
With a Little Help from My Conversational Agent:
Towards a Voice Assistant for Improved Patient Compliance and
Medication Therapy Safety
Jan Schulte to Brinke
1
, Christian Fitte
2a
, Eduard Anton
2b
, Pascal Meier
1
and Frank Teuteberg
2c
1
Smart Enterprise Engineering, German Research Center for Artificial Intelligence,
Parkstraße 40, 49080 Osnabrueck, Germany
2
Accounting and Information Systems, Osnabrueck University, 49069 Osnabrueck, Germany
Keywords: Conversational Agent, Medical Assistant, Patient Compliance, Medication Therapy Safety.
Abstract: The chronically ill and the elderly often need to take several drugs, which increases the complexity of
medication management. This frequently results in a decrease in patient compliance and raises the risks of
their drug therapy. To support patients in medication management, we developed a multimodal assistant that
includes a conversational agent supplied with data from a database managed by healthcare professionals via
a web service. The developed artifact analyzes medication plans, identifies adverse drug reactions and side
effects, and reminds patients to take their medication correctly and on time. Applying the design science
research paradigm, we systematically identified 16 issues, derived eight meta-requirements, and elaborated
three design principles. Based on this, the artifact was implemented and evaluated by three experienced
pharmacists, who highlighted the usefulness of the solution and provided feedback for further improvements.
Finally, we present an evaluation concept for potential users and discuss the implications of the medication
assistant. Overall, the medical assistant comprises valuable functionalities to support patients, and it increases
medication therapy safety and patients’ compliance.
1 INTRODUCTION
Given global demographic change, the share of the
elderly is increasing worldwide. Senior citizens are
much more likely to suffer from chronic or multiple
diseases and rely on several medications
simultaneously (Peters et al. 2010). With high
quantities of drugs, the complexity of medication
management rises, thereby increasing the risk of
medication errors and patient non-compliance
(Schäfer 2011; Vrijens et al. 2008). Taking the wrong
medication, an incorrect dosage, or a combination of
several drugs can lead to dangerous adverse drug
reactions (ADRs) (Reimers and Klein 2015). An
ADR is a “response to a medicinal product which is
noxious and unintended” (EMA 2017, p. 8), causing
severe side effects and even leading to 25,000 annual
deaths in Germany (Dormann et al. 2017). In addition
a
https://orcid.org/0000-0002-2637-1198
b
https://orcid.org/0000-0002-5676-710X
c
https://orcid.org/0000-0003-3870-6642
to ADR-induced medical implications, economic
consequences are mirrored in the costs of ADR-
related hospital admissions, which are estimated for
only Germany at around 434 million euros per year
(Rottenkolber et al. 2011), excluding the associated
indirect costs (Stark, John, and Leidl 2011).
Yet, most ADRs are considered avoidable
(Schurig et al. 2018), particularly with patients’
adhering to their prescribed medication plans
(Tafreshi et al. 1999). In many cases, patients do not
take their drugs because they forget to take them (Kim
et al. 2018), they fear side or adverse effects (Flávio
Ferreira et al. 2014; W. T. Hsieh et al. 2018; Teixeira
et al. 2017), or they are ignorant to the benefits of the
medication (Flávio Ferreira et al. 2014; Sebillo et al.
2017; Teixeira et al. 2017). Therefore, to achieve
effective patient compliance, the provision of
information on drugs and their medication is of
central importance (Grube, Dehling, and Sunyaev
Schulte to Brinke, J., Fitte, C., Anton, E., Meier, P. and Teuteberg, F.
With a Little Help from My Conversational Agent: Towards a Voice Assistant for Improved Patient Compliance and Medication Therapy Safety.
DOI: 10.5220/0010411707890800
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 5: HEALTHINF, pages 789-800
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
789
2017). In this context, eHealth technologies can
support patients to comply with their therapy
(Mertens et al. 2015; Sedlmayr 2018). In particular,
conversational agents (CAs) offer promising
potential for improving medication self-management
and reducing intake errors (EmmaHome 2020; Flavio
Ferreira et al. 2013; Jesús-Azabal et al. 2020; Teixeira
et al. 2017). A CA in the healthcare domain is an
“artificial intelligence program that can conduct an
intelligent conversation via auditory or textual
methods regarding healthcare issues” (Wang and Siau
2018, p. 1). The use of CAs for medication
management can allow for avoiding medication
errors by providing the necessary information in a
more natural human-computer interaction, thus
improving the usability and accessibility, especially
for the elderly who have less technological know-how
(Teixeira et al. 2017). However, available solutions
often offer only reminder functions without providing
comprehensive information about the medication
(EmmaHome 2020; Jesús-Azabal et al. 2020).
Moreover, the functionalities often primarily utilize
information that is based on data managed by the
patient (MyTherapy 2020; Sebillo et al. 2017; Silva
et al. 2013). Thus, most CAs reflect the error-prone
input of the patient, without any monitoring by
healthcare professionals who can assure data quality
and the safety of drug therapy. Drawing on these
shortcomings in assuring the safety of drug therapy
for patients, we pose the following research question:
RQ: How Can CAs Be Designed and Implemented to
Increase Medication Therapy Safety for Patients?
To answer this research question, we design and
implement a multimodal assistant comprising a CA to
increase medication therapy safety by allowing a
patient to use voice commands to inquire about the
side effects or reactions of their medication. The
system is complemented by a web portal for
healthcare professionals which receives input from a
central pharmacy data service and allows for the
management of patients’ medication plans. Driven by
the design science paradigm (DSR) (Gregor and
Hevner 2013), we structure the remainder of this
paper as follows: We introduce our DSR multistep
research approach and the applied methods for the
development and evaluation of the application in
Section 2. Then, outlining the theoretical background,
we summarize the related work and derive issues for
medication assistants in Section 3. Based on the
identified issues, we derive meta-requirements and
elaborate design principles in Section 4. The
subsequent Sections 5 is devoted to describing the
designed and developed artifact and the evaluation
concept. Finally, the paper concludes by discussing
the implications for research and practice, presenting
the limitations, and providing an outlook for future
research.
2 RESEARCH APPROACH
The design, development, and evaluation of the
artifact in response to our research question follows
the design science research (DSR) methodology by
Peffers et al. (2007). DSR aims to address important
real-world problems that remain unsolved or require
further investigation via a technological artifact
(Hevner et al. 2004). In this paper, we address the
issues of the current available solutions for
medication management to increase patient
compliance and medication therapy safety by
developing a multimodal assistant comprising a CA
and a web service that handles patient queries by
accessing a managed medical database. The process,
in pursuit of realizing the solution, involves the
identification of an observed problem, followed by
the design, implementation, and evaluation of the
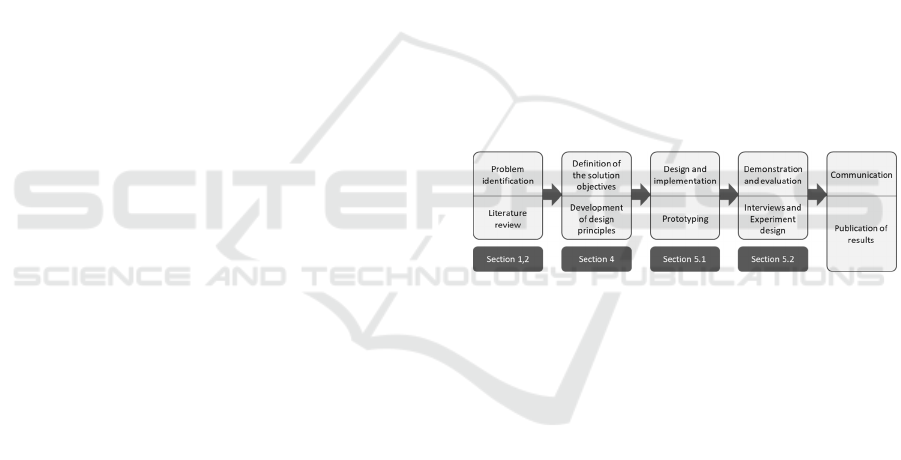
artifact (cf. Figure 1) (Peffers et al. 2007).
Figure 1: DSR Approach of this Study (Peffers et al. 2007).
As a starting point for problem identification, we
conducted a systematic literature analysis (vom
Brocke et al. 2009). We queried titles, abstracts, and
keywords in the interdisciplinary databases
SpringerLink, ScienceDirect, AISeL, IEEE Xplore,
Emerald insight, JSTOR, and EBSCOhost using the
search string ("requirements" OR "design principles"
OR "Anforderungen") AND ("medication assistant"
OR "health assistant" OR "medical agent" OR
"Gesundheitsassistent" OR "Medikationsassistent").
Ever since Apple introduced the voice assistant Siri
in 2011, the research and general interest in CAs have
increased significantly (Luger and Sellen 2016);
hence, we included papers from 2011 onward and
filtered for the languages English and German. The
resulting 621 publications were analyzed based on the
following inclusion and exclusion criteria: We
considered any theoretical and practical work
involving medication assistants or their development
and excluded studies that have a broader research
focus in the eHealth field without focusing on drug
Scale-IT-up 2021 - Workshop on Scaling-Up Healthcare with Conversational Agents
790

management. After applying the inclusion and
exclusion criteria, the remaining eight studies served
for a forward and backward search that yielded
another nine studies, resulting in a literature corpus of
17 studies. An analysis of the literature yielded the
identification of 16 issues (Is), from which we derived
nine meta-requirements (MRs) for the solution, which
we finally combined into three design principles
(DPs). Based on the systematically derived DPs, we
developed a multimodal system consisting of three
sub-systems, as presented in Section 5. Finally, we
evaluated the artifact by interviewing three
experienced pharmacists regarding their assessment
of its usefulness and their evaluation of areas for
improvement of the artifact (Myers and Newman
2007). We analyzed the interviews according to
Mayring (2010), and incorporated the experts’
suggestions for improvements in order to prepare the
CA for the next evaluation cycle with patients, which
will be presented as an experimental design.
3 THEORETICAL
BACKGROUND
3.1 Complexity of Patient Compliance
and Medication Therapy
Management
In Germany alone, more than 100,000 approved
medications exist, which may contain a variety of
ingredients (ABDA 2019). Nearly half of these
medications are available only by prescription (Rx).
In contrast, over-the-counter medications (OTC) are
available to every customer without restriction.
Approximately 23% of German citizens take more
than three medications simultaneously (ABDA
2019). This can lead to a high risk of ADRs,
especially if patients are treated by several healthcare
professionals without a central medication plan
(Reimers and Klein 2015), thus necessitating that the
patients notify the treating physician of their
medications themselves. Patients might forget to
mention medications, assume they are not relevant, or
deliberately withhold information out of shame.
In addition to the high risks of polymedication,
many patients must change their way of life due to
their drugs. The challenge of integrating their
medication intake into a daily routine poses a barrier
for many people causing them to forget or even refuse
the medication. This results in a reduction in patient
compliance, which “[...] describes the extent to which
a person's behavior (in terms of taking medications,
following diets or executing lifestyle changes)
coincides with medical or health advice” (Haynes
1979). The concept of compliance has evolved from
a directive model (obedience to therapy) to a passive
model (loyalty to therapy) and from an active model
(cooperation in therapy) to an interactive model, in
which the patient participates in the treatment and
cooperates with the doctor as a means of
empowerment (Schäfer 2011). Patients can be
divided into three categories when assessing
compliance: If patients follow at least 80% of their
therapy guidelines, they are described as “compliant”
(Schäfer 2011). A compliance level between 20% and
80% categorizes a patient as “partially compliant.” It
can be assumed that this patient group can best be
persuaded to adhere to therapy measures. Compliance
levels below the threshold of 20% are called “non-
compliant.” Non-compliance may be intentional
(e.g., due to religious reasons, exaggerated fears,
general distrust of medicine, reservations about side
effects, or convenience), or it can occur
unintentionally by accidentally choosing the wrong
dosage or changing the duration or frequency
(Petermann and Mühlig 1998).
3.2 Issues for Medication Assistant
Application
Based on the literature review described in Section 2,
we derived issues of and requirements for medication
assistant applications. Of the 17 identified studies, we
found eight papers that focus specifically on
applications developed for seniors, while nine papers
present solutions engineered for patients in general.
The user interfaces of the identified applications
include either CAs, web interfaces, specific hardware
components, or a mixture of multiple components.
We identified 16 issues to be addressed using our
medical assistant. First, patients are often confronted
with an information overload (I1) (e.g., in form of
endless information from leaflets), which renders it
difficult to find relevant information regarding the
proper medication intake (Dehling and Sunyaev
2013; Tiwari et al. 2011). On the other hand,
medication errors can occur due to missing
information (I2) (e.g., concerning the medication
storage) (Flávio Ferreira et al. 2014; Mira et al. 2014;
Sebillo et al. 2017; Teixeira et al. 2017). Regardless
of the information quantity, a language that is too
complex or contains technical terminology (I3) can
cause misinterpretations (Chang et al. 2019; Dehling
and Sunyaev 2013; Farhadyar and Safdari 2018;
Flavio Ferreira et al. 2013; Flávio Ferreira et al. 2014;
Jesús-Azabal et al. 2020; Kim et al. 2018; Teixeira et
With a Little Help from My Conversational Agent: Towards a Voice Assistant for Improved Patient Compliance and Medication Therapy
Safety
791

al. 2017). This can then lead to situations in which the
identification of medications is based on appearance
or storage rather than the technical product name. A
too-small illustration of information (I4) causes
comparable problems (Chang et al. 2019; Dehling
and Sunyaev 2013; Farhadyar and Safdari 2018;
Flavio Ferreira et al. 2013; Flávio Ferreira et al. 2014;
Silva et al. 2013; Teixeira et al. 2017; Tiwari et al.
2011). Numerous studies indicate that medical
assistants are generally too complex to use (I5)
(Flávio Ferreira et al. 2014; P. J. Hsieh 2016; Tiwari
et al. 2011). In particular, a complex initial set-up or
a required adjustment of the medication plan can
reduce acceptance among users (Sebillo et al. 2017).
Additional issues arise in the registration of
medications (I6) (Dayer et al. 2013; Flavio Ferreira et
al. 2013; Flávio Ferreira et al. 2014; W. T. Hsieh et
al. 2018; Sebillo et al. 2017; Silva et al. 2013;
Teixeira et al. 2017). The manual integration of
information can produce mistakes and might be
perceived by the patient as requiring too much effort
(Dayer et al. 2013; Silva et al. 2013). A prerequisite
for acceptance and thus an efficient application is for
patients to trust the CA without worrying about
misinformation (I7) (Flavio Ferreira et al. 2013;
Flávio Ferreira et al. 2014; Sneha and Varshney 2012;
Teixeira et al. 2017; Tiwari et al. 2011). In this
context, data security concerns (I8) may hinder such
acceptance (Dehling and Sunyaev 2013; Kim et al.
2018; Santo et al. 2016; Sneha and Varshney 2012).
In addition, many patients suffer from unintended
drug interactions due to polymedications (I9), which
can lead to serious health problems and avoidable
hospital admissions (Dayer et al. 2013; Dehling and
Sunyaev 2013; W. T. Hsieh et al. 2018; Kim et al.
2018; Mira et al. 2014; Silva et al. 2013). Further
health problems can occur due to side effects (I10)
(Dehling and Sunyaev 2013; Farhadyar and Safdari
2018; Flávio Ferreira et al. 2014; W. T. Hsieh et al.
2018; Teixeira et al. 2017; Tiwari et al. 2011).
Moreover, uncertainty of patients often results in a
false dosage of medications (I11) (Chang et al. 2019;
Dayer et al. 2013; Farhadyar and Safdari 2018; Mira
et al. 2014; Sebillo et al. 2017; Silva et al. 2013;
Sneha and Varshney 2012; Tang et al. 2011; Teixeira
et al. 2017). This could be addressed by increasing the
transparency and traceability of doctors’ visits (I12)
(Chang et al. 2019; Dayer et al. 2013). Fourteen
papers mention problems related to polymedications
(I13), and 16 of the 17 relevant papers identified that
patients forget or deny medication intake (I14).
Chronic patients often require long-term medication
(I15) (Chang et al. 2019; W. T. Hsieh et al. 2018;
Jesús-Azabal et al. 2020; Mira et al. 2014; Santo et al.
2016; Sneha and Varshney 2012; Teixeira et al. 2017;
Tiwari et al. 2011) which requires strong discipline
and well-organized medication management (I16)
(Chang et al. 2019; Dayer et al. 2013; Farhadyar and
Safdari 2018; Kim et al. 2018; Santo et al. 2016;
Sebillo et al. 2017; Tiwari et al. 2011).
Existing applications focusing on the safety of
drug therapy include hanahealth, Mytherapy, Emma
Home, and mediteo (EmmaHome 2020; hana health
2019; Mediteo 2020; MyTherapy 2020). Applications
such as SapoMed and Sedato, for the elderly and rural
areas, have been highlighted in the scientific literature
(Flavio Ferreira et al. 2013; Jesús-Azabal et al. 2020;
Sebillo et al. 2017; Silva et al. 2013). However, to the
best of our knowledge, the existing solutions focus on
specific target groups or offer rather limited
functionalities for patient safety and compliance, such
as medication reminders. This study aims to create an
age-independent assistant for the management of
multiple medications, which considers side effects,
ADRs, and medication reminders and enables a
combination of medication data with continously
measured vital signs. Our artifact represents an
enhancement of the FeelFit platform (Meier et al.
2019). FeelFit aggregates measured vital parameters
of several devices (e.g., weight, pulse, and blood
pressure) and visualizes the medical record for the
patient, authorized physicians, and pharmacists.
4 DESIGN PRINCIPLES
We categorized the identified issues into groups,
namely usability, information processing, and
medical issues to derive MRs and consolidate DPs
according to Gregor et al. (2020). By following these
guidelines, we ensure to consider aim, context, and
mechanism within the design of our artifact (Gregor,
Kruse, and Seidel 2020). The linked Is, MRs and DPs
are depicted in Figure 2.
To counteract the issues of information overload
(I1) and missing information (I2), the medical
assistant should present the information in a context-
sensitive manner (MR1) by emphasizing only
relevant information (e.g., appearance of the pill and
intake information). If necessary, it should provide
the user with additional information upon request.
Furthermore, terminology that is too specialized or
technical (I3) and a representation that is too small
(I4) should be avoided. Therefore, we derive MR2,
for which the information should be presented in an
understandable and clear way. To enable the intuitive
operation of the application (MR3), complex
interfaces that are difficult to use (I5) should be
Scale-IT-up 2021 - Workshop on Scaling-Up Healthcare with Conversational Agents
792
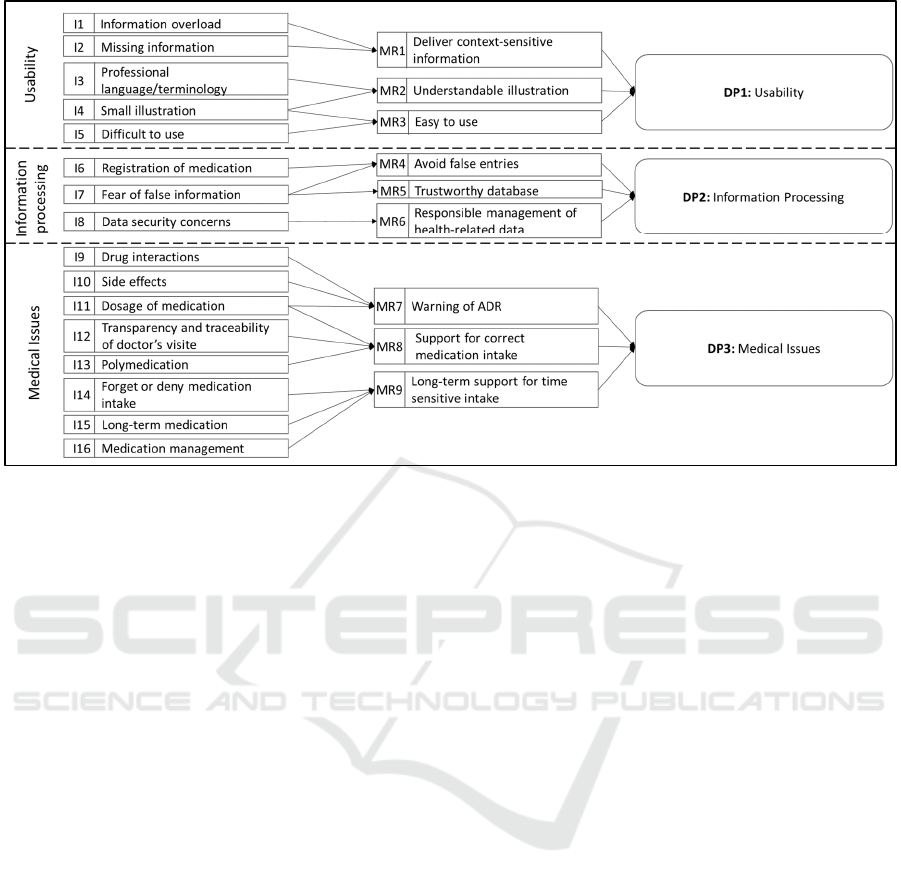
Figure 2: Issues, Meta Requirements, and Design Principles.
avoided alongside I3 and I4. As a result, the
medication assistant should be easy to use by users
who have little technical experience and during
exceptional situations. MRs 1-3 were assigned to the
category usability and can be consolidated into the
first design principle:
DP1: To provide users with comprehensive
information, provide an application with relevant
information in a context-sensitive and
understandable way, because this sparse and
manageable display of the information protects
the user from being overwhelmed and enables an
easy and efficient application of the relevant
information
Problems with the registration of medication (I6)
and false information provided by the medication
assistant (I7) should be avoided. For this purpose, the
input of incorrect data should be prevented through
the design of the application (MR4). Data entry
should be designed to be as simple as possible and
managed by medical professionals. Furthermore, to
avoid incorrect entries (I7), it must be ensured that the
basic information on the drugs is correct. Therefore,
a trustworthy drug database must be used (MR5).
Furthermore, the data security concerns of users must
be considered (I8), which requires responsible
handling of private health data (MR6). This implies
parsimonious data storage so that only the necessary
user data is saved in the databases. Furthermore, this
data should be managed securely. The guideline for
information processing can be summarized as
follows:
DP2: To enable trustworthy information
processing, provide an application that ensures
integrity, secure and reliable data management,
because health data are considered to be
particularly "sensitive" and are subject to special
protection and, in addition, false or incorrect data
can lead to health consequences.
The application is intended to prevent problems
pertaining to interactions (I9), side effects (I10) and
dosage (I11). To achieve this, the medication
assistant should warn patients about possible ADRs
(MR7). On one hand, new drugs must be checked for
interactions with already-recorded medications. In
this way, possible ADRs can be identified during the
prescription process. On the other hand, ADRs should
be identified as a possible cause of the existing
symptoms by analyzing recorded drugs based on their
side effects. In addition, issues of tracking visits to the
doctor (I12) and difficulties in taking several
medications (I13) should be avoided by helping
patients to take their medication correctly (MR8) by
raising the transparency of information. Moreover,
the application should make it easier to distinguish
drugs, and it should provide information regarding
the correct type and quantity of medication. In
addition, forgetting or refusing to take the medication
(I14), non-compliance with long-term medication
(I16), and problems in medication management (I17)
should be avoided. Therefore, users should be
With a Little Help from My Conversational Agent: Towards a Voice Assistant for Improved Patient Compliance and Medication Therapy
Safety
793
supported in the long term through taking all
medications at the right time (MR9). In this way, the
user should receive important information at regular
intervals and throughout the entire medication period.
Furthermore, the application should remind the user
to take the medication on time and obtain follow-up
prescriptions. From these MRs follows the DP3,
which focuses on the medical issues:
DP3: To protect users from medical problems,
provide an application that supports chronic
disease management by identifying ADRs and
reminding patients to take the right medications
at the right time, because these features help to
improve medication management and patient
compliance.
5 MEDICAL ASSISTANT
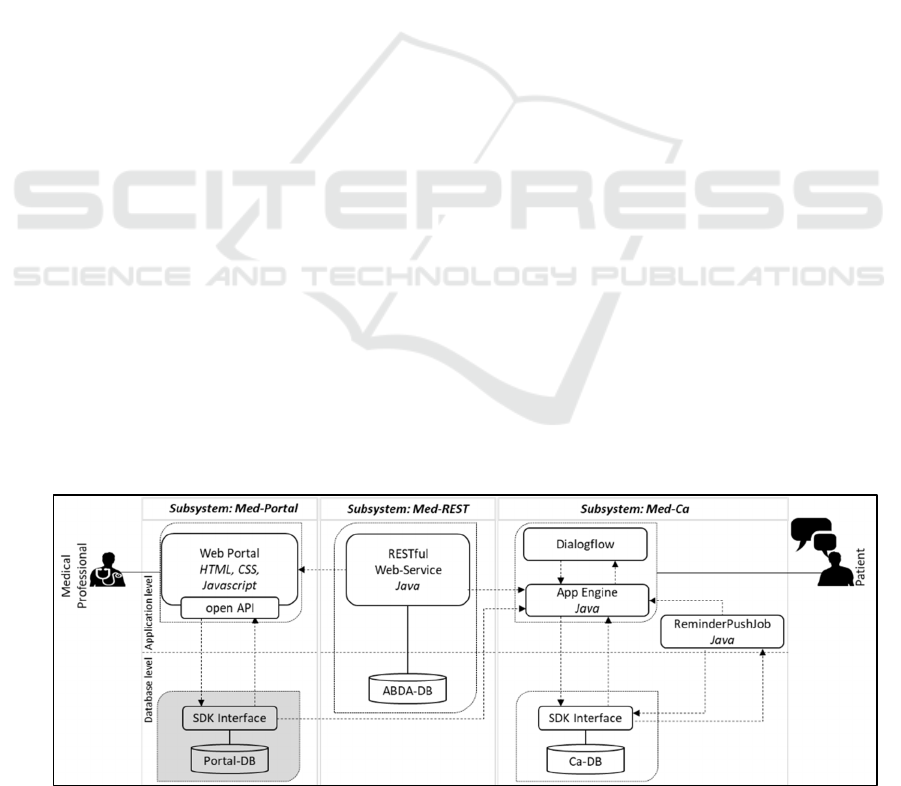
5.1 Software Architecture
An overview of the software architecture of the
multimodal assistant is provided in Figure 3. The
arrows depicted in the overview indicate the direction
in which data is transferred. The three main modules
of the application are the web application (Med-
Portal), the RESTful web service (Med-REST), and
the CA (Med-CA).
Med-REST: This subsystem serves as the interface
to the Med-Portal and Med-CA and functions as a
data communication link between the components.
An essential component of the web service is the
medication data provided by ABDATA Pharma-
Daten-Service, a division of Avoxa - Mediengruppe
Deutscher Apotheker GmbH. The data comprises all
available Rx and OTC medications in Germany with
various drug information including the ingredients,
storage and application methods, side effects and
possible interactions with other drugs. In addition, the
database includes economic and legal information
entailing the medications’ price, distribution
channels, or information on dispensing conditions
(Pharma-Daten-Service 2019). As we emphasize
drug safety and patient compliance in this study, we
neglect the economic and legal drug information. To
enable an enhanced query of the data, we imported
the relevant data into a relational database (ABDA-
DB) using the open-source H2 Database Engine
(Database 2019). Since we are determined to prevent
changes in the ABDA database to avoid
misinformation, we implement only HTTP-GET
methods for the individual resources of the web
service. In addition, we created an authentication
method for security purposes that acts as the interface
for using the web service. With each call, the system
first checks whether a valid token of the Med-Portal
or the Med-CA has been transferred, so that only
registered users can access the web service. If
authentication is successful, the request is forwarded
to the corresponding class in the application. All data
is transmitted encrypted via the Hypertext Transfer
Protocol Secure (HTTPS).
Med-Portal: To allow medical professionals to use
the Med-Rest interface and manage the medications
of their patients, we created an HTML-page. First, we
added a search field that allows for searching the web
service (Med-REST) for medication information. To
do so, a GET-function of the service is called by the
client. We implemented the possibility of using
wildcards (“%”) to enhance the search usability. The
method returns the trade name, potency, and
manufacturer of the queried drugs, displayed in a
table.
If a new drug must be added for a particular
patient, another GET-function is applied to check
whether possible interferences between the new drug
and the patient's existing medication plan can be
identified. If possible interactions occurred, a
Figure 3: Software Architecture of the Medication Assistant.
Scale-IT-up 2021 - Workshop on Scaling-Up Healthcare with Conversational Agents
794

Figure 4: User Interface: Med-Portal (left) and Med-CA (right).
warning message displays the trade names of the
interacting drugs, any recommended actions, and a
description of the effect (cf. Figure 4, left image).
Based on this, the medical professional can decide
whether to cancel the process or enter the drug despite
the possible interactions. To add the new drug, the
ingredients, trade name, dosage and shape of the drug
are retrieved from the web service via a dedicated
function. Furthermore, the physician can manually
enter the intake time (morning, noon, evening, night),
unit quantity, notes, reason for ingestion, reminder
time (morning, noon, evening, night), and remaining
stock for the patient.
Med-CA: This subsystem represents the module used
by the patient (cf. Figure 4, right image). The Med-
CA utilizes Google’s Dialogflow platform for speech
processing (Google 2020). The voice commands
issued by the patient are performed by the
corresponding functions on the server side on
Google’s App Engine platform. These functions are
fed by general medication information via the Med-
REST service and can be supplemented by patient-
specific data from a medical database (Portal-DB)
connected to the open API. As mentioned, we used
the FeelFit database (Meier et al. 2019) as the Portal-
DB for testing purposes, which stores various patient-
specific data such as blood pressure or patient
activity. In addition, a software development kit
(SDK) is used to access another database from the
App Engine (CA-DB). This database stores data that
is relevant only for the functions of the Med-CA and
serves as the foundation for the ReminderPushJob
application. The ReminderPushJob checks whether
there are reminders defined in the database for a given
time, which triggers the sending of a push notification
containing the medication information to the user.
The information comprises the medicine (product
name), doses, color of the drug, method, and intake
advice derived from the ABDA database. The
remaining stock stored in the Portal-DB is
automatically adjusted based on the applied
medication. If the remaining stock drops below a
threshold of 10 units, a warning message is stored in
the CA-DB and is issued to the patient.
5.2 Evaluation
The evaluation is aimed to validate whether the
system meets the proposed design and to analyze the
systems performance (Kuechler and Petter 2012). In
the first evaluation cycle, we interviewed three
experienced pharmacists. The second evaluation
cycle is supposed to generate insights on user’s
acceptance. To do so, we present an experimental
design for a future long-term study.
First Iteration: Expert Interviews.
To evaluate the medication assistant regarding its
usefulness, the quality of the information, and the
potential for improvement, we interviewed three
qualified, self-employed pharmacists based in
Germany (E1: 25, E2: 20 and E3: 29 years of
experience). After presenting the medical assistant
and the functionalities of the system to the
pharmacists, we conducted a semi-structured
interview (Myers and Newman 2007).
According to the experts, the target group of the
Med-CA are patients with chronic diseases suffering
from asthma, diabetes, or cardiovascular diseases
(E1, E2, E3). The CA could support patients in
organizing their medication management,
particularly by checking the remaining stocks and
With a Little Help from My Conversational Agent: Towards a Voice Assistant for Improved Patient Compliance and Medication Therapy
Safety
795

reminding them to reorder medications (E1). In
addition, reminders for medication intake and
respective advice (intake: oral, injection, liquid, and
so on) could increase patients’ compliance.
According to E2, an improvement in functionality
would be the confirmation of the patient’s drug intake
following a reminder. Furthermore, E2 remarks that
if a patient is not at home when receiving a
notification, the patient should be reminded on a loop
until the final intake is confirmed. The pharmacist E2
stressed that notifications of all types should avoid
complex and professional terminology, which our
artifact based on I3 considered. Therefore,
information on the medication intake, storage, and
side effects from the ABDA-DB should be translated
into generally understandable language. The process
of identifying certain drugs could be supported by
providing pictures of the respective pill on the
patient’s smartphone (E2). In addition to notifications
concerning medication intake, patients could also be
reminded to measure their vital signs, such as blood
pressure (E2). These measurements should be stored
directly in the patient’s profile to enable the
pharmacist or other healthcare professionals to check
whether the patient’s medication is correctly adjusted.
All pharmacists agreed that they could integrate
the Med-Portal into their daily work routine.
However, they indicated that additional effort should
be minimized (e.g., by interfaces to already-used
software) (E2, E3). In particular, compatibility with
nation-wide standardized medication plans should be
ensured (E3). Furthermore, pharmacists require
additional individual advice options on the dosage or
storage of medicines (e.g., in the form of notes to a
particular entry) (E1, E3). In case of questions,
patients should also have the ability to contact their
pharmacist directly via chat (E3). In addition to
automatic ADR analyses, pharmacists should be able
to perform regular manual assessments of the
medication plan to identify potential redundant
prescriptions or obsolete drugs (E3).
Regarding Med-REST, the experts mentioned
that the web service should feed on data that goes
beyond the ABDA-database, as not all potential
interactions might be listed, and patients could take
additional substances that are relevant to their
medication plan but are not included in the
pharmaceutical database (e.g., nutritional
supplements) (E1). Complementary health-related
data such as vital signs, blood-, liver- and kidney-
values, weight, allergies or food intolerances should
be integrated into the patient’s profile as they might
be relevant for the effective use of certain
medications (E1).
Overall, the basic functions of the assistant were
assessed by the experts as beneficial in terms of
supporting users in drug management and improving
patient compliance. The features for further
improvement can be categorized in data and
functional improvements and are summarized in
Table 1.
Table 1: Feedback of Interviewed Pharmacists.
No. Data Improvements Fre
q
uenc
y
1 Easy Language 1
2
Detailed advice to medication
intake
2
3 Information about stora
g
e 1
4 Pictures of drugs 1
5
Additional data: vital signs,
nutrition supplements, allergies,
intolerances, wei
g
ht
1
No. Functional Improvements Fre
q
uenc
y
6 Interfaces to existing software 1
7 Notifications for vital signs
measurement
1
8 Inte
g
ration of vital si
g
ns 3
9 Integration of laboratory values 2
10 Consideration of chronic diseases 1
11 Confirmation of intake 1
12 Notification-loo
p
1
13 Chat 1
Second Iteration: Experimental Design.
After evaluating the CA with experienced
pharmacists, the system needs to be tested and
evaluated by potential users. To prepare the second
evaluation cycle, we conceptualize a suitable
experimental design which will be operationalized in
a future study. The study design is supposed to be an
experiment. It will be held in a smart home
showroom, which creates a comfortable atmosphere
in which participants feel at ease. Thereby we can
avoid biases due to a laboratory setting.
First, we will survey participants in regards to
their demographics, pre-experience and general
attitude towards technology. Second, they will be
introduced to a scenario involving a specific person,
who suffers from multiple diseases, takes several
medications, lives alone and is not able to visit the
pharmacy on his or her own. Nevertheless, the person
obtains his or her medication supply through home
deliveries. In addition to the delivery service, the
pharmacy that supplies the person offers a CA for the
medication management. After being introduced to
the scenario, the study participants will be asked to
conduct the following three tasks:
Scale-IT-up 2021 - Workshop on Scaling-Up Healthcare with Conversational Agents
796

I. Ask the CA, which medications must be taken
today and ask a follow-up question about the
appearance of the pill.
II. Ask the CA, when you need to reorder
medication according to your therapy plan. If
necessary, order new medication from your
local pharmacy.
III. Report side effects to the CA, resulting from
taking medication, e.g. headache, and find out
causes for the symptoms.
After completing the tasks, participants have to
answer a user experience questionnaire. We will
apply the user experience questionnaire based on
Schrepp et al. (2014). Besides initial questions
towards the acceptance and the trust towards the
technology, participants have to share their
impression of the system by rating 26 items on a scale
of two contradictory features, e.g., good/bad,
slow/fast or enjoyable/annoying. The user experience
questionnaire provides insights in terms of novelty of
the artifact, stimulation, dependability, efficiency,
perspicuity and attractiveness. In addition, it allows to
establish a benchmark with comparable artifacts.
6 DISCUSSION
The management of the risks associated with
polymedication is of great medical and economic
importance (Taylor et al. 2013). Therefore, patients in
Germany, who take more than three different
prescribed medications have been eligible for a
standardized medication plan since 2016. In addition,
some pharmacies manage customer profiles to track
customers’ individual medication records (Reimers
and Klein 2015). However, most patients do not
request such a medication plan, as they are unaware
of their right to a managed plan and the possible
ADRs of their medication. Furthermore, medication
plans often do not consider OTC drugs, and ADRs
might just as well occur with fewer than three
prescribed drugs. To support medication
management, digital assistants such as CAs can be
found in many popular app stores and are designed to
support patients in their medication therapy
(EmmaHome 2020; MyTherapy 2020). However,
these applications often support only a reminder
function for medication intake (EmmaHome 2020;
Jesús-Azabal et al. 2020) and rely on the error-prone
input of the patient to create a medication plan
(MyTherapy 2020; Sebillo et al. 2017; Silva et al.
2013). As a result, the safety of medication therapy is
not emphasized in these particular solution
approaches. The CA presented in this study enters
into dialog with patients using simple language to
provide them with important information concerning
the medication to be ingested, and it considers side
effects and ADRs with other drugs. The system
enables patients to self-manage their medications and
provide access to selected healthcare professionals,
who can access their medication plan via a web
service. Through the integration of healthcare
professionals such as pharmacists or doctors, it is
possible to strengthen the intersectoral cooperation, to
send secured notifications about medication intake to
the patient and to reorder medications directly,
thereby simplifying the integration of therapy plans
into everyday life and increasing patient compliance
on several levels. Moreover, the ADR check function
and the associated notification of interferences and
information concerning the composition of drugs,
side effects, and symptoms increases the health
competence and health awareness of patients.
All interviewed pharmacists emphasized the
relevance of vital parameters for medication therapy
safety. We connected the FeelFit database to our
system to integrate vital signs from various devices
(Meier et al. 2019). On one hand, this enables
pharmacists to check whether the medication doses
are correctly adjusted. On the other hand, a
continuous visualization of vital signs can
demonstrate the consequences of non-compliance to
the patient (Meier et al. 2019). This is especially
relevant for non-compliant patients who do not take
their chronic diseases seriously, intentionally refuse
adherence to their therapy plan, or secretly deny
medications because of inconvenience (Petermann
and Mühlig 1998).
Implications for Research and Practice: All the
pharmacists involved in the evaluation agreed that our
multimodal prototype has a high value for medication
management safety and patient compliance. From the
perspective of a practitioner who works in the
healthcare sector, the application could provide more
transparency into the ongoing therapy of patients and
thereby intervene in the treatment more quickly and
proactively, thereby increasing the overall quality of
healthcare supply. This is particularly important,
since ADRs could often be avoided by increasing the
transparency for healthcare professionals and thus
reducing healthcare expenses caused by avoidable
hospital admissions due to medication errors (Salvi et
al. 2012; Taylor et al. 2013). Furthermore, the active
management of patients’ data (e.g., by pharmacists)
can increase the importance of local healthcare
experts and enhance the relationship with patients
(Mossialos et al. 2015). Given the challenges of
With a Little Help from My Conversational Agent: Towards a Voice Assistant for Improved Patient Compliance and Medication Therapy
Safety
797

increasing e-commerce and the loss of rural
infrastructure due to urbanization and the shortage of
physicians, the number of German pharmacies has
decreased over the past 10 years (ABDA 2019). Our
artifact could provide a new interface in the patient-
pharmacist relationship (Volland 2015). In this
context, the combination with blistering business
models might be a valuable future service for local
pharmacies. From the patient’s perspective, the
medication assistant can not only support the safety
of drug therapy but also increase the integration into
everyday life and thus promote patient compliance.
As a result, this facilitates healing and supports health
prevention. However, to establish integrity to our
system, it requires a trustworthy and reliable data
management and compliance standards for all
involved users.
Our findings contribute to theory, as we shed light
on guidelines to design and develop multimodal
medical assistants for medication management. With
the derived DPs in this study, we provide context-
oriented guidelines for medical assistants that
complement more generic DPs of CAs. With our
modular approach, we enable the connection to other
applications, such as FeelFit and thus the
enhancement of functionality in the realm of personal
medical health assistants. On this basis, further
research can be initiated, and additional
functionalities and improvements can be developed
based on our evaluation.
Limitations: As with any study, our research is
subject to limitations. First, we conducted only one
iteration of the DSR cycle. The feedback of the expert
interviews must be implemented into the prototype
during the next iteration cycle. Especially the
readability of a standardized medication plan by
scanning the QR code, should be added in the next
development cycle. Second, we have not yet
evaluated the CA form the perspective of potential
patients. Since evaluations with patients require a
comprehensive study design, we presented an
evaluation concept which will be operationalized in a
future study. Third, we have not yet analyzed the
integration of the system into existing healthcare
initiatives or patient portals. Future studies with
patients should investigate the connection to current
systems like advanced patient portals or virtual health
coaches. Finally, our CA is partially focused on the
German healthcare system. The prototype and its
evaluation might be biased by legal, structural, and
cultural influences. Nevertheless, the demand for
medical assistants rises internationally, and the results
of our study can be transferred to any other healthcare
system.
7 CONCLUSION
Our study aimed to develop a multimodal assistant
that supports and secures patients in their medication
management, thereby increasing safety and
facilitating adherence to therapy plans. Within a DSR
project, we identified 16 Is to describe problem areas
that must be considered when developing an
application to enhance medication therapy safety, and
we derived nine MRs and consolidated them into the
three DPs focusing on usability, information
processing and medical issues. Those were built in a
prototype consisting of three components.
First is the Med-Portal component, which can be
accessed by medical professionals to manage
patients’ medication plans and provide individual
advice. The second is the Med-REST web service,
which is the interface of the German ABDA database
that contains all available Rx and OTC medications.
Last is the Med-CA component, which enables
patients to follow their medication plan and obtain
additional information regarding the correct intake,
storage, and side effects. Notifications about
medication intake and reordering can support patients
in integrating therapy management into their
everyday lives. An evaluation with experienced
pharmacists has demonstrated the high relevance and
usefulness of our developed medication assistant.
Finally, an evaluation with patients is conceptualized
for the application within a future study. So far, the
designed artifact offers the potential to increase
patients’ compliance and medication therapy safety
and to improve the relationship between patients and
pharmacists.
REFERENCES
ABDA. 2019. ‘DIE APOTHEKE - Zahlen - Daten - Fakten
2019’. : 84.
vom Brocke, Jan et al. 2009. ‘Reconstructing the Giant: On
the Importance of Rigour in Documenting the
Literature Search Process’. 17th European Conference
on Information Systems 9: 2206–2217.
Chang, Danni, Zhenyu Gu, Fan Li, and Rong Jiang. 2019.
‘A User-Centric Smart Product-Service System
Development Approach: A Case Study on Medication
Management for the Elderly’. Advanced Engineering
Informatics 42: 100979.
Database, Engine H2. 2019. H2 Documentation.
Dayer, Lindsey et al. 2013. ‘Smartphone Medication
Adherence Apps: Potential Benefits to Patients and
Providers’. Journal of the American Pharmacists
Association 53(2): 172–81.
Scale-IT-up 2021 - Workshop on Scaling-Up Healthcare with Conversational Agents
798

Dehling, Tobias, and Ali Sunyaev. 2013. ‘Improved
Medication Compliance Through Health IT: Design
and Mixed Methods Evaluation of the Application
EPill’. ICIS 2013 Proceedings.
Dormann, Harald, Michael Bangemann, Hans-Ulrich
Prokosch, and Jürgen Zerth. 2017. ‘Digitalisierte
Arzneimittelversorgung Am Beispiel Des
Bundeseinheitlichen Patientenbezogenen
Medikationsplans – Eine Frage Der
Stakeholderakzeptanz’. In Digitale Transformation von
Dienstleistungen Im Gesundheitswesen I, Springer
Fachmedien Wiesbaden, 149–64.
EMA. 2017. ‘Guideline on Good Pharmacovigilance
Practices (GVP) Annex I - Definitions (Rev 4)’. Heads
of Medicine Agencies (October): 1–33.
EmmaHome. 2020. ‘Emma - EmmaHome - Emma’.
https://www.emma-hilft.com/emma-home/.
Farhadyar, Kiana, and Reza Safdari. 2018. ‘Requirements
of MHealth-Based Medication Management Systems’.
International Journal of Innovative Research in
Computer Science & Technology 6(2): 12–17.
Ferreira, Flavio et al. 2013. ‘Multimodal and Adaptable
Medication Assistant for the Elderly: A Prototype for
Interaction and Usability in Smartphones’. In Iberian
Conference on Information Systems and Technologies,
CISTI,.
Ferreira, Flávio et al. 2014. ‘Elderly Centered Design for
Interaction - The Case of the S4S Medication
Assistant’. In Procedia Computer Science, Elsevier
B.V., 398–408.
Google. 2020. ‘Dokumentation Zu Dialogflow | Google
Cloud’. https://cloud.google.com/dialogflow/docs/.
Gregor, Shirley, and Alan R Hevner. 2013. ‘Positioning and
Presenting Design Science Research for Maximum
Impact’. MIS quarterly: 337–55.
Gregor, Shirley, L Chandra Kruse, and Stefan Seidel. 2020.
‘The Anatomy of a Design Principle’. Journal of the
Association for Information Systems.
Grube, Anton, Tobias Dehling, and Ali Sunyaev. 2017.
‘How Do Patients Expect Apps to Provide Drug
Information?’ Hawaii International Conference on
System Sciences 2017 (HICSS-50).
hana health. 2019. ‘Medikation Neu Gedacht’.
https://www.hana-health.de/index.html (August 11,
2020).
Haynes, R Brian. 1979. ‘Determinant of Compliance: The
Disease and the Mechanics of Treatment’. Compliance
in health care.
Hevner, Alan, S.T. March, J. Park, and S. Ram. 2004.
‘Design Science in Information Systems Research’.
MIS Q 28: 75–105.
Hsieh, Pi Jung. 2016. ‘An Empirical Investigation of
Patients’ Acceptance and Resistance toward the Health
Cloud: The Dual Factor Perspective’. Computers in
Human Behavior 63: 959–69.
Hsieh, Wen Ting, Yung Cheng Su, Hsin Lien Han, and
Ming Yuan Huang. 2018. ‘A Novel MHealth Approach
for a Patient-Centered Medication and Health
Management System in Taiwan: Pilot Study’. JMIR
mHealth and uHealth 6(7): e154–e154.
Jesús-Azabal, Manuel et al. 2020. ‘Voice Assistant to
Remind Pharmacologic Treatment in Elders’. In
Communications in Computer and Information
Science, Springer, 123–33.
Kim, Ben Y B et al. 2018. ‘Consumer Mobile Apps for
Potential Drug-Drug Interaction Check: Systematic
Review and Content Analysis Using the Mobile App
Rating Scale (MARS)’. JMIR mHealth and uHealth
6(3): e74–e74.
Kuechler, Bill, and Stacie Petter. 2012. ‘Design Science
Research in Information Systems’. (1): 1–66.
Luger, Ewa, and Abigail Sellen. 2016. ‘Like Having a
Really Bad PA: The Gulf between User Expectation
and Experience of Conversational Agents’. In
Proceedings of the 2016 CHI Conference on Human
Factors in Computing Systems, ACM, 5286–97.
Mayring, Philipp. 2010. ‘Qualitative Inhaltsanalyse’.
Handbuch qualitative Forschung in der Psychologie:
601–13.
Mediteo. 2020. ‘Mediteo App |
Medikamentenerinnerungen Und Pillenwecker’.
https://www.mediteo.com/de/.
Meier, P. et al. 2019. ‘FeelFit – Design and Evaluation of a
Conversational Agent to Enhance Health Awareness’.
In Proceedings International Conference on
Information Systems (ICIS 2019), Munich.
Mertens, Alexander et al. 2015. ‘Influence of Mobile ICT
on the Adherence of Elderly People with Chronic
Diseases’. Lecture Notes in Computer Science
(including subseries Lecture Notes in Artificial
Intelligence and Lecture Notes in Bioinformatics) 9194:
123–33.
Mira, José Joaquín et al. 2014. ‘A Spanish Pillbox App for
Elderly Patients Taking Multiple Medications:
Randomized Controlled Trial’. Journal of Medical
Internet Research 16(4): e99–e99.
Mossialos, Elias et al. 2015. ‘From “Retailers” to Health
Care Providers: Transforming the Role of Community
Pharmacists in Chronic Disease Management’. Health
Policy 119(5): 628–39.
Myers, Michael D., and Michael Newman. 2007. ‘The
Qualitative Interview in IS Research: Examining the
Craft’. Information and Organization 17(1): 2–26.
MyTherapy. 2020. ‘Tabletten Erinnerung Leichtgemacht –
MyTherapy App’. https://www.mytherapyapp.com/de.
Peffers, Ken, Tuure Tuunanen, Marcus A Rothenberger,
and Samir Chatterjee. 2007. ‘A Design Science
Research Methodology for Information Systems
Research’. Journal of management information systems
24(3): 45–77.
Petermann, Franz, and Stephan Mühlig. 1998. ‘Grundlagen
Und Möglichkeiten Der Compliance-Verbesserung’.
Petermann, F.(Hg.): Compliance und
Selbstmanagement. Göttingen: Hogrefe: 73–102.
Peters, E, R Pritzkuleit, F Beske, and A Katalinic. 2010.
‘Demografischer Wandel Und
Krankheitshäufigkeiten’. Bundesgesundheitsblatt -
Gesundheitsforschung - Gesundheitsschutz 53(5): 417–
26.
With a Little Help from My Conversational Agent: Towards a Voice Assistant for Improved Patient Compliance and Medication Therapy
Safety
799

Pharma-Daten-Service, ABDATA. 2019. ABDA-
Datenbank.
Reimers, Kai, and Stefan Klein. 2015.
Arzneimitteltherapiesicherheit Im Spannungsfeld von
Vollständiger Medikationsübersicht, Mündigem
Patienten Und Individualisierter Medikation. 3rd ed.
Cuvillier Verlag Göttingen.
Rottenkolber, Dominik et al. 2011. ‘Adverse Drug
Reactions in Germany: Direct Costs of Internal
Medicine Hospitalizations’. Pharmacoepidemiology
and Drug Safety 20(6): 626–34.
Salvi, Fabio et al. 2012. ‘Adverse Drug Events as a Cause
of Hospitalization in Older Adults’. Drug Safety 35:
29–45.
Santo, Karla et al. 2016. ‘Mobile Phone Apps to Improve
Medication Adherence: A Systematic Stepwise Process
to Identify High-Quality Apps’. JMIR mHealth and
uHealth 4(4): e132–e132.
Schäfer, Christian. 2011. Patientencompliance : Durch
verbesserte Therapietreue Effizienzreserven
ausschöpfen TT - Patient compliance: Using efficacy
reserves by improvement of treatment compliance.
Schäfer, Christian: Universität Mainz; Gutenberg
School of Management and Economics (Germany):
Gabler.
Schrepp, Martin, Andreas Hinderks, and Jörg
Thomaschewski. 2014. ‘Applying the User Experience
Questionnaire (UEQ) in Different Evaluation
Scenarios’. In International Conference of Design,
User Experience, and Usability, Springer, 383–92.
Schurig, A. Marlen et al. 2018. ‘Adverse Drug Reactions
(ADR) and Emergencies-the Prevalence of Suspected
ADR in Four Emergency Departments in Germany’.
Deutsches Arzteblatt International 115(15): 251–58.
Sebillo, Monica, Giuliana Vitiello, Danilo Cuciniello, and
Serena Carrabs. 2017. ‘Human-Centered Design of a
Personal Medication Assistant - Putting Polypharmacy
Management into Patient’s Hand!’ In Lecture Notes in
Computer Science, Springer Verlag, 685–99.
Sedlmayr, Martin. 2018. ‘EHealth Als Schlüssel Für
Bessere Patientencompliance-Technische
Möglichkeiten Und Medizinische Herausforderungen’.
GesundheitsRecht 17(1): 17.
Silva, Bruno M et al. 2013. ‘A Mobile Health Application
for Outpatients Medication Management’. In IEEE
International Conference on Communications, Institute
of Electrical and Electronics Engineers Inc., 4389–93.
Sneha, Sweta, and Upkar Varshney. 2012. ‘Strategies
Towards Chronic Disease Management via Medication
Compliance’. AMCIS 2012 Proceedings.
Stark, Renee G, Jürgen John, and Reiner Leidl. 2011.
‘Health Care Use and Costs of Adverse Drug Events
Emerging from Outpatient Treatment in Germany: A
Modelling Approach’. BMC Health Services Research
11.
Tafreshi, Mohammad J, Michael J Melby, Keith R Kaback,
and Teresa C Nord. 1999. ‘Medication-Related Visits
to the Emergency Department: A Prospective Study’.
Annals of Pharmacotherapy 33(12): 1252–57.
Tang, Lei et al. 2011. ‘MHS: A Multimedia System for
Improving Medication Adherence in Elderly Care’.
IEEE Systems Journal 5(4): 506–17.
Taylor, Robert, Joseph V Pergolizzi, R Amy Puenpatom,
and Kent H Summers. 2013. ‘Economic Implications of
Potential Drug-Drug Interactions in Chronic Pain
Patients’. Expert Review of Pharmacoeconomics and
Outcomes Research 13(6): 725–34.
Teixeira, António et al. 2017. ‘Design and Development of
Medication Assistant: Older Adults Centred Design to
Go beyond Simple Medication Reminders’. Universal
Access in the Information Society 16(3): 545–60.
Tiwari, Priyesh et al. 2011. ‘Feasibility Study of a Robotic
Medication Assistant for the Elderly’. In Conferences
in Research and Practice in Information Technology
Series, , 57–66.
Volland, Dirk. 2015. Spring ‘Extending Pharmacist-Patient
Communication with ICT’. St. Gallen.
Vrijens, Bernard et al. 2008. ‘Adherence to Prescribed
Antihypertensive Drug Treatments: Longitudinal Study
of Electronically Compiled Dosing Histories’. Bmj
336(7653): 1114–17.
Wang, Weiyu, and Keng Siau. 2018. ‘Trust in Health
Chatbots’.
Scale-IT-up 2021 - Workshop on Scaling-Up Healthcare with Conversational Agents
800
