
E-ORDERING IN THE PHARMACEUTICAL SUPPLY CHAIN
Explaining Standardisation from a Collective Action Perspective
Stefan Schellhammer, Kai Riemer and Stefan Klein
European Research Center for Information Systems (ERCIS)
Department of Information Systems, The University of Münster
Leonardo-Campus 3, Münster, Germany
Keywords: Pharmaceutical Industry, Collective Action, Standardisation, Interorganisational Information System (IOIS).
Abstract: In this paper, we discuss a unique case of industry-wide standardisation, i.e. the proliferation of an elec-
tronic ordering protocol across wholesalers and community pharmacies in the Republic of Ireland. The exis-
tence of multiple parties involved in the standardisation process and the nature of the standard lead us to
study the case from a collective action perspective. In doing so, the emergence and the diffusion of industry-
wide standards are being studied as distinct but connected set of dilemmas. The case leads us to theorise that
strong industry associations play a significant role in the initiation and success of such standardisation ef-
forts on the industry level. Due to space restrictions this short paper can only provide a snapshot of our en-
tire argument; a long version can be obtained from the authors.
1 INTRODUCTION
The formation of interorganisational information
systems (IOIS) has been widely studied in the IS
literature. Typically, the purpose of an IOIS lies in
supporting, facilitating, or improving inter-
organisational (business) transactions, with elec-
tronic data interchange as the core building block. In
this paper, we specifically focus on vertical informa-
tion systems (VIS) that promote data exchange and
business process coordination between business
partners along the supply chain. In doing so, we
concentrate on the development of core standards as
a prerequisite for the development of interoperable
systems among the business partners. The core stan-
dards encompass communication protocols, message
syntax and semantics, such as product codes. De-
spite the large body of literature on standardisation
and IOIS it still remains unclear why in some indus-
tries open standardised IOIS have emerged whereas
in others competing systems developed.
IOIS are sometimes regarded as a strategic de-
vice to improve customer retention through lock-in,
while in other cases IOIS may serve as a means to
collectively reduce transaction costs. In this paper,
we will concentrate on the second type and briefly
juxtapose it with an example of the former type. Our
case covers the standardisation process in the crea-
tion of a universal electronic ordering system in the
Irish pharmaceutical distribution, which took place
during the 1980s. In contrast to the standardised
Irish solution, in the British pharmaceutical distribu-
tion industry several competing electronic ordering
system have emerged.
Our data shows that the resulting differences
cannot be explained by environmental factors like
regulation or by the needs of their users; most of
these factors are strikingly similar. The question
arises what triggered the set up of the Irish system in
this unique way. We will argue that an industry as-
sociation has played a significant role in this proc-
ess, and that the role and importance of associations
for the emergence of IOIS has not been studied suf-
ficiently (Damsgaard & Lyytinen, 2001).
We are facing two challenges: (1) How can we
explain that the initiators or sponsors of electronic
ordering solutions (in our case the wholesalers) par-
tially suspended their competition and agreed on
standards to be used for electronic ordering? (2)
How can we explain the adoption and diffusion
across a heterogeneous and fragmented group of
business partners, i.e. the pharmacies. We will ap-
proach this effort from a collective action theory
perspective. In our analysis we draw on earlier work
conducted in this field (Markus et al., 2006).
20
Schellhammer S., Riemer K. and Klein S. (2008).
E-ORDERING IN THE PHARMACEUTICAL SUPPLY CHAIN - Explaining Standardisation from a Collective Action Perspective.
In Proceedings of the First International Conference on Health Informatics, pages 20-25
Copyright
c
SciTePress

In the next section we present the Irish case. Af-
ter this we introduce collective action theory before
we analyse and discuss the case.
2 IRISH PHARMACEUTICAL
DISTRIBUTION INDUSTRY
In this section we document how EDI standards
emerged in the Irish pharmaceutical distribution
between wholesalers and community pharmacies.
We begin by introducing our method and give a
brief overview of the pharmaceutical industry in
Ireland. This is followed by a description of the
emergence of the standard itself and its subsequent
use by the actors.
2.1 Method
A case study design has been chosen to conduct the
research, because of the complexity of the research
question and its focus on a rich real-life context
(Yin, 2003). Four semi-structured interviews have
been conducted, two with a representative of one of
the Irish pharmaceutical wholesalers, one with lead-
ing managers at the Irish body of community phar-
macists (IPU) and one with a manager of a large
software system vendor. All interviews were tape
recorded, transcribed, coded and analysed. The data
was evaluated independently by two researchers.
Furthermore, several other data sources were used
for the study – among these are web sites, standards
documents, systems documentations etc.
2.2 Market Structure
The task of the pharmaceutical wholesalers is to
provide a national wholesale service for pharma-
ceuticals for community pharmacies and hospitals.
The relevant market is demarcated by the national
borders. As a result of a consolidation process over
the past 10-15 years, the market in the Republic of
Ireland (R.I.) is nearly evenly divided between three
wholesalers. All Irish wholesalers operate as na-
tionwide full-line suppliers.
On the customer side more than 1400 community
pharmacies exist in Ireland. Irish pharmacists are
represented by a professional body, the Irish Phar-
maceutical Union (IPU), which represents 90% of
all Irish pharmacists. The mission of the IPU is to
promote the professional and economic interest of its
members. This incorporates conducting negotiations
on behalf of the members and the development and
maintenance of a “constructive dialogue with gov-
ernment, agencies and other groups in relation to
matters of mutual interest.” (IPU, 2006)
Three software vendors serve the pharmacy mar-
ket. Their product, the “patient medication record”
(PMR) incorporates the EDI standards as basis for
the ordering module. Around 1300 pharmacies work
with computer systems (typically with electronic
point of sales systems (EPOS) and dispensary soft-
ware).
The wholesale prices for drugs are fixed as is the
margin for wholesalers. However, the wholesalers
de facto pass on a significant part of their margin to
the pharmacies via rebates, bonus schemes and other
price incentives (Fingleton et al., 2002). These pre-
scription drugs are paid by the patients or they are
reimbursed to the pharmacies through a variety of
state-administered schemes (GMS, DPS, LTI).
As a result of these regulations the use of pricing
policies by wholesalers and pharmacies is very re-
stricted. As wholesalers typically offer volume dis-
counts, pharmacies generally use one wholesaler as
the primary supplier and a second one to split pur-
chases and as a fall-back when supply of a particular
product cannot be obtained (Fingleton et al., 2002).
While the wholesalers compete for becoming a
pharmacy’s preferred supplier, the pharmacies use
their bargaining power as a result of low switching
costs: The possibility to switch between wholesalers
alleviates the problem of stock shortage on the
wholesalers’ side, because they risk loosing pharma-
cies in case of repeated stock shortages. Swift deliv-
ery is a crucial element of the pharmaceutical supply
chain. Consequently, most pharmacies operate on
very small stock. This is made possible by extensive
logistics operations. Pharmacies can rely on very
short delivery times and deliveries two times a day
by each of the wholesalers. Wholesalers will ship on
the same day all orders that are received by the cut-
off time late in the morning.
2.3 Emergence of the Standard
In 1984 United Drug (UD), one of the wholesalers,
studied the emergence of electronic ordering sys-
tems (McKesson, see (Johnston & Vitale, 1988)) and
intended to adapt one of the U.S. solutions for the
Irish market. The Irish pharmacies, in dispensing
medicines, only kept handwritten records at the time.
Because pharmacy market regulation varies sig-
nificantly across countries, UD decided to develop a
new solution from scratch. In doing so, UD played
with the idea of developing a proprietary ordering
system; the strategic rationale being to lock-in
E-ORDERING IN THE PHARMACEUTICAL SUPPLY CHAIN - Explaining Standardisation from a Collective Action
Perspective
21
pharmacies and subsequently to increase market
share. The idea was to take the UK market as a
blueprint where American Hospital Supply had suc-
cessfully established ordering software to lock-in
hospitals.
While the wholesalers claim that they ultimately
realised the shortcomings of a proprietary solution it
was the Irish Pharmaceutical Union (IPU) who
strongly engaged in the process and came out with
an open solution. While the IPU regarded the move
from placing orders over the phone to submitting
electronic order files as a clear administrative advan-
tage for the pharmacies (faster, less errors etc.), key
actors at the IPU emphasised the benefits of a stan-
dardised solution over a number of proprietary solu-
tions for the pharmacies.
As a result, the IPU facilitated negotiations
among the wholesalers to develop both a standard-
ised order data transfer protocol and a common
numbering system (based on EAN). While whole-
salers in the UK were introducing product number-
ing based on the PIP code, IPU had been negotiating
with EAN (at that stage it was EAN UK, today it is
GS1 Ireland) to use its numbering scheme. The idea
was to administer a central product identification
number that does not differ between wholesalers, as
was (and is) the case in the UK (Chemist&Druggist,
2007). In order to facilitate the introduction of EAN
numbering codes, the IPU itself applied for and was
granted manufacturer status in order to be able to
assign EAN numbers to pharmaceuticals.
In parallel, the IPU facilitated negotiations
among the wholesalers and system vendors. Consen-
sus on a common protocol was achieved after about
6 months. At the time, half a dozen system vendors
developed pharmacy solutions and all of them in-
cluded the electronic ordering standards into their
packages. Conceptually, this led to the proliferation
of different IOIS, all of which are using the same
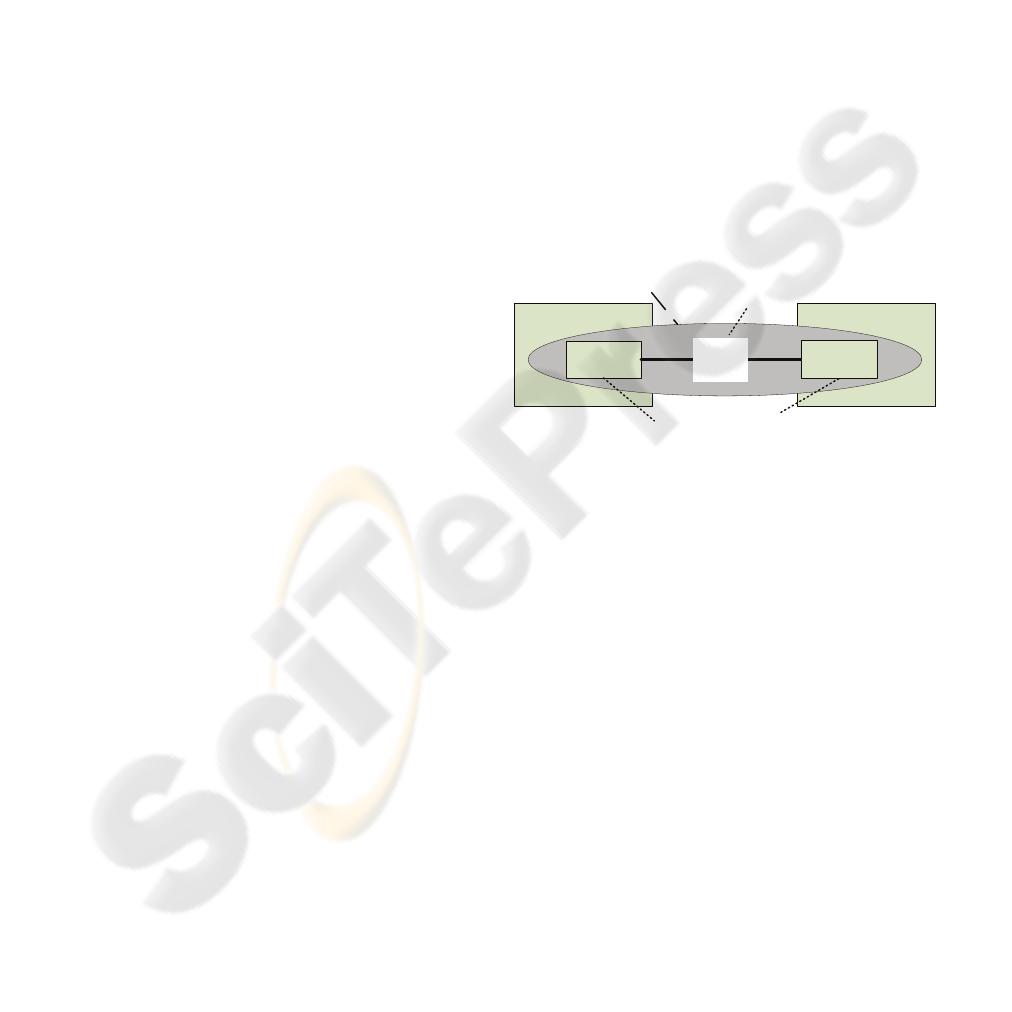
standardised EDI components (see Figure 1).
2.4 System Usage and Maintenance
The diffusion of electronic ordering happened
gradually over a 10 year period; today, all pharma-
cies are able to order electronically and the transac-
tions between wholesalers and pharmacies are still
based on the same system. Surprisingly, even the
modem-based communication protocol has survived
virtually unchanged. Electronic ordering (eOrdering)
accounts for 90 percent of all orders.
The wholesalers’ systems accept incoming or-
ders (wants lists) via a modem connection and send
back an order confirmation. If all items on the
“wants list” can be supplied no response is sent. In
any other case the systems send back a list with un-
available items. Furthermore, bonus items are re-
ported back to the pharmacist in this way. The
pharmacy can then immediately turn the list of out
of stock items into an order with one of the other
wholesalers.
The IPU-product file is a complete list of all
products available in an Irish pharmacy. It identifies
each item by an IPU-number. Furthermore, the file
also contains characteristics of the product (package
size, ingredients and toxic class), consultation ad-
vices, prices and additional codes for reimburse-
ment. For maintenance and administration of the
file, the IPU operates a dedicated department. The
IPU distributes the product file on a monthly basis to
all pharmacies, wholesalers and system vendors.
The order protocol uses the IPU product file for
product identification and the IPU communication
protocol called IPUCOMMS v 2.4a for the modem
dial-up link from the pharmacy to the wholesalers.
order
module
codes,
protocols,
messages
order
module
IOIS
wholesaler system pharmacy system
non-standardised
components
standardised
components
Figure 1: Components of the IOIS.
3 COLLECTIVE ACTION
The case presented above shows the development of
common EDI standards, which are the cornerstone
of the electronic ordering system that is still used in
Ireland today. We argue that the presented phe-
nomenon of joint standard development can be in-
terpreted as a case of collective action.
The Theory of Collective Action deals with the
provision of public goods. It explores the market
failures where individual rationality and self-interest
do not lead to an efficient provision of public or col-
lective goods. Please note that, in our case, we are
looking at standards as an instance of non-pure pub-
lic goods, i.e. club goods that belong to a sub group
of the market (they are excludable (Kindleberger,
1983)), but share similar characteristics.
Markus et al. have studied the consequences that
collective good characteristics have on standardisa-
tion. (Markus et al., 2006) They differentiate the
HEALTHINF 2008 - International Conference on Health Informatics
22

development and the diffusion of standards as two
distinct dilemmas. They come to the conclusion that
for a successful standardisation both need to be
solved at the same time.
The dilemma of the development of a standard
refers to the different areas of conflict that exist
when a standard is developed in a consortium: Con-
flicts of interest refer to the heterogeneity of inter-
ests among the actors; conflicts of alignment refer to
the cost of aligning internal systems to the new stan-
dard; and conflicts of appropriation result from dif-
ferent cost/ benefit structures of the actors (Müller-
Tengelmann, 1995). Mechanisms have to be found
to solve these conflicts to ensure successful stan-
dards development.
The dilemma of the diffusion of standards refers
to the incentive to delay the adoption of a standard
until a critical mass has been reached. The diffusion
of public goods (e.g. communication standards) is
often hampered by what is called the penguin effect
(Farrell & Saloner, 1987): Early adopters cannot
capitalise fully on the standard as long as no other
actor adopts the standard (negative network effect).
The interconnection between both dilemmas has
been described by Markus et al.: “…standards con-
tent can be seen both as an outcome of the mecha-
nisms employed by a VIS standards-setting consor-
tium to resolve collective action dilemmas and as an
input to diffusion on the VIS standards devel-
oped.”(Markus et al., 2006)
The successful development of a standard is the
conditio sine qua non for its diffusion. However, it
does not guarantee the success of the standard in the
market. To the contrary, strategies to solve the de-
velopment dilemma can turn out to be counterpro-
ductive for its diffusion. Hence, the dual dilemma
situation can only be solved if both dilemmas are
targeted simultaneously.
4 CASE ANALYSIS
In this section we analyse the driving factors behind
the standardisation process in the Irish case using
collective action as the conceptual framework.
4.1 Standards Development
While different actors claim ownership of the idea of
a standardised product code, the IPU certainly has
been one of the most vocal advocates of standard-
ised solutions at a time when some of the wholesal-
ers were still considering proprietary solutions. Their
rationale was threefold: a single standard in the Irish
market would facilitate the existing practice of order
splitting and thus maintain the lever by which the
pharmacies executed their (small) power vis-à-vis
the much bigger wholesalers. Secondly, standards-
based electronic ordering would be the most effi-
cient way of ordering and would help to reduce
transaction costs. Thirdly, the Irish market is too
small to justify competing solutions.
Moreover, the IPU clearly played a crucial role
in facilitating and moderating the negotiations be-
tween the wholesalers. The IPU provided a neutral
venue and drove the negotiations without being per-
ceived as partisan. In effect they facilitated joint
action of the wholesalers. The negotiations took
about six months which were regarded as efficient
by the participants.
In doing so, the IPU played a triple role: Next to
the moderator or broker role, the IPU represented
the pharmacists and their interests. Given that they
represented the overwhelming majority of pharma-
cists, they had a strong mandate to articulate their
constituents’ interests. Through this representative
participation, they not only pushed hard for a stan-
dardised solution but also shaped the design of the
standards. Moreover, the IPU provided some initial
assurance that the solution would be adopted by the
pharmacies, the prospective adoptors: the IPU de
facto overcame the fragmentation of the pharmacies
and ensured that the new system would perpetuate
and indeed facilitate the established practices of or-
der splitting. Thirdly, the IPU became the secretary
of the standardisation process; in particular they
agreed to take the role of developing and maintain-
ing the product codes (IPU product file). Thereby
they ensured the sustainability of the chosen solution
and reinforced their role in the market by securing
an additional revenue stream based on the license
fees for the product file.
All in all the IPU managed to contain the con-
flicts of interest among the wholesalers (horizontal
conflicts of interest) as well as potential conflicts of
interest between the wholesalers and the pharmacies
(vertical conflicts of interest).
The alignments required of the internal systems,
as another potential area of conflict, could be kept to
a minimum by developing a product code that con-
forms to the EAN-13 standard that was already be-
ing processed by the wholesalers. Thus, no major
alignments were required. With respect to the order-
ing protocol, the negotiating process has been de-
scribed as very cooperative in the sense that differ-
ent needs of the participants were accommodated.
Under the prime aim to hammer out a common pro-
tocol, the wholesalers tried to make sure that it
E-ORDERING IN THE PHARMACEUTICAL SUPPLY CHAIN - Explaining Standardisation from a Collective Action
Perspective
23

would dove-tail with their systems. Furthermore, the
scope of the solution was kept lean so that align-
ments were kept to a minimum.
Conflicts of appropriation are the third area of
potential conflict. Our data reveals no indication of
this type of conflict. Ordering protocol and product
file are administered and maintained by the IPU. The
IPU refinances these activities through licensing fees
from wholesalers and manufactures. Our data does
not indicate that this division of tasks has ever been
challenged by the actors.
The case shows a successful development of a
standard. This is especially interesting as in other
countries with similar characteristics like the UK
(but also Australia) proprietary solutions exist. Good
reasons can be found for either option: competitive
advantages and customer lock-in on the one side,
efficiency and swift diffusion on the other.
However, in the Irish case it was the IPU who
facilitated the development of a joint solution. As
the wholesalers are the main beneficiaries from elec-
tronic ordering, the IPU was in a position to articu-
late requirements on behalf of the pharmacies while
providing assurances of the adoption and diffusion
of the solution. Today, the product numbering
scheme can be seen as a new power basis for the
IPU. The IPU succeeded to preserve that power ba-
sis against the PIP-code solution of the wholesalers.
Thereby the IPU was able not only to satisfy needs
of its members but also to bring itself in a better po-
sition for future negotiations. In the end, the whole-
salers made themselves dependent on the IPU and its
product code.
4.2 Standards Diffusion
While the literature reports cases where the parties
involved in the standard development later blocked
or delayed their diffusion, the Irish pharmaceutical
wholesalers embraced and supported the new stan-
dards. All wholesalers have integrated the new or-
dering standards into their own systems. The same is
true for the system vendors supplying the pharma-
cies with the software.
While the IPU encouraged its members to install
and use the new electronic ordering facility, it is
reported that in the beginning pharmacists have been
reluctant to use the new technology; they saw the
advantages of the new system on the wholesalers’
side. The wholesalers initially responded with dis-
counts on electronic orders. Today, about ninety
percent of all orders reach wholesalers electroni-
cally.
The high implementation ratio of wholesalers
can be explained by the low costs of alignment and a
lack of feasible alternatives due to the bargaining
power of the pharmacists represented by the IPU. No
wholesaler tried to impede the development process
openly. And any attempt to promote a proprietary
system by an individual wholesaler afterwards
would not have been tolerated by the pharmacies,
but would likely have encouraged them to switch to
the other wholesalers. Furthermore, all system ven-
dors took part in the negotiations. A multiplicity of
proprietary standards would not have been in their
interest, because pharmacists would have pressured
them to build software incorporating all different
standards.
Several factors can be identified that favoured
the industry-wide standard and ultimately triggered
its success: First, the IPU has a strong standing to-
wards the wholesalers. The wholesalers were very
aware that anything that would run against the inter-
est of the pharmacists would face strong and painful
opposition by the IPU. Furthermore, the IPU was not
taken by surprise when the wholesalers started to
develop electronic ordering solutions. Rather, the
IPU was aware and attentive towards these new
technologies and their implications for its members.
This set the IPU in a position to intervene at an early
stage, where the different stakeholders had not yet
firmly committed themselves nor invested into a
particular technology. Timing was critical and the
IPU clearly used it to its advantage.
Another factor that facilitated the industry-wide
standardisation can be seen in the relatively small
group of wholesalers operating in a small and con-
fined market. Regulated prices and margins pose a
high pressure on wholesalers to optimise their proc-
esses and save costs in warehousing, delivery and
order processing. Therefore a high incentive existed
to get such a system working and thereby streamlin-
ing the order process.
5 CONCLUSIONS
While we are interested in the ordering system as a
VIS or IOIS, our analysis has focused on the under-
lying standards as core prerequisites of interorgani-
sational solutions. The Irish case reinforces the no-
tion that standardisation processes for VIS are in-
deed precarious. Proprietary solutions have been
considered by the initiators and have been chosen in
other countries with plausible strategic motives.
Our data supports the notion of a dual dilemma
of standardisation, which needs to be addressed si-
HEALTHINF 2008 - International Conference on Health Informatics
24

multaneously. The dilemma implicitly also ad-
dresses the need to overcome horizontal competition
(among wholesalers) and vertical conflicts of inter-
est (among wholesalers and pharmacies).
With respect to our research questions we have
found theoretical explanations for the unique case
situation. The single most important part of our ex-
planation rests on the role, power and reputation of
the IPU. The IPU facilitated to initiate and moderate
collective action initially among the wholesalers and
subsequently with an increasing mandate and role
for themselves as party to the negotiations. By repre-
senting the overwhelming majority of the pharma-
cists they overcame the fragmentation of the market
and changed the power dynamics in the negotiations.
Moreover, they provided assurances with respect to
the adoption of the standards. The IPU established
themselves successfully as standards keeper. The
availability of widely accepted standards like EAN
product coding schemes clearly helped to build
credibility and to enhance the acceptance of the cho-
sen solution.
The wholesalers as initiators of ordering systems
agreed on common standards because they saw (or
were alerted to) the obvious interests of the pharma-
cies as potential adopters.
However, the constellations of actors, historical
and regulatory environment has been quite unique.
While we have found theoretical explanations for the
outcome ex post, similar developments are still far
from predictable. The notion of causality remains
contested: too many contingencies and considera-
tions are at play, which could have lead to another
outcome. Hence we have tried to establish plausible
reasons.
The specific actor constellation, in particular the
multiple roles which the IPU played successfully,
explain the achieved consensus. However, there
were a number of facilitating contingencies, which
have not determined the outcome but help to explain
it: The historical coincidence of standard develop-
ment in a technological “virgin market”, where the
business partners had not yet invested in their own
systems, convinced all parties – including the soft-
ware vendors – to pursue the chosen standards. The
area of consensus building (product code, order
message, communication protocol) facilitated the
consensus building. The standards guaranteed inter-
operability between the applications yet in a model
of loose coupling.
In the end, the strong role(s) of the IPU, in com-
bination with the economic benefits (and incentives)
of electronic ordering and the existence of one un-
contested standard, lead to wide adoption and a sus-
tainable solution.
ACKNOWLEDGEMENTS
The study described in this paper is part of a re-
search project (no. 1328/2-2) funded by a research
grant of the DFG (German National Science Foun-
dation) and is concerned with the question of how
institutional and national factors influence structures
and evolutionary paths of IOISs.
REFERENCES
Chemist&Druggist. (2007). The PIP code in the pharma-
ceutical supply chain. Retrieved 16.07., 2007, from
http://www.dotpharmacy.co.uk/pip.html
Damsgaard, J., & Lyytinen, K. (2001). The Role of Inter-
mediating Institutions in the Diffusion of Electronic
Data Interchange (EDI): How Industry Associations
Intervened in Denmark, Finland, and Hong Kong. The
Information Society, 17(3), 195-210.
Farrell, J., & Saloner, G. (1987). Competition, Compatibil-
ity and Standards: The Economics of Horses, Penguins
and Lemmings. In H. L. Gabel (Ed.), Product Stan-
dardization and Competitive Strategy (11 ed., pp. 1-
21). Amsterdam: Elsevier.
Fingleton, J., Purcell, D., & Goggin, I. (2002). Report of
Investigation (pp. 1-64): Competition Authority.
IPU. (2006). About the IPU. Retrieved 16.07, 2007, from
http://www.ipu.ie/index.php?option=com_content&tas
k=view&id=17&Itemid=42
Johnston, H. R., & Vitale, M. R. (1988). Creating Com-
petitive Advantage with Interorganizational Informa-
tion Systems. MIS Quarterly, 12(2), 153-165.
Kindleberger, C. P. (1983). Standards as Public, Collective
and Private Goods. Kyklos, 36, 377-396.
Markus, M. L., Steinfield, C. W., Wigand, R. T., &
Minton, G. (2006). Industry-wide IS Standardization
as Collective Action: The Case of the US Residential
Mortgage Industry. Management Information Systems
Quarterly, 30(Special Issue), 439-465.
Müller-Tengelmann, H. (1995). Kollektive
Investitionsstrategien - Der elektronische
Datenaustausch als überbetriebliche Infrastruktur.
Frankfurt am Main; Berlin; Bern; New York; Paris;
Wien: Lang.
Yin, R. K. (2003). Case Study Research: design and
methods: Sage Publications Inc.
E-ORDERING IN THE PHARMACEUTICAL SUPPLY CHAIN - Explaining Standardisation from a Collective Action
Perspective
25
