
Using Resource-saving Wastewater Treatment Technology in an
Industrial City
Natalia V. Barabash
a
and Valeria N. Orobinskaya
b
Institute of Service, Tourism and Design (Affiliate), North-Caucasus Federal University, Pyatigorsk, Russia
Keywords: Waste Water, Treatment Methods, Coagulation, Coagulant "Bopak-E", Resource Saving.
Abstract: Rapid demographic growth of the population has led to the fact, that the renewable water resources are not
renewable over time. Already, today there is a shortage of fresh water. Methods for purification and resource
saving technology of water is the only alternative directions in coming decades. Therefore, there is a great
need for the development of suitable, inexpensive and fast wastewater treatment and reuse or conservation
methods in this century. Various types of water treatment and recycling methods were discussed in terms of
their fundamentals, applications, costs, maintenance and suitability. In addition, a systematic approach to
water purification and recycling was presented, including their understanding, assessment and selection of
parameters. A quick guide on the selection of appropriate technologies for specific applications was evaluated.
The article discusses not only global issues related to solutions to the problem of water shortage, but also the
results of experimental studies showing the feasibility of using a purification method using coagulants.
1 INTRODUCTION
One of the most important international political
agendas is the conservation of resources, today
conditionally renewable fresh water resources – so in
July 28, 2010 The UN General Assembly has
included the right to water in the list of basic human
rights. The aggravation of global and regional
problems related to freshwater water resources has
led, according to the UN, to the fact that in the 21st
century water is a strategically valuable resource,
gradually displacing oil and gas from the market.
According to the research of Petukhova E.O.
(Petukhova, 2018) “… In many countries of the
world, programs are being developed to ensure water
security, since a ton of clean water in an arid climate
is already more expensive than oil. Today this
problem is relevant in various areas and industries.
Conservation, efficient using (recycling) and
securing of clean freshwater render focus of
sustainable development policies and economic
growth in the countries including the Russian
Federation, as Russian input in Top 5 largest
a
https://orcid.org/0000-0001-5361-1081
b
https://orcid.org/0000-0002-6484-3109
countries in the world by fresh water reserves (Fig.
1).
In terms of fresh water reserves, the Russian
Federation ranks second after Brazil, more than 2.5
million rivers and 2.7 million lakes and freshwater
depots are glaciers (Gupta et al, 2012, Danilov-
Danilyan, 2009, Sazhin et al, 2012).
In order to preserve water resources in the main
drainage areas, it is necessary to solve the following
problems, considered on the example of the North
Caucasian Federal District, the solution of which will
stabilize the economic situation associated with this
region (Gupta et al, 2012, Danilov-Danilyan, 2009,
Sazhin et al, 2012).
Barabash, N. and Orobinskaya, V.
Using Resource-saving Wastewater Treatment Technology in an Industrial City.
DOI: 10.5220/0010586500810088
In Proceedings of the International Scientific and Practical Conference on Sustainable Development of Regional Infrastructure (ISSDRI 2021), pages 81-88
ISBN: 978-989-758-519-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
81
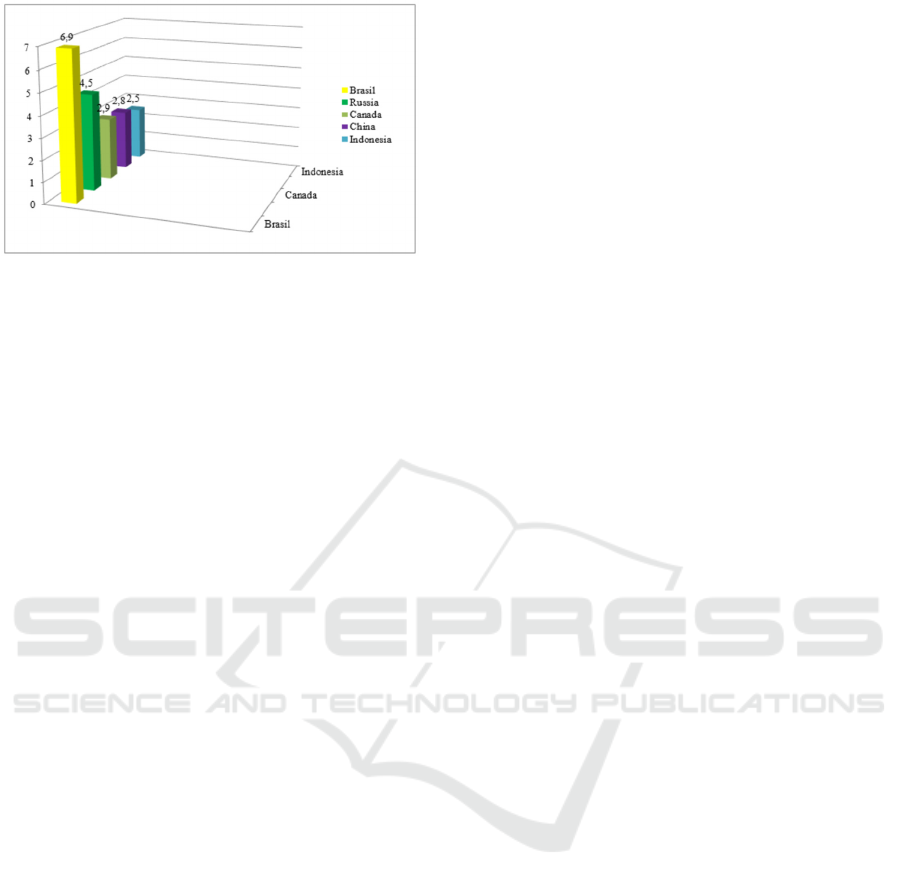
Figure 1: Countries of the world rich in fresh water
reserves.
Pollution Pool and Don River sewage led to
shortages of freshwater sources, depletion and
degradation of small rivers.
Kuban River basin - one the of the strategic
objects of water resources in the region, has led to
water use restrictions for the needs of the population,
agricultural organizations and industry.
This situation is a consequence of the presence of
a fairly large number of problems related to water use
in Russia and the North Caucasian Federal District in
particular.
One of the main problems of water use in Russia
is excessive pollution of water bodies. On average,
per year in the Russian Federation, wastewater
discharge into surface water bodies is 54,712 million
m3, of which polluted wastewater is 36%, including
21% without any treatment (Gupta et al, 2012,
Danilov-Danilyan, 2009, Sazhin et al, 2012).
The demand for water is enormous and increases
every year. But, even realizing the importance of the
role of water, a person still continues to toughly
exploit water bodies, irrevocably changing their
natural regime with discharges and waste.
At present, the problem of water pollution is
perhaps the most urgent. And one of the most
important aspects of environmental protection is the
protection from pollution of water resources and
ensuring the needs of the population and the national
economy with clean water.
“… .According to Rosstat, economic growth in
Russia is accompanied by a disproportionate
deterioration in the environmental situation, Fig. 1 . ,
it is noted that in third place, as a pollutant, is energy,
more precisely, “production and distribution of
electricity, gas and water” (21.2%) ... ” (Sazhin et al,
2012).
To reduce the negative impact on the
environment, it is necessary to implement the
necessary environmental protection measures, such
as the treatment of waste, industrial and storm water.
This is possible at sewage treatment plants, where
wastewater used in the process of life is cleaned and
neutralized to such an extent that it does not have a
harmful effect on the environment. But despite all the
measures taken to protect water bodies, treatment of
waste water, which are formed as a result of
household and industrial activities, not always is so
effective event and requires continuous improvement.
Disposal of waste water and neutralization is a
one second of the most important ecological
problems of the present time and in this direction
continuously carried out a wide variety of research,
which are based on physico-chemical or biochemical
degradation processes harmful components of waste
water.
2 MATERIALS AND METHODS
What do we mean by the concept of "waste water"? It
is the water, the former in the domestic, industrial or
agricultural use, as well as passing through any
contaminated areas. Depending on the conditions of
formation, wastewater is divided into domestic or
household fecal, atmospheric (storm) and industrial.
Domestic water formed from Stock s showers,
baths, kitchens, toilets, washing floors and from
others. They contain impurities, of which
approximately contains 58% organic matter and a
42% mineral (Gupta et al, 2012).
Atmospheric waters are formed as a result of
atmospheric precipitation and flowing down from the
territories of enterprises. They are contaminated with
organic and mineral substances. Industrial
wastewaters are liquid wastes which arise during
extraction and processing organic and inorganic
materials. The quantity and quality composition of
industrial wastewater depends on the type of
production.
The WHO committee recommended the
following classification of chemical water pollutants:
1) biologically unstable organic compounds;
2) low toxic e inorganic salts;
3) petroleum products;
4) biogenic compounds;
5) substances with specific toxic properties,
including heavy metals.
There are several ways to reduce the amount of
contaminated wastewater, including such as:
development and implementation of anhydrous
technological processes; improvement of existing
processes; development and implemented equipment;
introduction of air coolers; reuse of treated
wastewater in recirculating and closed systems.
ISSDRI 2021 - International Scientific and Practical Conference on Sustainable Development of Regional Infrastructure
82

The lack of clean natural waters and the high
demand of the industry for water determine the need
to continue work on the further improvement of
treatment systems.
Under these conditions, the development of new
technological solutions that ensure high and stable
quality of wastewater treatment is relevant and in
demand.
The choice of one or another treatment method is
carried out taking into account the sanitary and
technological requirements for treated wastewater for
the purpose of their further discharge into a water
body and use, as well as taking into account the
volume of wastewater and the concentration of
pollution in them, the necessary material and energy
resources, and economic efficiency. process.
The use of physicochemical methods for
wastewater treatment has a number of advantages
over biochemical ones:
1) the ability to remove toxic biochemically non-
oxidizable organic pollutants from wastewater;
2) achieving a deeper and more stable degree of
cleaning;
3) smaller structures;
4) less sensitivity to load changes;
5) the possibility of full automation;
6) a deeper study of the kinetics of some
processes, as well as issues of modeling,
mathematical description and optimization, which is
important for the correct choice and calculation of
equipment;
7) methods are not related to control over the
activity of living organisms;
8) the possibility of recovering various
substances.
Of the most common methods of physicochemical
purification, it is necessary to single out, first of all,
the use of various reagents that cause coagulation of
contaminants, which are indispensable in cases where
it is necessary to remove finely dispersed suspended
substances from wastewater.
What is coagulation? Coagulation is the process
of conglomeration of suspended particles through
interaction and aggregation. Used in clearing
(recycling) of waste water with the aim of
accelerating the precipitation of fine impurities and
emulsified substances under the influence of special
compounds – coagulants, forming flakes of metal
hydroxides, precipitate. Aggregation occurs due to
the difference in charges of colloidal and suspended
particles (weak negative charge) and coagulating
agents (weak positive charge), which leads to
aggregation (Joss et al, 2006).
The process of hydrolysis of coagulants and the
formation of flocs occurs in the following stages:
Me
3+
+ HOH → Me (OH)
2+
+ H
+
Me (OH)
2+
+ HOH → Me (OH)
2
+
+ H
+
Me (OH)
2
+
+ HOH → Me (OH)
3
+ H
+
Me
3+
+ 3 HOH → Me (OH)
3
+ 3 H
+
In reality, the hydrolysis process is much more
complicated. The metal ion forms a number of
intermediates through reactions with hydroxide ions
and polymerization. The formed compounds have a
positive charge and are easily adsorbed by negatively
charged colloidal particles (Bernet et al, 2000,
Rodionov et al, 2000).
As coagulants, salts of aluminum, iron or their
mixtures are usually used. The choice of coagulant
depends on its composition, physicochemical
properties and cost, the concentration of impurities in
the water, on the pH and salt composition of the
water.
As coagulants used aluminum sulfate Al
2
(SO
4
)
3
×18 H
2
O; sodium aluminate NaA1O
2
;
oxyaluminum chloride Al
2
(OH)
5
Cl ; tetraoxo c
ulphates of aluminum-potassium and aluminum-
ammonium [alum - potassium alum KAl (SO
4
)
2
×
12 H
2
O and ammonia NH
4
Al ( SO
4
)
2
× 12 H
2
O ].
Of these, the most widespread is aluminum sulfate,
which is effective in the range of pH = 5-7.5. It is
highly soluble in water and has a relatively low cost.
It is used in dry form or as 50% - of the solution.
Of iron salts, iron sulfates Fe
2
(SO
4
)
3
× 2 H
2
O,
Fe (SO
4
)
3
× 3 H
2
O and FeSO
4
× 7 H
2
O, as well as
ferric chloride FeCl
3,
are used as coagulants . The
greatest clarification occurs when using ferric salts.
Ferric chloride is used in dry form or in the form of
10 - 15% solutions. Sulfates are used in the form of
powders.
The coagulant dose depends on the pH of the
wastewater. Fe
3+
the рН is 6-9, and for Fe
2+
the рН
is 9, pH is 9.5 and higher. For alkalinization of
wastewater, NaOH and Са(ОН)
2
. Water treatment
with coagulants is the most common method for
purifying large volumes of water from coarsely
dispersed and colloidal contaminants. The use of
the coagulation method has increased in recent
years, and this trend continues. In this regard, the
assortment of coagulants and related reagents,
offered for the purification of natural waters in
order to improve the quality of treated water, is
rapidly growing. In currently increasingly
widespread coagulants are highly BASIC spine-oxy
chlorides of aluminum, which until recently only
been used in water purification obtained for
drinking purposes.
Using Resource-saving Wastewater Treatment Technology in an Industrial City
83

Recently, the variety of impurities in wastewater
has increased significantly with significant
fluctuations in their composition and color. This
requires a lot of effort in wastewater treatment, for
which aluminum sulphate is traditionally used, which
no longer always meets the requirements.
To provide a comprehensive solution to these
problems, we propose to use a coagulant - aluminum
oxy chloride (polyaluminium- hydrochloride) of the
highest quality category with a basicity index of
"5/6", which received 1st place in the international
competition for coagulants.
Development of production and application of
technology oxy chloride and aluminum "BOPAK" to
purify water in the cities of Russia took place in the
framework of inter-regional environmental programs.
Production hydroxy chloride and aluminum for
organizations - water channels carried in Azov,
Rostov region and Ekaterinburg on TU 216350-002-
39928758-02.
Coagulant " Bopak-E " is a low-hazard compound
of the 3rd hazard class, the limiting hazard indicator
is sanitary-toxicological, maximum concentration
limit is 0.5 mg / l (for aluminum).
Guaranteed quality indicators water treatment
technical and economic advantages of using oxy
chloride Aluminum:
ꞏ Causes highly effective coagulation of colloidal-
dispersed particles and organic substances from
water, as a result of which a rapidly precipitating and
well-filtered flock is formed;
Decrease in the content of organochlorine
compounds;
Ensuring the content of residual aluminum is less
than 0.2 mg / l;
Consumption of the reagent in the range of 0.3 -
3.0 mg Al / liter of water;
Stability of the coagulation process, including at
low water temperatures;
When introduced into water, it practically does
not reduce the alkalinity and pH of the treated water,
as in comparison with traditionally used coagulants,
which contributes to: a decrease in the rate of
corrosion of metals in water supply and heat supply
systems, by eliminating the formation of aggressive
carbon dioxide and the possibility of refusing to use
alkaline agents;
In comparison with traditional coagulants, it
reduces the amount of anions introduced into the
water by 10 times;
Easy-to-use and stored solution, which is easily
diluted to the required degree before dosing;
Transition to a new reagent in the conditions of
operating stations, as a rule, does not require
reconstruction of the existing reagent facilities,
greatly facilitates the work of the service personnel;
Approved for use in drinking and hot water supply
systems.
Application hydroxy chloride and aluminum has
many advantages, directly affecting the economics of
its use (including and in comparison with a
conventional aluminum sulphate):
being a partially hydrolyzed salt, aluminum
oxy chloride has a greater ability to polymerize,
which accelerates flocculation and
precipitation of coagulated suspension;
work confirmed hydroxyl chloride and
aluminum in a wider pH range as compared
with aluminum sulfate, which leads to a
complete hydrolysis and, consequently, reduce
the concentration of residual aluminum in the
drinking water;
reducing alkalinity when coagulating oxy
chloride ohm aluminum is substantially smaller
that, along with the absence of the addition of
sulphate results in a reduction of corrosion of
water activity, exclusion stabilization
treatment, to improve water pipelines status
urban distribution network and preserve
consumer properties of water during
transportation and also allows to completely
abandon from the use of alkaline agents and
leads to savings of those at the middle station
up to 20 tons per month;
low residual aluminum content at high doses
administered;
reduction of the working dose of the coagulant
by 1.5 - 2.0 times in comparison with
aluminum sulfate;
delivery in a ready-made working solution,
which makes it possible to abandon the process
of dissolving the coagulant, leading to energy
savings for stirring at the middle station up to
100 thousand kW / h annually;
during the transition to oxy chloride, aluminum
does not require changes in the technology of
the station with the drinking water;
reduction of labor intensity and operating costs
for storage, preparation and dosing of the
reagent, improvement of sanitary and hygienic
working conditions.
Inorganic cationic polymer coagulant " Bopak-E
" has the ability to form complex compounds with a
wide range of organic and inorganic substances in
water.
It fundamentally differs from ordinary aluminum
salts in that it has a so-called surface acidic shell,
ISSDRI 2021 - International Scientific and Practical Conference on Sustainable Development of Regional Infrastructure
84
which ensures the highest possible efficiency of water
purification from suspended solids and metals.
According to sanitary characteristics, it
corresponds to the quality required for food products
and is made from pure hydrochloric acid and metallic
aluminum.
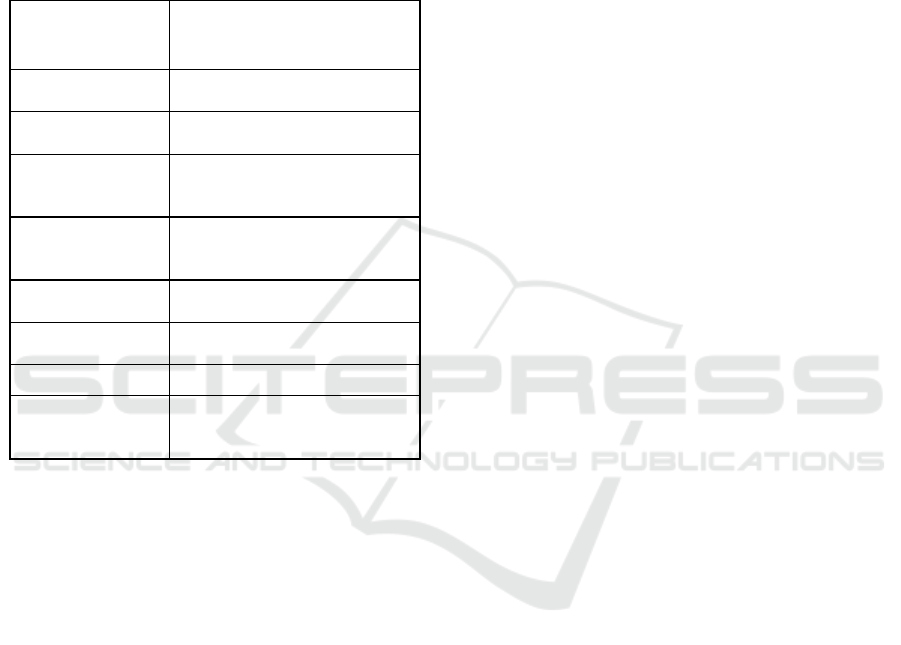
The technical characteristics of the coagulant are
shown in Table 1.
Table 1: Specifications hydroxy chloride and aluminum.
Formula is the
product
Al
2
( OH )
5
CI , aqueous
solution
basicity
5/6
mass fraction of
aluminu
m
(10 ± 1)%
mass fraction of
Al
2
O
3
(17.0 - 20.8)%
mass fraction of
chlorides
(6.2 ± 0.5)%
specific gravity (1.27 ± 0.03) kg / dm
3
pH
4.5 ± 0.5
viscosity 30 ± 10 cps
freezing point
minus 18
о
С , after defrosting
does not lose coagulation
p
ro
p
erties
Application area:
in drinking water treatment systems;
treatment of municipal, industrial waste water;
preparation of water for technical needs, for
heat power engineering.
in perfumery, pulp and paper, leather industry
(Chong et al, 2010, Zapolsky, Baran, 1987).
Oxychloride, aluminum " Bopak -E" can deliver
any chemically resistant containers: cans, barrels,
containers, tanks and other containers.
The coagulation rate depends on the concentration
of the electrolyte. At low electrolyte concentrations,
the efficiency of particle collisions, that is, the ratio
of the number of collisions that ended in adhesion to
the total number of collisions, is close to zero. As the
concentration increases, the rate of coagulation
increases, but not all collisions are effective; such
coagulation is called slow. Further, rapid coagulation
occurs, in which all collisions of particles end in the
formation of aggregates.
In polydisperse systems, coagulation occurs faster
than in monodisperse systems, since coarse particles,
when settling, entrain smaller ones. Particle shape
also affects the rate of coagulation. For example,
elongated particles coagulate faster than spherical
ones.
The size of the flakes (in the range of 0.5-3 mm)
is determined by the ratio between the molecular
forces holding the particles together and the
hydrodynamic forces of detachment, which tend to
destroy the aggregates.
The strength of the flakes depends on the particle
size distribution of the resulting particles and
plasticity. Agglomerates of particles that are
inhomogeneous in size are stronger than
homogeneous ones. Due to the release of gases from
the water, as well as a result of aeration and flotation,
gas saturation of the flocs occurs, which is
accompanied by a decrease in the density of the flocs
and a decrease in the sedimentation rate.
The process of wastewater treatment by
coagulation consists of the following stages: dosing
and mixing of reagents with wastewater; flocculation
and sedimentation.
Various mixers are used to mix coagulants with
water. In hydraulic mixers, mixing occurs due to
changes in the direction of movement and the speed
of water flow. In mechanical mixers - devices with a
stirrer, the stirring process should be uniform and
slow so that the particles, when approaching, form
flakes that would not collapse when the mixer rotates.
After mixing the waste water with the reagents,
the water is sent to the flocculation chambers.
Cloisonne, vortex, and mechanical-agitated chambers
are used. The formation of flakes in the chambers
proceeds slowly - within 10-30 minutes. They are
reservoirs, divided by partitions into a series of
corridors, in succession, passable by water, in which
the coagulant is mixed with waste water. The speed
of the water in the corridors is 0.2-0.3 m / s.
The settling of flakes occurs in sedimentation
tanks and clarifiers. Hour then mixing step of
coagulation and precipitation carried out in a single
apparatus. Coagulant flakes are formed in the annular
zone. Suspended particles with flakes settle to the
bottom and are removed from the apparatus. Clarified
water through the hole enters the tray, from where it
is sent for further purification (Babenkov, 1983.
Danilov-Danilyan, Bolgov, 2009, Sazhin et al, 2012)
The optimal dose of the mouth of the reagent - the
time here to probe based coagulation laboratory
Company, successively changing the dose of
coagulant. At the same time, the properties of its
macromolecules and the nature of dispersed particles
are considered.
Using Resource-saving Wastewater Treatment Technology in an Industrial City
85
3 RESULTS AND DISCUSSSIONS
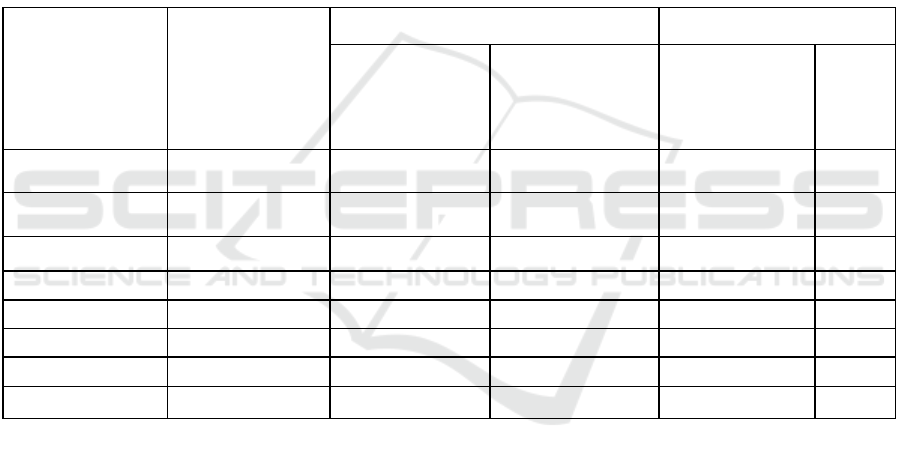
Taking into account the composition and properties
of wastewater entering the Lermontov wastewater
treatment plant, we proposed to use as a trial
coagulant aluminum oxychloride with a
concentration of 2 mg / l instead of aluminum sulfate
determined by the technological regulations with an
optimal dose of 20 mg / l. The results of water analyze
before and after vertical sedimentation tanks using
aluminum oxychloride are shown in Table 2.
The complex of treatment facilities in the city of
Lermontov consists of two technological lines with a
capacity of 25 thousand m3 / day.
Wastewater from the municipal and industrial
sewage system is pumped from the sewage pumping
station to the receiving chamber of the treatment
plant. The receiving chamber with the help of surface
shield gates directs part to the first stage of treatment
facilities, and the remaining part to physical and
chemical treatment facilities.
The first technological line is designed for
wastewater treatment in the amount of 10 thousand m
3 / day. The operation of this line is based on the
method of biological wastewater treatment. The
second technological line is designed to receive and
purify 15 thousand m 3 / day of waste water. The
operation of this line is based on the method of
physical and chemical cleaning. It is on this
technological line that the coagulation process is
used.
Table 2: The results of analyzes of the use of coagulant about aluminium oxychloride in vertical settling tanks.
The substance to be
determined
Incoming for
cleaning, mg / l
After settling tank, mg / l Cleaning effect
Using Al
2
(OH)
5
CI
Without using Al
2
(OH)
5
CI
Using Al
2
(OH)
5
CI
Without
using
Al
2
(OH)
5
CI
Suspended
substances
158.6 3.97 14.9 97.5 90.6
Ammonia nitrogen 30.4 21.1 24.7 30.7 18.75
Nitrite 0.57 0.34 0.36 40.4 36.8
Phosphates 8.2 3.5 7.8 56.7 4.9
Aluminum 0.022 0.0134 0.0154 39.0 30.0
BOD
5
178.89 60.3 75.7 66.2 57.6
Copper 0.052 0.019 0.021 64.0 59.6
Fluoride 0.45 0.31 0.36 31.1 20.0
It can be seen from the results obtained that the
use of aluminum oxychloride as a coagulant makes it
possible to obtain a higher cleaning effect in terms of
such indicators as suspended solids, ammonium
nitrogen, nitrites, phosphates, aluminum, BOD 5 ,
copper and fluorides.
Wastewater, after being cleaned on two
technological lines, goes to the post-treatment
section, where it is additionally processed on sand
filters and disinfected in tanks by chlorination.
Comparative results of complete wastewater
treatment at the wastewater treatment plant in the city
of Lermontov are shown in Table 3.
ISSDRI 2021 - International Scientific and Practical Conference on Sustainable Development of Regional Infrastructure
86
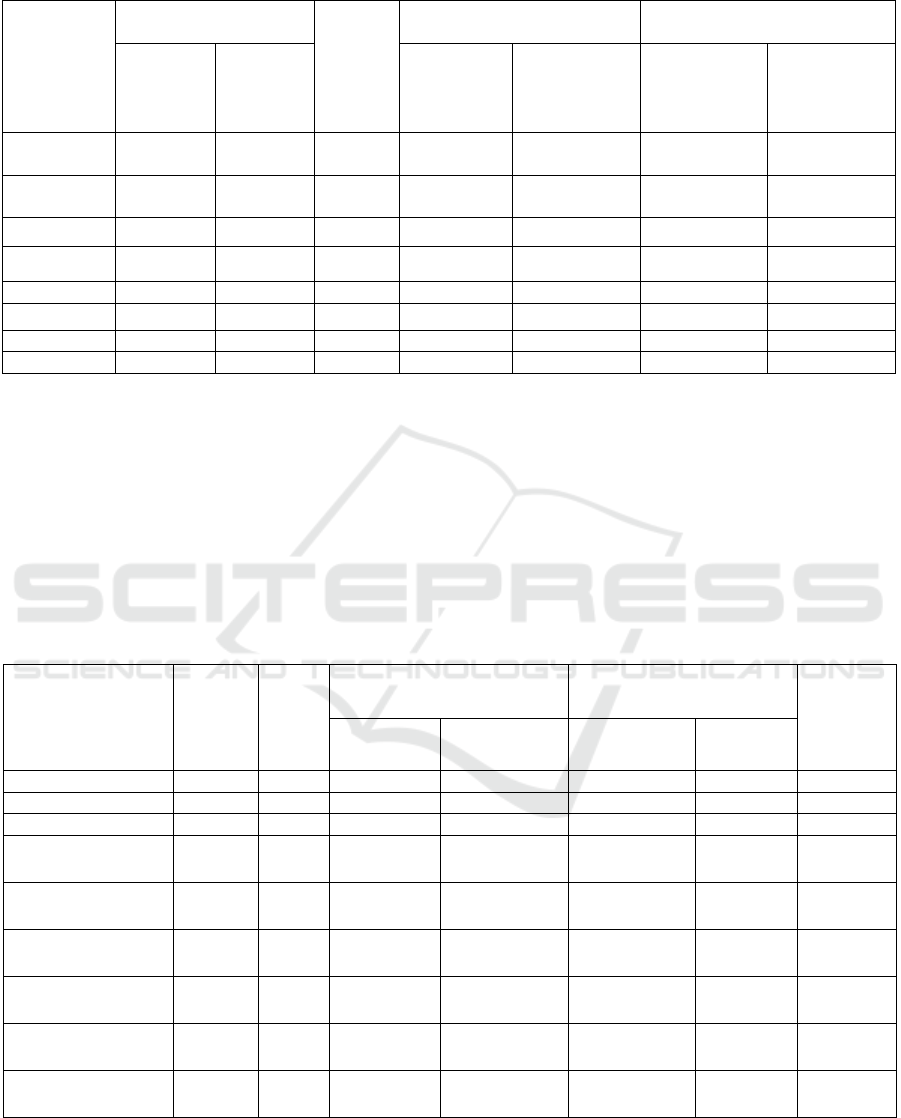
Table 3: Wastewater analysis results.
Substance to
be
determined
After PCH, mg / l
After
biologic
al
treatme
nt, mg /
l
After cleaning, mg / l Cleaning effect,%
Using
Al
2
( OH
)
5
CI
Without
using
Al
2
( OH
)
5
CI
Using
Al
2
( OH )
5
CI
Without
using
Al
2
( OH )
5
CI
Using
Al
2
( OH )
5
CI
Without
using
Al
2
( OH )
5
CI
Suspended
substances
3.97 14.9 53.53 2.7 4.2 92.1 87.71
Ammonia
nitrogen
21.1 24.7 6.4 1.58 2.5 89.87 83.97
Nitrite 0.34 0.36 0.279 0.11 0.13 65.62 59.37
Phosphates 3.5 7.8 2.37 0.061 0.27 98.91 95.17
Aluminu
m
0.0134 0.0154 0.0176 0.003 0.005 75.75 69.69
BOD
5
60.3 75.7 24.5 1.25 3.28 97.5 93.45
Co
pp
e
r
0.019 0.021 0.0312 0.003 0.004 88.46 84.61
Fluoride 0.31 0.36 0.44 0.19 0.22 52.5 45.0
4 CONCLUSION
The use of a new technological solution will
significantly reduce the content of pollutants in the
discharged wastewater into the receiving water within
the limits of the MPD, which will significantly reduce
the amount of payments for pollution of the Gorkaya
River and, thereby, improve the financial
performance of the enterprise. An approximate
calculation of the prevented annual economic damage
is shown in Table 4.
Table 4: Ecological characteristics of discharges before and after the implementation of environmental protection measures
and the prevented annual economic damage.
Pollutant name
MPC
i
p/x,
mg/l
А
i
Reduced discharge mass
(М
i
), t/year
Specific annual damage
(D
i
)
Averted
economic
annual
damage
(D)
Before events After the events Before events
After the
events
Sus
p
ende
d
substances 3,20 0,31 4,657 2,9938 268062,26 172326,55 95735,71
Ammonia nitro
g
en 0,500 2,0 18,384 11,303 1058204,27 650613,72 407590,55
Nitrite 0,080 12,5 5,8123 4,9181 334562,71 283091,51 51471,2
Phosphates 0,020 5 4,82868 1,145824 277944,40 65954,94
211989,4
6
Aluminum 0,005 200 3,5768 2,14608 205884,74
123530,8
2
82353,92
BOD
5
3,00 0,33 3,8715 1,4754 261885,46 84925,71
176959,7
5
Copper 0,001 1000 14,3072 10,7304 1386270,94
617654,1
9
768616,7
5
Fluoride 0,180 5,55 4,36727 3,77173 251385,10
217105,1
1
34279,99
Total output - - 59,80475 38,484334 4044199,88
2215202,
55
1828997,
33
Thus, the use of coagulant aluminium oxychloride
in urban wastewater treatment plants instead of
traditional aluminum sulfate will allow achieving a
higher degree of wastewater treatment and reducing
the negative impact on the environment.
Using Resource-saving Wastewater Treatment Technology in an Industrial City
87

REFERENCES
Gupta, V.K., Ali, I., Saleh, T.A., Nayak, A. and Agarwal,
S. (2012). Chemical treatment technologies for waste-
water recycling - an overview. RSCAdv, 2: 6380-6388.
Petukhova, E.O. (2018). Causes of shortage of fresh water.
Innovative methods and projects for obtaining drinking
water. Bulletin of PNRPU. Construction and
architecture, 9(3): 141-151
Joss, A., Zabczynski, S., Göbel, A., Hoffmann, B., Löffler,
D., McArdell, C.S., Ternes, T.A. and Siegrist, H.
(2006). Water Res. 40: 1686-1696.
Talarposhti, A.M., Donnelly T., Anderson, G.K. (2001).
Water Res., 35: 425-432.
Bernet, N., Delgenes, N., Akunna, J.C., Delgenes, J.P.,
Moletta, R. (2000). Water Res., 34: 611-619.
Rodionov, A.I., Klushin, V.N., Sister, V.G. (2000).
Technological processes of ecological safety.
Publishing house "N. Bochkarevoy", Kaluga, 792 p.
Chong, M.N., Jin, B., Chow, C.W.K., Saint, C. (2010).
Water Res., 44: 2997–3027.
Zapolsky, A.K. and Baran, A.A. (1987). Coagulants and
flocculants in water purification processes. Properties.
Receiving. Application.-L.: Chemistry. 208 p.
Babenkov, E.D. (1983). Water is purified by coagulants.
Moscow: Znanie, 64 p.
Danilov-Danilyan V.I. (2009). Water resources of the
world and prospects of the water management complex
of Russia. OOO "Typography LEVKO", Institute for
Sustainable Development, Center for Environmental
Policy of Russia.
Danilov-Danilyan V.I. and Bolgov M.V. (2009). On the
water strategy of the Russian Federation for the period
up to 2020. Water problems of large river basins and
ways to solve them. Collection of scientific papers.
Barnaul: LLC "Agency of advertising technologies",
pages 59-81.
Sazhin, V.B., Seldinas, I., Kochetov, O.S., Kochetov, L.M.,
Belousov, A.S., Sazhina, M.B. and Dmitrieva, L.B.
(2008). Problems of rational use of water resources in
Russia. Advances in chemistry and chemical
technology. XXII 11 (91): 56-70.
ISSDRI 2021 - International Scientific and Practical Conference on Sustainable Development of Regional Infrastructure
88
