-
tion 5 concludes the paper.
2 STOCHASTIC INTEGRATE
AND FIRE MODEL
A typical approximation for the Hodgkin and Hux-
ley neuron model assumes a leaky integrate and fire
model. By adding a noise component to the model,
it is possible to simulate the variability of real neu-
rons (Keat et al., 2001). For a linear first order in-
tegrate and fire mechanism, the SLIF model is de-
scribed by the stochastic differential equation (SDE):
dv(t) = −
1
τ
v(t)dt + i(t)dt + σξ(t) (1)
where τ is a constant variable, ξ(t) is standard white-
noise and σ is a multiplicative term which defines the
power of the noise source. The above SDE is valid un-
til the subthreshold potential surpasses a given thresh-
old V
th
. Whenever this happens a spike is fired and
the neuron enters a refractory period where no spike
can be fired. Accordingly with the SLIF model, at the
end of this period, which typically lasts a couple of
528
Tomás P., Martins J. and Sousa L. (2008).
TOWARDS A UNIFIED MODEL FOR THE RETINA - Static vs Dynamic Integrate and Fire Models.
In Proceedings of the First International Conference on Bio-inspired Systems and Signal Processing, pages 528-533
DOI: 10.5220/0001067905280533
Copyright
c
SciTePress
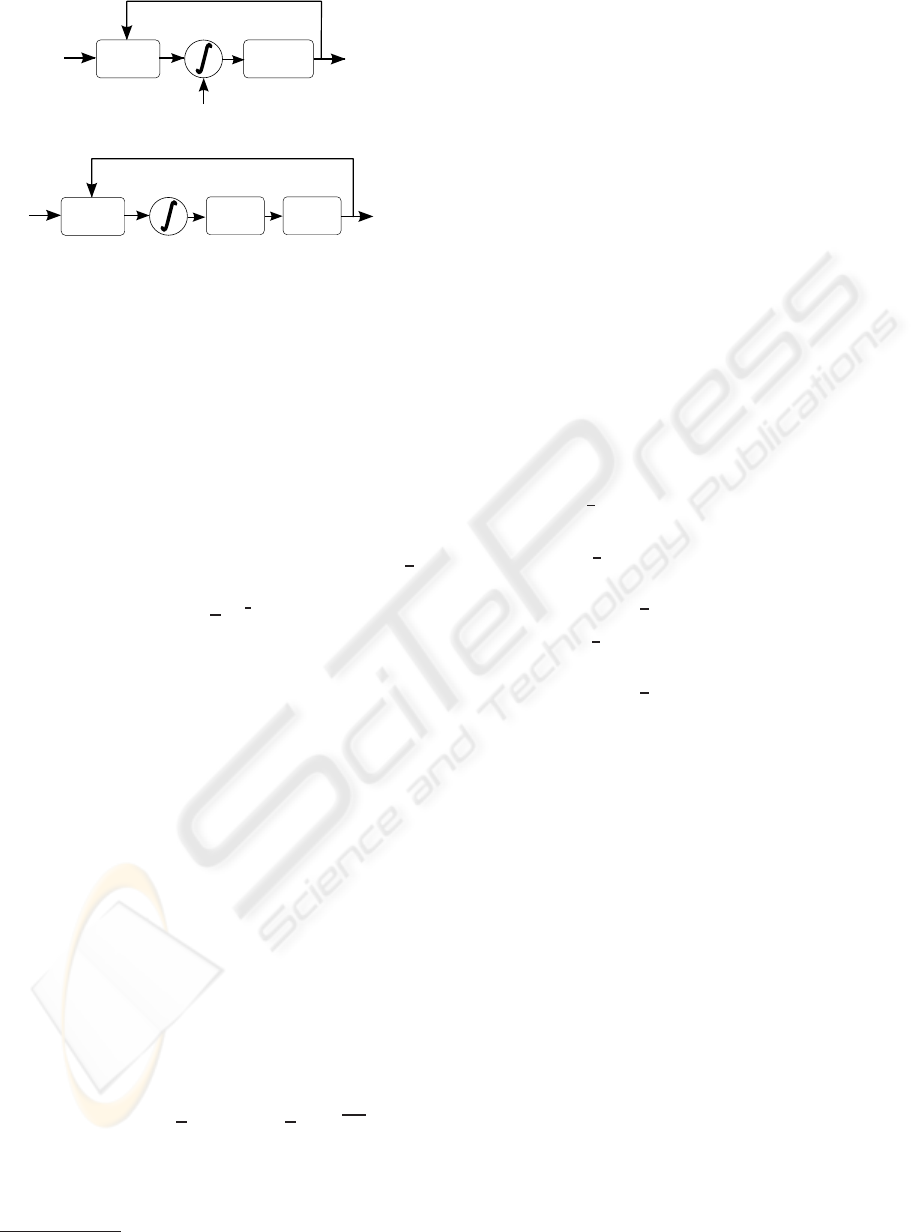
Black-box
G(s,y)
Threshold
block
Visual
Stimuli
Spike
Sequence
v(t)
y(t)
s(t)
i(t)
W(t)
(a) General Stochastic Integrate and Fire model.
Black-box
G(s,y)
Sigmoid
Visual
Stimuli
Spike
Sequence
v(t)
y(t)
s(t)
i(t)
Poisson
Process
(b) Generalised Poisson model.
Figure 1: Changing the noise source in Integrate and Fire
models.
milliseconds, the neuron is reset to a state where the
subthreshold potential is v
0
. Considering that the last
spike was fired at time t = t
0
, the solution to (1) is
1
:
v(t) =h
IF
(t − t
0
) ∗
h
i(t) + v
0
δ(t −t
0
) +W(t)
i
(2a)
W(t) ∼N(0, σ
t
) , σ
t
≡ σ(t) (2b)
where ∗ is the convolution operator and h
IF
(t) is the
transfer function of a low pass filter with a pole in
1
τ
:
h
IF
(t) =
1
τ
e
−
t
τ
H(t) (3)
where H(t) is the Heaviside step function.
2.1 Equivalence between the Slif Model
and the Poisson Based Model
Let us consider a SLIF model as shown in Figure 1(a),
where s(t) represents the visual stimulus, y(t) is the
spike response, and G(s, y) is an arbitrary function
that transforms the pair {stimulus, spike response se-
quence} into the SLIF input i(t). The integrator block
in the figure represents the linear filter h
IF
(t).
Under the SLIF model assumption, the noise
source can be moved to the integrator output by modi-
fying its statistics: v(t) = u(t) +W
′
(t) ∼ N(u(t), σ
2
t
).
Considering that after the refrationary period the sub-
threshold potential v follows a Gaussian distribution
with standard deviation σ
0
, and noting that the con-
volution is a linear operator, the output noise source
remains Gaussian, with standard deviation:
σ
2
t
= H(t − t
0
)
h
σ
2
τ
2
+
σ
2
0
− σ
2
τ
2
e
−2
t−t
0
τ
i
(4)
where t
0
represents the time instant of the last spike
and σ is the standard deviation of the noise during the
integration period, i.e. the Interspike Interval (ISI).
1
Notice that (1) is similar to the Langevin’s SDE.
Therefore, the probability for eliciting a spike at a
given time instant t is given by the probability for the
potential v(t) = u(t) +W
′
(t) to surpass V
th
:
P
y(t) = 1
= P
u(t) +W
′
(t) ≥ V
th
=
= P
W
′
(t) ≥ V
th
− u(t)
=
= 1− N
cdf
(V
th
− u(t)|0, σ
2
t
) =
= N
cdf
(u(t)|V
th
, σ
2
t
) (5)
where N
cdf
(x|µ, σ
2
t
) is the normal cumulative distribu-
tion function with mean µ and variance σ
2
t
, evaluated
at point x. The function N
cdf
defines a sigmoid where
V
th
controls the translation (centre of the sigmoid) and
σ
t
controls the expansion of the sigmoid.
Since the spiking probability of the SLIF model
is given by a sigmoid function, the model in Fig-
ure 1(a) is equivalent to the Poisson based model in
Figure 1(b), where the shape of the sigmoid depends
on the noise statistic during the refractionary and in-
tegration periods; three cases can be considered:
(1) if σ
2
0
= σ
2
τ
2
, the noise variance throughout the in-
tegration period is always the same;
(2) if σ
2
0
< σ
2
τ
2
, the noise variance increases expo-
nentially from σ
2
0
, just after the refractory period,
converging to σ
2
τ
2
;
(3) if σ
2
0
> σ
2
τ
2
, the noise variance decreases expo-
nentially from σ
2
0
, just after the refractory period,
converging to σ
2
τ
2
;
Equivalently, for a neuron described by the Pois-
son spike generation process presented in Figure 1(b),
the change in noise variance is translated into a vary-
ing slope of the sigmoid – except in case (1). In case
(2)/(3), the model variability increases/decreases as
time from last spike progresses.
However it is worth to notice that this is not a true
Poisson model, as the firing of a spike at a time in-
stant depends on the recent spiking history. Since the
integrator is reset whenever a new spike is fired, this
is true even without the feedback path.
The above conversion thus shows that integrate-
and-fire (IF) models improve the precision of neu-
ron models by adding three important functions to the
typical Poisson based model: i) a natural refractionary
period given by the time for the integrator to recharge
and fire a second spike; ii) a feedback mechanism;
and iii) a sigmoid-like nonlinearity. While the feed-
back mechanism is not absolutely required to achieve
a reasonable precision – see (Capela et al., 2007) –,
the modelling of the feedback mechanism with a suf-
ficiently variable filter allows for a considerable im-
provement on the precision of the model – see (Tom´as
and Sousa, 2007).
TOWARDS A UNIFIED MODEL FOR THE RETINA - Static vs Dynamic Integrate and Fire Models
529
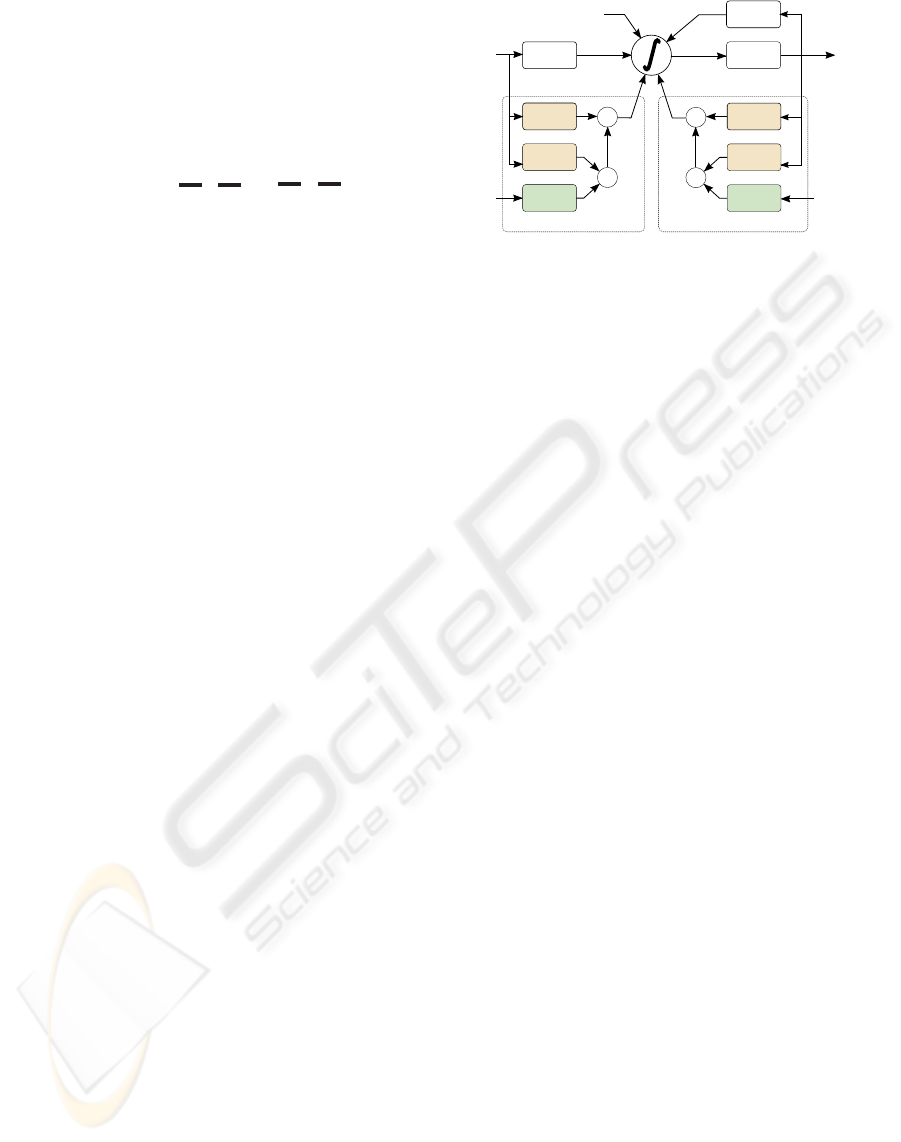
3 DYNAMIC MODEL
The typical modelling of neurons represents G(s, y)
in Figure 1 by a linear system dependent on the input
stimuli s(t) and on spike history y(t):
G(s(t), y(t)) = (h
f
∗ s)(t)
| {z }
i
f
(t)
+(h
b
∗ y)(t)
| {z }
i
b
(t)
(6)
where h
f
(t) and h
b
(t) represent the feedforward and
feedback linear filters, respectively.
In some cases, driven by the necessity to model
the contrast adaptation mechanisms existent in the
retina, non-linear functions are included in the de-
scription of G(s,y) (Baccus and Meister, 2002).
These non-linearities can be, for instance, approxi-
mated by a Taylor series:
G(s(t), y(t)) = f
f
(i
f
(t)) + f
b
(i
b
(t)) (7a)
f
x
(y) =
∑
k
a
k
y
k
(7b)
Nevertheless, the inclusion of dynamics in neuron
model is usually avoided, eventhough recent research
suggest that they are required for effectively mod-
elling the precise timing of neurons (Gerstner et al.,
2006). This is particularly verified in IF neuron mod-
els where its natural non-linearities introduce local
minimums, making the training process harder. In or-
der to model the temporal dynamics of the retina cir-
cuitry, a model must be constructed where the trans-
fer function changes with time, for instance by means
of a modulation process. Typically, neural dynamics
are considered only as a function of the stimuli – e.g.
motion detection (Bialek and van Steveninck, 2005).
However, as referred in section 2, if the precision of
neuron models is improved by the inclusion of a feed-
back path, one should also consider the spike history
for modelling neural dynamics.
The proposed model is divided into two parts: a
static part and a dynamic part. Both parts have depen-
dencies on the stimuli and the spike history. The static
part is composed by a static forward filter h
sf
(t) and
a static backward filter h
sb
(t):
i
sf
(t) = (h
sf
∗ s)(t) (8a)
i
sb
(t) = (h
sb
∗ y)(t) (8b)
The dynamic part is composed by a set of dynamic
forward linear filters h
(k)
d f
(t) and dynamic backward
linear filters h
(k)
db
(t), whose amplitude is dynamically
controlled by linear functions dependent of both the
Threshold
v(t)
x
+
Visual
stimuli
Feedforward dynamic block 'k'
h (t)
sf
h (t)
df
h (t)
fdf
h (t)
fdb
h (t)
sb
h (t)
db
h (t)
bdb
h (t)
bdf
x
+
Spike
Sequence
s(t) y(t)
y(t)
s(t)
i (t)
sf
i (t)
sb
i (t)
df
i (t)
db
Feedback dynamic block 'k'
W(t)
Figure 2: Dynamic model.
stimulus and the spike history:
i
(k)
d f
(t) = c
(k)
d f
(t)(h
(k)
d f
∗ s)(t) (9a)
i
(k)
db
(t) = c
(k)
db
(t)(h
(k)
db
∗ y)(t) (9b)
c
(k)
d f
(t) =
h
(h
(k)
fd f
∗ s)(t)+ (h
(k)
fdb
∗ y)(t)
i
(9c)
c
(k)
db
(t) =
h
(h
(k)
bd f
∗ s)(t)+ (h
(k)
bdb
∗ y)(t)
i
(9d)
where h
(k)
x
(t) represents the linear filter x for compo-
nent k and i
(k)
d f
(t) and i
(k)
db
(t) are the outputs of the dy-
namic component k. The complete model is depicted
in Figure 2.
The outputs of both the static and the dynamic
components are then added in the integrator block:
i(t) =i
sf
(t) + i
sb
(t) +
∑
k
i
(k)
d f
(t) +
∑
k
i
(k)
db
(t) (10)
and the subthreshold potential, v(t), follows (2).
3.1 Model Implementation
To implement the model and estimate its parameters,
it was discretised in time steps of T
s
= 1 ms, leading
to a discrete representation x
n
≡ x[n] of the continuous
signal x(t). Basis functions were used for describing
each of the filters in the model. While one can sim-
ply estimate the value of the filter for each sample
nT
s
, the use of basis functions allows: i) to reduce
the dimensionality of the problem, thus accelerating
convergence; and ii) to decrease overfitting of the pa-
rameters to the data used during the training step.
Each of the filters in the static component
h
x
(t), x ∈ {sf, sb}, or in its discretised form,
h
x
=
h
x
[1], ··· , h
x
[M]
, where M is the filter mem-
ory, is defined by means of the basis functions
B = [B
T
1
, ··· , B
T
B
]
T
, B
k
= [b
k1
, ··· , b
kM
] as:
h
x
=
B
∑
m=1
a
xm
B
m
= A
T
x
B (11)
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
530
where A
x
= [a
x1
, ··· , a
xB
]
T
. In the case of the fil-
ters in the dynamic components, h
(1)
x
(t), · ·· , h
(C)
x
(t),
x ∈ {d f, db, fd f, fdb,bd f, bdb}, one can organ-
ise them in a matrix H
x
=
h
(1)T
x
, ··· , h
(C)T
x
T
,
h
(k)
x
=
h
(k)
x
[1], ··· , h
(k)
x
[M]
, where the filters are or-
ganised in rows and the samples in columns. The fil-
tering matrix H
x
can be computed as:
H
x
= A
T
x
B (12)
For implementing the integrator linear filter, an in-
finite impulse response (IIR) description is used:
H
IF
(z) =
1− β
1− βz
−1
(13)
where β defines a pole in s
pole
=
1
T
logβ and log(x) is
the natural logarithm of x. For the system to remain
stable, β ∈ ℜ
]0,1[
, such that s
pole
< 0.
Notice that unlike in the continuous version of the
model, the filter is now normalised with unitary DC
gain. Thus, the variance of the noise, σ
2
n
, at instant n,
given that the last spike event was fired at instant n
0
,
becomes:
σ
2
n
= β
2(n−n
0
)
σ
2
0
+ (1 − β)
2
σ
2
n−n
0
−1
∑
k=0
β
2k
=
= β
2(n−n
0
)
σ
2
0
+ (1 − β)
2
1− β
2(n−n
0
)
1− β
2
σ
2
(14)
3.2 Model Tuning
The complete set of parameters for the dynamic
model in Figure 2 is:
n
A
sf
, A
sb
, A
d f
, A
db
, A
fd f
, A
fdb
, A
bd f
, A
bdb
,
β, σ, σ
0
,V
th
,V
0
o
(15)
However, some parameters depend on others.
Namely, V
th
and V
0
depend on the general gains of the
model, A
x
. Changing their values implies changing
the filter gains such that the total integration time for
firing a spike remains the same. Also, modifying the
value of β is similar to changing the shape of the other
linear filters. Moreover, from our experience the ini-
tial noise variance σ
2
0
(the variance of the noise after
the refractionary period) does not significatively in-
fluence the model tuning. Thus this parameter was re-
moved from the learnable parameter set as well. The
complete set of trainable parameters is therefore:
n
A
sf
, A
sb
, A
d f
, A
db
, A
fd f
, A
fdb
, A
bd f
, A
bdb
, σ
o
(16)
which corresponds to the basis functions coefficients
for each filter and the noise standard deviation. The
non-trainable parameters were set to: β = 0.9, σ
0
= 0,
V
th
= 1 and V
0
= 0.
For the optimisation of the model parameters, a
Bayesian approach was applied to compute the proba-
bility of the spike sequence (Tom´as and Sousa, 2007).
Afterwards, gradient ascent was applied to maximise
this probability. Following (15) from (Tom´as and
Sousa, 2007), the non null gradients in order to the
parameters in (16) are:
du
n
dA
sf
= s
n
∗ B ∗ h
IF
(17a)
du
n
dA
sb
= y
n−1
∗ B ∗ h
IF
(17b)
du
n
dA
d f
=
(s
n
∗ B)(c
d f
)
T
n
∗ h
IF
(17c)
du
n
dA
db
=
(y
n−1
∗ B)(c
db
)
T
n
∗ h
IF
(17d)
du
n
dA
fd f
=
(s
n
∗ B)(s
n
∗ A
T
d f
B)
T
∗ h
IF
(17e)
du
n
dA
fdb
=
(y
n−1
∗ B)(s
n
∗ A
T
d f
B)
T
∗ h
IF
(17f)
du
n
dA
bd f
=
(s
n
∗ B)(s
n
∗ A
T
d f
B)
T
∗ h
IF
(17g)
du
n
dA
bdb
=
(y
n−1
∗ B)(y
n−1
∗ A
T
d f
B)
T
∗ h
IF
(17h)
1
σ
n
dσ
n
dσ
= 2(1− β)
2
1− β
2(n−n
0
)
1− β
2
σ (17i)
where (c
x
)
n
= [c
(1)
x
[n], ··· , c
(C)
x
[n]]
T
; the convolution,
represented by the symbol ∗, operates along the
columns of the operands; and the convolution with
h
IF
is performed using the IIR filter in (13), resetting
the integration output to V
0
whenever a spike is fired.
However, unlike in static models, such as those
described in (Tom´as and Sousa, 2007), carefull ini-
tialisation is required in dynamic models. A method
for doing this is to apply spike triggered analy-
sis (Schwartz et al., 2002; Simoncelli et al., 2004).
Eventhough this algorithm is valid under true Pois-
son neuron model, under IF models it becomes bi-
ased (Pillow and Simoncelli, 2003). This is mostly
due to the IF natural non-linearities. However, since
the estimation process of IF models has local mini-
mums, it provides a good starting point.
Since the model includes feedforward and feed-
back dynamic mechanisms, Spike Triggered Average
(STA) and Spike Triggered Covariance (STC) anal-
ysis were performed using both the stimuli and the
spike history. The static filters h
sf
and h
sb
in Figure 2
were initially set with the shape of the feedforward
and feedback STA, respectively. To adjust the initial
TOWARDS A UNIFIED MODEL FOR THE RETINA - Static vs Dynamic Integrate and Fire Models
531
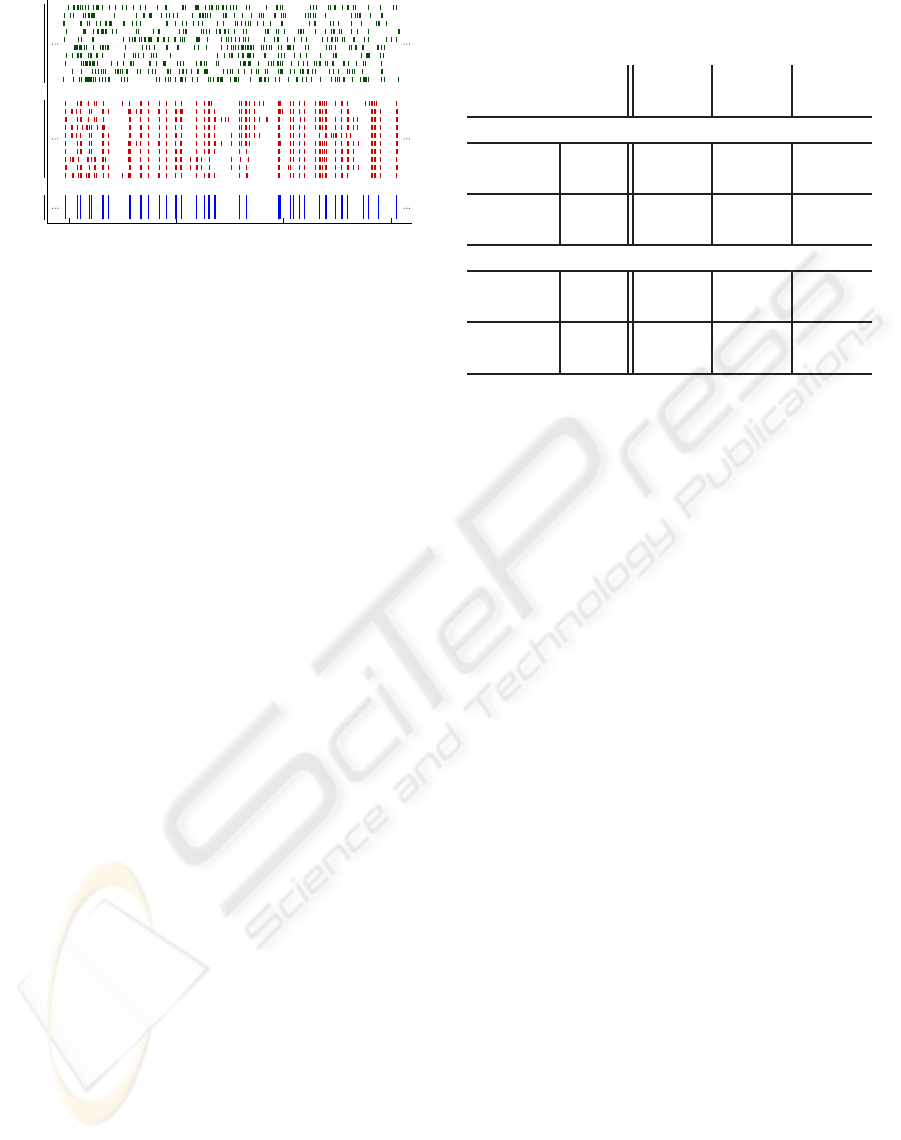
15 20 25 30
Time [s]
Real
Data
Dynamic
Model
Static
Model
Figure 3: Spike response of the dynamic and static model
vs. the response of real data (vertical lines represent the
timing of the elicited spikes).
shape of the dynamic filters h
(k)
d f
and h
(k)
db
, excitatory
and inhibitory directions can be extracted using STC
analysis.
4 EXPERIMENTAL RESULTS
The proposed training algorithm was implemented
and tested to estimate the responses of real rabbit
retina ganglion cells. The data set consists of four
trials of full field white noise stimulus, where each
trial has a duration of ≈ 50s with an average count of
6.58 spikes per second. While the stimuli values for
these four trials is the same, small differences exist in
the stimulation time: on average stimulation changed
with a new random value at every 152 ms; the stan-
dard deviation of stimuli change was 238 ms. The
visual stimuli was normalised by subtracting its mean
value and then by dividingit by the standard deviation
of itself. The resulting stimuli, which corresponds to
the inputs
n
given to the model, is therefore a sequence
of normally distributed random values with zero mean
and unitary standard deviation.
To estimate the dynamic model, many basis func-
tions can be used to describe the linear filters. Typi-
cal examples are the Laguerre bases (Akc¸ay and Nin-
ness, 1999; Tom´as and Sousa, 2007) or sinusoidal ba-
sis (Keat et al., 2001). However, they are typically un-
able to describe delayed filters well and, for the used
data, it considerably deteriorated the results. Thus, in
the present work simple Gaussian kernels were used.
While these bases are not orthogonal, they allow to
significatively reduce the number of trainable param-
eters, whilst allowing to achieve good results. A total
of 31 kernels were employed each separated by 10 ms,
and having a standard deviation of 5.6 ms.
As depicted in subsection 3.2, for model tuning
Table 1: Error measures between trained models and real
responses.
Spike Inter
NMSE
Time Spike
Static Model
Training mean 420.54 317.69
0.882
trial std 10.39 7.05
All four mean 444.02 337.49
0.903
trials std 12.03 6.95
Dynamic Model
Training mean 251.63 242.62
0.625
trial std 22.13 21.71
All four mean 325.85 281.32
0.808
trials std 20.55 16.68
mean - mean result std - standard deviation
the static filters h
sf
and h
sb
were initially set with the
shape of the STA applyed to the stimuli and spike his-
tory, respectively. The dynamic filters h
d f
and h
db
were set with the 5 most excitatory directions (ex-
tracted by using STC analysis); the experimental data
showed no strong inhibitory directions. The modulat-
ing filters h
fd f
, h
fdb
, h
bd f
and h
bdb
were initially set
to a small, non-zero value.
To compare the performance of the proposed dy-
namic model, we also performed fitting with a static
model (the number of dynamic blocks in Figure 2 was
set to zero). The training procedure was the same for
both the static and the dynamic model. One of the
ganglion cells’ response trials was used for training.
The other three were used for comparison. Again,
stimulation times are not exactly the same for all tri-
als, which leads to slightly different neuron responses.
For evaluating the performance of the models, 30
spike response trials were produced by using both
the trained static and dynamic models. In Figure 3,
we present the first 10 response trials obtained us-
ing the training data set. The figure also presents the
real retina ganglion cells’ response. Analysing the re-
sponses one can clearly see that the static model is
unable to acquire the structure of the ganglion cells’
response. On the other hand, the dynamic model is
able to accurately reproduce the spike response pat-
tern. However it does tend to fire 15% more spikes
than the ganglion cell (average on the four trials).
For a better assessment of the response of the
models, two error metrics proposed in (Victor and
Purpura, 1997) were used. The first metric accounts
for the cost associated with the absolute time of occur-
rence of neuronal events (Spike Time Metric). The
second metric accounts for the cost of changing the
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
532

intervals between two spikes (Inter Spike Metric).
The movement cost q was set to 50 s
−1
– see (Vic-
tor and Purpura, 1997). A firing rate metric based on
the normalised mean squared error (NMSE) (Berry II
and Meister, 1998) was also applied. For that purpose,
the firing rates where estimated for both the real and
the estimated data, by convolving their PeriStimulus
Time Histogram (PSTH) (Berry II and Meister, 1998)
with a gaussian window of zero mean and 20ms of
standard deviation.
Cross-evaluation between the models’ responses
and the real ganglion cells responses using the de-
scribed error metrics are presented in Table 1. This
table shows the results when comparing the training
trial and the other trials. The presented error met-
rics confirm our analysis: the dynamic model is able
to capture the dynamics of real retina ganglion cells
which cannot be described by a simple set of a feed-
forward filter and a feedback filter. This can be seen
by noticing that the mean values for all error met-
rics are much lower for the dynamic model than for
the static model. The dynamic model also tends to
achieve a higher degree of variability than the static
model. However, this is due to the natural variability
of the real data.
In the presented work, the number of dynamic
components was fixed to five. However, for obtain-
ing a more general model, one could start by using a
larger number of dynamic components and then us-
ing feature selection – such as in (Tom´as and Sousa,
2007) – to remove all unnecessary components.
5 CONCLUSIONS
Many researchers tend to classify SLIF models and
Poisson-based models into different groups. How-
ever, as show in this paper, the former can be trans-
lated into the latest. One of the most important fea-
tures of the SLIF model is the presence of feed-
back mechanisms. Given that this property helps to
increase the spiking precision, we present a model
whose coefficients are dynamic in time. Moreover we
present a method to estimate its coefficients: it uses
eigen-analysis to estimate the initial parameters and a
gradient ascent technique for tuning the model. Pre-
sented results show that the proposed model is able to
achieve better results than the simpler static models.
REFERENCES
Akc¸ay, H. and Ninness, B. (1999). Orthonormal basis func-
tions for modelling continuous-time systems. Signal
Processing, 77(1):261–274.
Baccus, S. and Meister, M. (2002). Fast and Slow Contrast
Adaptation in Retinal Circuitry. Neuron, 36(5):909–
919.
Berry II, M. J. and Meister, M. (1998). Refractoriness and
neural precision. The Journal of Neuroscience.
Bialek, W. and van Steveninck, R. (2005). Features and
dimensions: Motion estimation in fly vision. Arxiv
preprint q-bio.NC/0505003.
Capela, S., Tom´as, P., and Sousa, L. (2007). Stochas-
tic integrate-and-fire model for the retina. In
15th European Signal Processing Conference (EU-
SIPCO’2007).
Chichilnisky, E. J. (2001). A simple white noise analysis of
neuronal light responses. Network: Computation in
Neural Systems, 12(2):199–213.
Gerstner, W., Jolivet, R., Brette, R., Clopath, C., Rauch, A.,
and Luscher, H. (2006). Predicting Neuronal Activ-
ity with Simple Models of the Threshold Type. Time
(msec), 100:50.
Keat, J., Reinagel, P., Reid, R. C., and Meister, M. (2001).
Predicting every spike: A model for the responses of
visual neurons. Neuron, 30:803–817.
Paninski, L. (2006). The most likely voltage path and
large deviations approximations for integrate-and-fire
neurons. Journal of Computational Neuroscience,
21(1):71–87.
Pillow, J. and Simoncelli, E. (2003). Biases in white noise
analysis due to non-Poisson spike generation. Neuro-
computing, 52(54):109–115.
Schwartz, O., Chichilnisky, E. J., and Simoncelli, E. P.
(2002). Characterizing neural gain control using
spike-triggered covariance. In Dietterich, T. G.,
Becker, S., and Ghahramani, Z., editors, Advances in
Neural Information Processing Systems, volume 14,
pages 269–276, Cambridge, MA. MIT Press.
Simoncelli, E. P., Paninski, L., Pillow, J., and Schwartz, O.
(2004). Characterization of Neural Responses with
Stochastic Stimuli, chapter 23, pages 327–338. MIT
Press.
Tom´as, P. and Sousa, L. (2007). Feature selection for the
stochastic integrate and fire model. In 2007 IEEE In-
ternational Symposium on Intelligent Signal Process-
ing (WISP’2007).
Victor, J. D. and Purpura, K. P. (1997). Metric-space analy-
sis of spike trains: theory, algorithms and application.
Computation Neural Systems, 8:127–164.
TOWARDS A UNIFIED MODEL FOR THE RETINA - Static vs Dynamic Integrate and Fire Models
533
