
CAPILLARY ELECTROPHORESIS ELECTROCHEMICAL
DETECTOR WITH NOBLE MICROCHANNEL STRUCTURE
FOR MINIATURIZATION
Development of a Capillary Electrophoresis Microchip Format Electrochemical
Detector for Endocrine Disruptors Sensing
Kon Ha
1*
, Gi-sung Joo
2
, Grace Nisola
3
, Wook-Jin Chung
3
, C. J. Kang
1
and Yong-Sang Kim
1,2
1
Dept. of Nano Science and Engineering, Myongji University, Gyeonggi 449-728, Korea
2
Dept. of Electrical Engineering, Myongji University, Gyeonggi 449-728, Korea
3
Dept. of Environmental Engineering and Biotechnology, Myongji University, Gyeonggi 449-728, Korea
Keywords: Capillary electrophoresis, Electrochemical detector, Lab-On-a-Chip, Miniaturization, Endocrine disruptors,
PDMS, ITO, bisphenol A (BPA).
Abstract: Numerous researches have been focused on capillar
y electrophoresis (CE) and amperometric detection
(AD) using a double-T micro-channel configuration. The combination of these two techniques becomes a
powerful analytical tool due to enhanced features in terms of sensitivity and selectivity. The developed CE-
AD chip is low cost and requires less power consumption. Its high compatibility with micro-fabrication
technology has made it popular for analysis of different compounds. However, due to the need to further
miniaturize the CE-AD device, a twisted CE micro-channel configuration is fabricated and tested in this
study. Furthermore, enhanced analyte separation due to delayed response time can be achieved using a
serpentine CE separation micro-channel. Phenolic compounds were used as testing analytes to confirm the
results using different types of running buffers. Also, the data gathered from the new micro-channel
configuration is compared with the previously gathered results obtained from double-T separation micro-
channels.
1 INTRODUCTION
Established analytical methods require sophisticated
equipment in order to obtain accurate and reliable
results. However, available apparatuses are bulky,
expensive and require sample pre-treatment steps in
order to minimize interferences. In lieu of these
conventional techniques, the micro-scale lab-on-a-
chip (LOC) devices could provide better
performances and other benefits. One in particular is
analytical cost reduction due to LOC cheaper
production, lower reagent volume requirement and
shorter analytical time. Though micro-fabrication
has become successful to miniaturize capillary
electrophoresis amperometric detector (CE-AD)
LOC devices, numerous problems are yet to be
solved. One of the common predicaments is the poor
selectivity of the detector which is related to the
unsatisfactory separation at the CE component.
Double T configuration has been commonly used in
CE microchannels. However, the effective length of
a CE microchannel may not be sufficient to
complete the separation process or may require
longer channel in order to attain an effective
separation, which defies the goal of miniaturization.
Other ways include proper tuning of separation
fields in order to provide an appropriate migration
time for different analytes in the sample but this
technique could not aid in further miniaturization of
the device. Changing the microchannel
configuration is another way to resolve separation
problem. In this study, a twisted or serpentine CE
microchannel configuration is investigated. An LOC
device is fabricated with twisted microchannels with
equivalent length of the previously reported double
T configuration. In a twisted configuration, the size
of the device can be further reduced. The influence
of the separation field is much less at curved channel
regions hence it is expected to have a more effective
separation as it slightly increases migration time of
different analytes. Interest in the use of polymeric
materials such as poly-dimethylsilioxane (PDMS)
130
Ha K., Joo G., Nisola G., Chung W., J. Kang C. and Kim Y. (2008).
CAPILLARY ELECTROPHORESIS ELECTROCHEMICAL DETECTOR WITH NOBLE MICROCHANNEL STRUCTURE FOR MINIATURIZATION -
Development of a Capillary Electrophoresis Microchip Format Electrochemical Detector for Endocrine Disruptors Sensing.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 130-133
DOI: 10.5220/0001046901300133
Copyright
c
SciTePress

and poly-methylmethacrylate (PMMA) has
increased over the past few years. PDMS has been
widely discussed due to good optical transparency
for detection, to cure at low temperatures, easily
replicates molding and good adhesion. Many PDMS
applications in CE microchip and microfluidic
channel design have been reported. This study
utilized PDMS as a substrate for formulating the
twisted CE microchannel for CE-AD microchip
device. As previously reported, a 3-microelectrode
system was fabricated on a glass substrate using bare
indium tin oxide (ITO) and Prussian blue (PB) as
catalyst on the working electrode.
2 EXPERIMENTAL
2.1 Materials and Chemicals
The chemicals used for PB electroplating solution
were composed of ferric chloride hexahydrate
(97%), potassium ferricyanide (99%), potassium
chloride, and hydrochloride acid (32%). The testing
analyte bisphenol A (BPA) was supplied by Wako.
All of which were diluted to get the desired
concentrations. Reagents used for microchannels
include Sylgard 184 PDMS from Dow Corning
Corp. (Midland, MI, USA). In order to mold PDMS
microchannels, negative photoresist SU-8 and XP
SU-8 developer from MicroChem Company were
used. Throughout the study, deionized water (DIW)
was used.
2.2 Microchip Fabrication
Figure 1 shows the simple process flow diagram for
the fabrication of the CE/ECD microchip. For the
electrodes in CE/ECD system, the ITO layer was
deposited on a glass substrate by R.F. magnetron
sputtering system. The thickness of the ITO film is
3400 Å. The 1.8 µm thick photoresist (AZ1512) was
spin-coated on the ITO-coated glass and patterned
for ITO electrodes. The sputter deposited ITO layer
was etched with FeCl
3
/HCl solution. In order to
avoid the interference of a high electric field with
EC detection, one decoupling-ground electrode as
the cathode of CE electric field was positioned in
front of the three-electrode electrochemical system.
The PB film was electrodeposited on the working
electrode. Electroplating solution is consisted of 20
mM FeCl3, 20 mM K
3
[Fe(CN)
6
], 0.2 M KCl and 0.1
M HCl. All the PB electrode surfaces were cleaned
with acetone and dried with N
2
gas. In order to
fabricate microchannels, 40 µm thick photoresist
(SU-8) was spin-coated and patterned on the silicon
wafer. The height of the positive patterns on the
moulding masters, which is equal to the channels
depth created on the PDMS layer, was 40 µm when
measured with a surface profiler. The PDMS layers
were fabricated by pouring a degassed mixture of
Sylgard 184 silicone elastomer and curing agent
(10:1) onto a molding master, followed by curing for
at least 1 hour at 72 °C. The cured PDMS was
separated from the mold, and reservoirs were made
at the end of each channel using a 3 mm circular
punch. The channel width is 80 µm. The double-T
injector channel had an offset of 170 µm with 5 cm
long separation channels and 1 cm long injection
channel. Also, the channel width is 80 µm. The
twisted injector channel had an offset of 170 µm
with 7 cm long separation channels and 1 cm long
injection channel. Before bonding the PDMS layer
to ITO-coated glass substrate containing the
electrodes, the two components were UV-Ozone
exposed to improve their bonding strengths.
2.3 Microchip Configuration
The microchip consists of four reservoirs and micro-
channels from PDMS (Fig. 2(a)), three electrodes
and electrodes for applying injection / separation
electric field on the glass substrate (Fig. 2(b)). The
width of the working electrode (W) is 100 µm, 50
µm for reference electrode (R) and 200 µm counter
electrode (C). In order to lower noise level, the
working electrode is surrounded by the reference
electrode.
2.4 CE/ECD Procedure
Each microchannel was preconditioned prior to use.
Acetone solution was flushed through the
microchannel for about 40 min to clean the
microchannels. Next, DIW and buffer solution were
flushed through the capillary for an hour
respectively. The running buffer solution was 10
mM 2-(N- morpholino) ethanesulfonic acid (MES)
fixed to pH 6.5 using 10 N NaOH. After
preconditioned, the entire microchannels would be
full of buffer solution. For CE process, no bubbles in
the capillary were permitted. The testing analytes
were injected by applying electric field of + 50 V/cm
from sample reservoir to sample waste reservoir.
With this process, the testing analytes are placed at
the head of the separation channel after about 7 sec
and buffer solution is still inside the microchannels.
Separation of the analytes was performed by
applying electric field of + 60 V/cm between buffer
CAPILLARY ELECTROPHORESIS ELECTROCHEMICAL DETECTOR WITH NOBLE MICROCHANNEL
STRUCTURE FOR MINIATURIZATION - Development of a Capillary Electrophoresis Microchip Format
Electrochemical Detector for Endocrine Disruptors Sensing
131
and detection reservoir. Amperometric detection was
performed in a three-electrode configuration. The
potential between working and reference electrode
was +700 mV DC in case of ECD for PB/ITO
electrode and +600 mV DC for bare ITO and Au
electrodes. When redox reaction between electrodes
and testing analytes happened, peak currents were
detected, recorded and stored directly on a computer.
Figure 1: Process flow for the fabrication of the CE/ECD
microchip.
3 RESULTS AND DISCUSSION
BPA was used to demonstrate the performance of
CE-AD microchip. Measurements were repeated for
several times. Figure 3 shows electropherogram of 1
mM BPA and butylphenol mixture by PB/ITO
electrode using double-T channel. Figure 4 shows
electropherogram of 1 mM BPA by PB/ITO
electrode using twisted channel. Especially figure 4
shows reproducibility of BPA detection. At the same
flow rates, the transport of ionic species to the
working electrode by double-T channel is slightly
faster than the twisted channel. Due to the curved
corners in twisted channels, the ionic transport rate
is slightly decreased. These zones are generally not
Sample Waste
Reservoir
Buffer
Reservoir
Sample
Reservoir
Detection
(a) (b)
(c) (d)
Figure 2: Configuration of (a) PDMS molding containing
microchannels and reservoirs and (b) electrodes on the
glass substrate. (c) Microchip side view and (d) Microchip
top view.
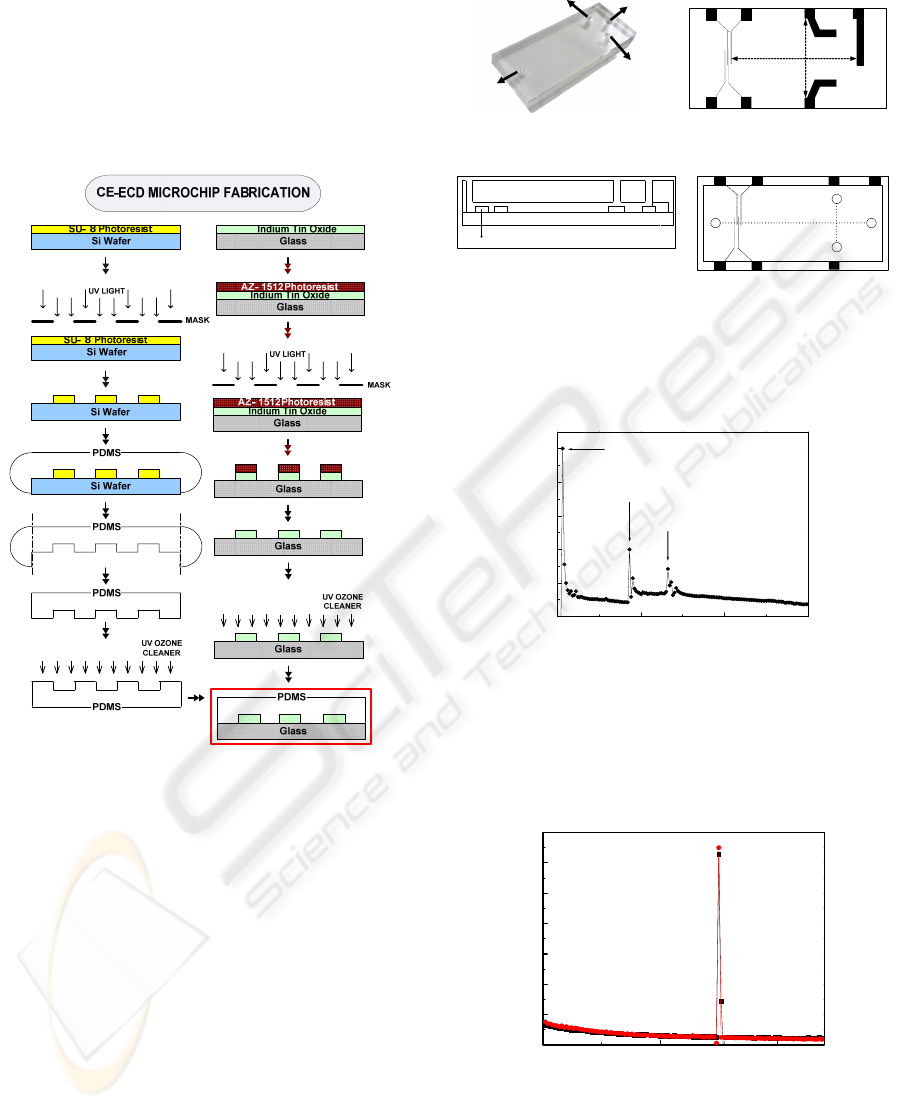
0 50 100 150
0
200
400
600
800
1000
Switching Peak
1 mM Butylphenol (64 sec, 146 nA)
1 mM Bisphenol A (41 sec, 315 nA)
Current (nA)
Time (sec)
Figure 3: Electropherogram of ECD using double-T
channel. Analytes are 1 mM BPA and 1 mM butylphenol
mixture. (PB/ITO electrode); Condition: Separation
voltage, 300 V; injection time, 7 sec; injection voltage, 60
V; detection voltage, 0.7 V
0 50 100
0
50
100
150
200
250
300
350
1 mM Bisphenol A (76 sec, 320 nA)
1 mM Bisphenol A (76 sec, 327 nA)
Current (nA)
Time (sec)
Figure 4: Electropherogram of ECD using twisted channel.
Analytes are 1 mM BPA. (PB/ITO electrode); Condition:
Separation voltage, 300 V; injection time, 7 sec; injection
voltage, 60 V; detection voltage, 0.7 V.
Reservoir
Sample Waste
Reservoir
Buffer
Reservoir
Sample
Reservoir
Detection
ervoir
R
C
W
Separation
E-field
Injection
E-
field
Res
C W
R
S
S
I
I
GLASS
ITO
PDMS
MICROCHANNEL
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
132
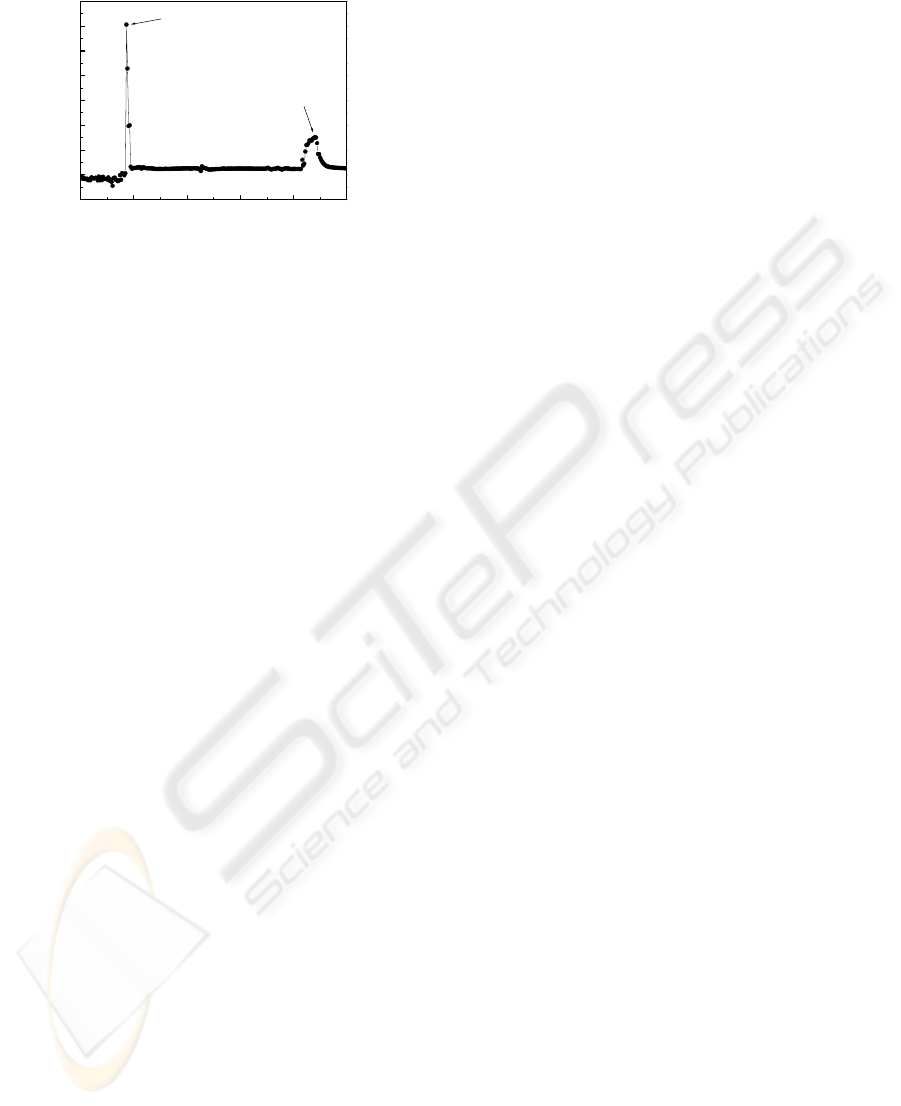
0 50 100 150 200 250
-100
0
100
200
300
400
500
600
700
1 mM Bisphenol A (208 sec, 130 nA)
Swiching Peak
Current (nA)
Time (sec)
Figure 5: Electropherogram of ECD using sodium-borate
buffer (pH 8.5). Analytes are 1 mM BPA. (bare ITO
electrode); Condition: Separation voltage, 300 V; injection
time, 40 sec; injection voltage, 60 V; detection voltage,
0.6 V.
affected by the separation electric field. The new
CE-AD chip is a fused PDMS-glass substrate. With
the CE-AD chip using twisted channel, 1 mM BPA
was detected. Results revealed that twisted channels
are effective to further miniaturize the exiting
double-T channel CE-AD chip; it also has a more
distinguished selectivity or analyte separation. After
a sample injection, the EC detector current rapidly
reached its steady-state background level with the
pH 6.5 buffer solutions and the BPA was easily
separated. The flat baseline and low noise level
indicate an effective isolation from the driving
voltage. Figure 5 show electropherogram of 1 mM
BPA by bare ITO electrode using sodium-borate
buffer. CE-AD using basic buffer is slower to flow
than CE-AD using acid buffer. Even if working
electrode is different, amperometric detector has
same channel structure.
4 CONCLUSIONS
The performance of our CE-AD microchip using
twisted channel was compared with those of
conventional CE-AD microchip using double-T
channel. Moreover, our device has several benefits
such as fast separation, high sensitivity, and simple
fabrication, which makes our CE-AD microchip a
good candidate to substitute the conventional CE-
AD devices. Our results demonstrate that twisted
channel is an effective technique to further reduce
the size of the current CE-AD LOC devices.
ACKNOWLEDGEMENTS
This work was supported by grant No. ROA-2006-
000-10274-0 from the National Research Laboratory
Program of the Korea Science & Engineering
Foundation.
REFERENCES
Y. Watabe, K. Hosoya, N. Tanaka, T. Kondo, H. Imai, M.
Morita, 2003. “ng/l Level of BPA Determination
Existing in Natural Water with HPLC-Electrochemical
Detection”, Japan analyst, Vol. 52, pp. 1167-1172
A. D'Antuono, V. C. Dall'Orto, A. L. Balbo, S. Sobral, I.
Rezzano, 2001. “Determination of Bisphenol A in
Food-Simulating Liquids Using LCED with a
Chemically Modified Electrode”, Journal of
agricultural and food chemistry, Vol. 49, pp. 1098-
1101
J. H. Kim, C. J. Kang and Y. S. Kim, 2004. “A disposable
polydimethylsiloxane-based diffuser micropump
actuated by piezoelectric-disc”, Microelectronic
Engineering, Vol. 71, pp. 119-124
J. H. Kim, C. J. Kang and Y. S. Kim, 2004. “A disposable
thermopneumatic-actuated microvalve stacked with
PDMS layers and ITO-coated glass”, Microelectronic
engineering, Vol. 73/74, pp. 864-869
R. S. Martin, A. J. Gawron, S. M. Lunte, C. S. Henry,
2000. “Dual-Electrode Electrochemical Detection for
Poly(dimethylsiloxane)-Fabricated Capillary
Electrophoresis Microchips”, Anal.Chem. Vol. 72, pp.
3196–3202
A. J. Gawron, R. S. Martin, S. M. Lunte, 2001.
“Fabrication and evaluation of a carbon-based dual-
electrode detector for poly(dimethylsiloxane)
electrophoresis chips”, Electrophoresis, Vol. 22 , pp.
242-248
J. Wang, M. P. Chatrathi, B. Tian, 2001.
“Microseparation Chips for Performing ultienzymatic
Dehydrogenase/Oxidase Assays: Simultaneous
lectrochemical Measurement of Ethanol and Glucose”,
Anal. Chem., Vol. 73, pp. 1296-1300
CAPILLARY ELECTROPHORESIS ELECTROCHEMICAL DETECTOR WITH NOBLE MICROCHANNEL
STRUCTURE FOR MINIATURIZATION - Development of a Capillary Electrophoresis Microchip Format
Electrochemical Detector for Endocrine Disruptors Sensing
133
