
Deep Visio-PhotoPlethysmoGraphy Reconstruction Pipeline for
Non-invasive Cuff-less Blood Pressure Estimation
Francesco Rundo
1 a
, Francesca Trenta
2 b
, Roberto Leotta
2
and Sebastiano Battiato
2 c
1
STMicroelectronics, ADG Central R&D, Catania, Italy
2
Department of Mathematics and Computer Science, University of Catania, Catania, Italy
Keywords:
Deep Learning, Computer Vision, PPG (PhotoPlethysmoGraphy).
Abstract:
In medical field, many cardiovascular and correlated diseases can be early treated by monitoring and analyzing
the subject’s blood pressure (BP). However, the measurement of blood pressure requires the use of invasive
medical and health equipment, including the classical sphygmomanometer or the digital pressure meter. In
this paper, we proposed an innovative algorithmic pipeline to properly estimate the systolic and diastolic
blood pressure of a subject through the visio-reconstruction of the PhotoPlethysmoGraphic (PPG) signal. By
means of an innovative method of face-motion magnification through Deep Learning, it is possible to visio-
reconstruct specific points of the PPG signal in order to extract features related to the pressure level of the
analyzed subject. The proposed approach can be used effectively in healthcare facilities for the fast and non-
invasive monitoring of the pressure level of subjects or in other similar applications. We compared our results
using a classic cuff-less blood pressure device with encouraging results that reach 92% in accuracy.
1 INTRODUCTION
Monitoring the systolic and diastolic pressure of both
healthy and hypertensive subjects is certainly one of
the most important aspects for safeguarding subject’s
health. Many cardiovascular diseases are strictly
caused by pressure dysfunctions and therefore can
be easily treated if the blood pressure level is kept
under control (Wu et al., 2015). In recent years,
there has been an increasing interest in measuring
blood pressure by taking advantage of simple and
non-invasive approaches, some of these are based
on the use of the PhotoPlethysmoGraphic (PPG) sig-
nal (Dastjerdi et al., 2017). Photoplethysmography is
a simple optical technique that can be used to detect
changes in the volume of blood in the microvascular
bed of tissues (Rundo et al., 2018c). It is not inva-
sive since it makes measurements on the surface of
the skin. Based on these assumptions, we designed a
Deep Learning (DL) architecture to perform the visio-
reconstruction of the PPG signal from the face-motion
analysis of the subject, with the aim to extract ad-hoc
features correlated to the level of systolic and dias-
a
https://orcid.org/0000-0003-1766-3065
b
https://orcid.org/0000-0003-2524-3837
c
https://orcid.org/0000-0001-6127-2470
tolic pressure. The proposed pipeline can be imple-
mented as an embedded firmware in any smartphone
equipped with a video camera and PPG sensing-
device (classical Light Emitting Diodes LEDs with
Silicon PhotoMultiplier (SiPM) photo-detector de-
vice (Rundo et al., 2018c)) and therefore can be ap-
plied daily and simply by any subject (Rundo et al.,
2018c). In Figure 1, we illustrated the method used
to sample the PPG signal as well as the physiological
correlation with heart activity and them with blood
pressure (Vinciguerra et al., 2018). As shown in Fig-
ure 1, the PPG sensing framework (PPG Sensing De-
vice) is composed by a coupled LEDs with SiPM de-
vice (photo-detector) with a STM32 based microcon-
troller for pre-processing the sampled physiological
raw data (Vinciguerra et al., 2018). More details
in (Rundo et al., 2018c; Vinciguerra et al., 2018).
In Figure 1, it is also evident how the PPG signal is
strongly correlated to the cardiac activity of a subject
(by measurement of changes in blood volume) and
then with blood pressure. The remainder of the pa-
per is structured as follows. In Section II we present
the related works. In Section III we describe the pro-
posed pipeline while in Section IV we report the ex-
periments we made for validating the proposed ap-
proach. Finally, in Section V we report the conclu-
sions.
Trenta, F., Rundo, F., Leotta, R. and Battiato, S.
Deep Visio-PhotoPlethysmoGraphy Reconstruction Pipeline for Non-invasive Cuff-less Blood Pressure Estimation.
DOI: 10.5220/0010380900750080
In Proceedings of the International Conference on Image Processing and Vision Engineering (IMPROVE 2021), pages 75-80
ISBN: 978-989-758-511-1
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
75
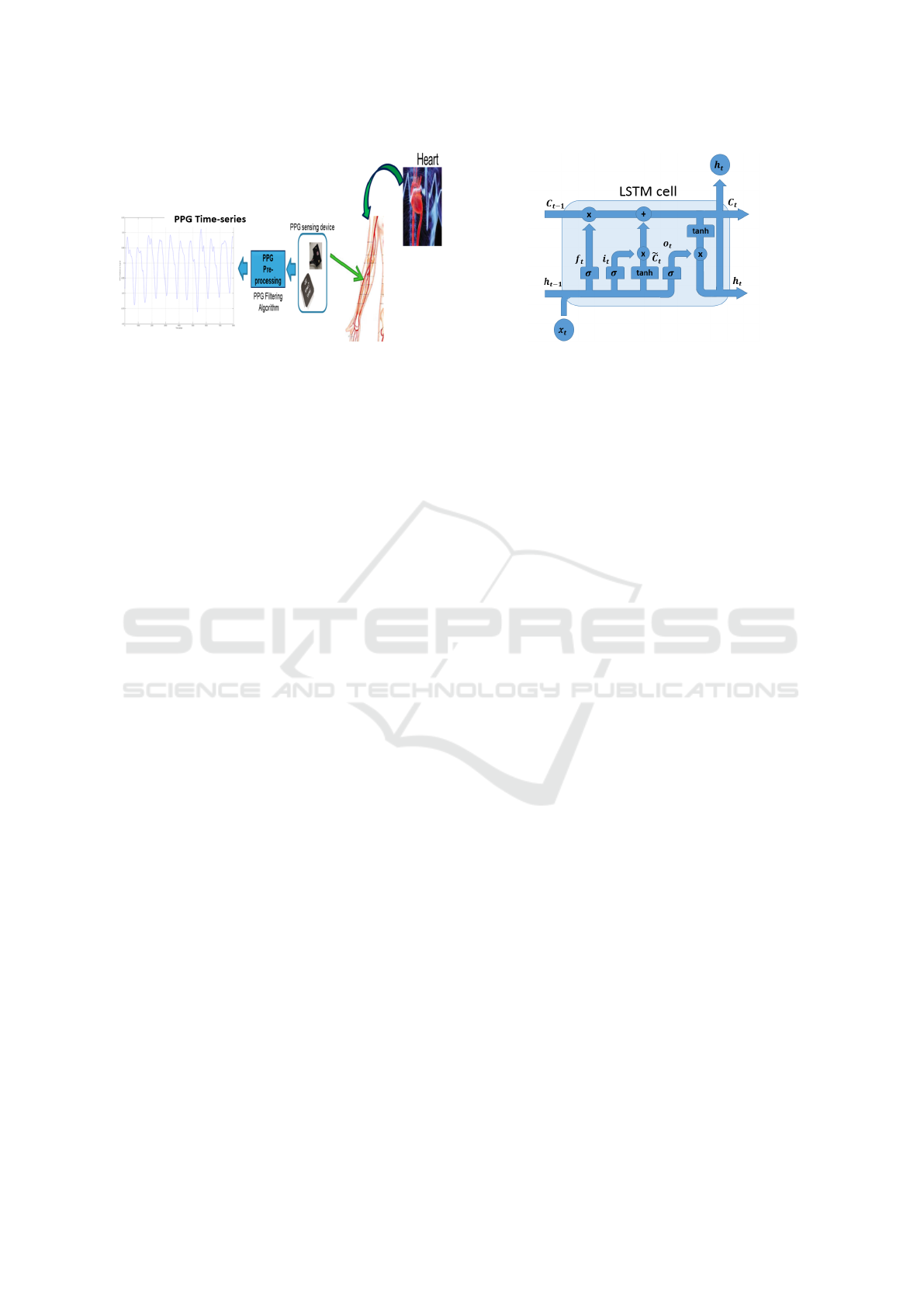
Figure 1: The PPG signal sampling pipeline.
2 RELATED WORKS
A considerable amount of literature focused on esti-
mating blood pressure or arterial stiffness taking ad-
vantage of Deep Learning (DL) methods. In (Monte-
Moreno, 2011), the author proposed a non-invasive
approach to estimate the systolic and diastolic blood
pressure by using PPG. The experimental results shed
light on relationship between blood pressure, glu-
cose levels and PPG waveform confirming the ef-
fectiveness of Machine Learning (ML)-based tech-
niques. In addition to these techniques, special-
ized ML-based methods have been proposed with
the recent emergence of Deep Learning. Slapnicar
et al. (Slapni
ˇ
car et al., 2019) investigated the prob-
lem of detecting Blood Pressure (BP) using a ML-
based architecture. In order to overcome limitations
derived from using cuff-based devices, the authors
used PPG and its first and second derivative to feed a
novel spectro-temporal Deep Neural Network (DNN).
Finally, they performed a leave-one-subject-out ex-
periments confirming the advantage of the proposed
model in computing dependency between PPG wave-
forms and blood pressure. In (Alty et al., 2007),
the authors proposed a ML-based pipeline to predict
arterial stiffness i.e. and an indicator correlated to
blood pressure. It is reliable for the classification of
subjects into high and low aortic pulse wave veloc-
ity (PWV) aiming to analyze cardiovascular disease.
Their work highlights the effective results achieved
by Support Vector Machine (SVM) not only for clas-
sification purpose but also in performing multilinear
regression. In (Rundo et al., 2018b), the authors de-
scribed an innovative approach to estimate cardiovas-
cular disease risk via blood pressure. The proposed
method measures BP by analyzing the PPG signal
without requiring any user calibration. In (Huynh
et al., 2018), the authors proposed an interesting ap-
proach to estimate the blood pressure by using aver-
aging Impedance Plethysmography (IPG) for detec-
Figure 2: Example of a LSTM cell.
tion of Pulse Transit Time (PTT). The experimen-
tal estimation of blood pressure (BP) provided very
interesting results (RMSE: 8.47 ± 0.91 mmHg and
5.02 ± 0.73mmHg for systolic and diastolic level, re-
spectively). Despite providing effective results, the
aforementioned approaches require the use of inva-
sive medical devices throughout the measurement of
blood pressure levels. In this work, the authors pro-
posed a less-invasive and cuff-less approach for mea-
suring blood pressure.
3 BACKGROUND AND THE
PROPOSED PIPELINE
In this paper, a novel Deep Long Short-Term Mem-
ory (LSTM) based pipeline is presented. The
LSTM Vanilla architecture was originally proposed
by Hochreiter and Schmidhuber (Hochreiter and
Schmidhuber, 1997) for preventing the Vanishing gra-
dient problem which affects Recurrent Neural Net-
works (RNNs). The LSTM cell is able to select
what information to discard or store. In order to
produce effective results in real-applications, this se-
lective method requires three different mechanism to
read, store and discard information by taking advan-
tage of specific vectors called “gates”. Basically, the
“input gate”, “output gate” and “forget gate” decision
is implemented via activation functions which define
if a given information is relevant or not. More in de-
tails, given x
t
as input vector, h
t−1
as previous cell
output, C
t−1
previous cell memory, h
t
as current cell
output and C
t
as current cell memory, we define Equa-
tions (1)-(3) to determine what information to store.
Finally, we generate the output of LSTM by updat-
ing old cell state as per Equation (4) and merging the
previous output, the input and the bias vector as Equa-
tions (5)-(6).
f
t
= σ(W
f
◦ [h
t−1
, x
t
] + b
f
(1)
i
t
= σ(W
i
◦ [h
t−1
, x
t
] + b
i
(2)
IMPROVE 2021 - International Conference on Image Processing and Vision Engineering
76

Figure 3: The proposed Deep LSTMs pipeline.
e
C
t
= tanh(W
C
◦ [h
t−1
,x
t
] + b
C
(3)
C
t
= f
t
∗C
t−1
+ i
t
∗
e
C
t
(4)
o
t
= σ(W
o
◦ [h
t−1
, x
t
] + b
o
(5)
h
t
= o
t
∗ tanh(C
t
)
(6)
In Figure 2, a prototype of LSTM cell is reported.
In recent years, several LSTM-based approaches have
been developed with promising results. For instance,
LSTM architectures have been largely employed in
automotive field to visio-reconstruct such part of the
car-driver PPG signal (sampled by ad-hoc sensors
placed in the steering) when this physiological sig-
nal was no longer available (Trenta et al., 2019). In-
spired by recent literature, we designed a Deep LSTM
pipeline aiming to better visio-reconstruct the PPG
waveforms of a subject in order to estimate the cor-
related blood pressure. The proposed system archi-
tecture consists of a physical signal-acquisition mod-
ule (PPG sensing device) needed for preliminary cal-
ibration as well as a vision module to extract effec-
tive facial descriptors and a Machine Learning frame-
work that reconstruct such part of the PPG wave-
forms (extremes points such as minimum, maximum
and so on) in order estimate the blood pressure (BP).
During the calibration phase of the proposed system,
the PPG signal is collected by using a coupled LED-
SiPM sensing system available in several medical de-
vices or smartphones and able to generate the PPG
raw data (Rundo et al., 2018a; Rundo et al., 2017).
Then, we apply the patented Bi2PRS algorithm to ob-
tain the filtered compliant PPG signal. The Bi2PRS
algorithm (Rundo et al., 2018a) is able to properly
filter the collected PPG raw data (by means of an ad-
hoc Butterworth band-pass filter) and then to deter-
mine, in the filtered PPG waveforms, the right value
of the extreme points such as max, min, etc. More
details on Bi2PRS in (Rundo et al., 2018a; Rundo
et al., 2017). During the PPG sampling session, we
recorded a video sequence of the subject by using a
smartphone having a camera device with 30 fps as
frame-rate, under high light condition. As widely
known in scientific literature (Oh et al., 2018), the
face of the subject performs visual micro-movements
imperceptible at naked eye and closely related to the
cardiac pumping activity. These micro-movements
are strongly correlated to PPG signal as a cardiac re-
lated signal. Inspired by the work of Wu et al. (Wu
et al., 2012), in which the authors introduced Video
Magnification to amplify facial micro movements for
revealing the flow of blood, we proposed an approach
in which a group of video frames (face sequences of
the subject) are extracted and then analyzed in or-
der to identify such facial landmarks to be tracked
(through the pixel intensities over time). Specifically,
we designed input layer of the proposed Deep LSTM
model for processing facial landmarks of both eyes.
In Figure 3, we have summarized the overall scheme
of our proposed pipeline. More details in the next
paragraphs. The framework of the proposed Deep
LSTM architecture is composed by one input layer,
three hidden layers, and one output layer. Specifi-
cally, we designed three hidden layers which include
two regular layers and one dropout layer appointed to
boost the overall performance. The model was trained
with an initial learning rate of 10
−3
. The batch size
was set to 512 and the maximum number of train-
Deep Visio-PhotoPlethysmoGraphy Reconstruction Pipeline for Non-invasive Cuff-less Blood Pressure Estimation
77
ing epochs was set to 100. During the calibration
phase, we trained the designed Deep LSTM to ana-
lyze the correlation between the facial time-evolution
selected landmarks of the subjects with correlated ex-
treme points of the PPG signal. The output of the
LSTM pipeline represents predicted extreme points
of the PPG signal considering the facial landmarks
time series. When the proposed Deep LSTM frame-
work has learned the correlation between facial land-
marks and extreme points of the subject’s PPG sig-
nal, the calibration phase will be dropped and there-
fore the system will operate feed-forward in the vi-
sion part only linked to the acquisition of facial land-
marks only. The calibration phase of the device re-
quires few minutes of acquisition of the PPG signal
(and corresponding visual data) and it will be neces-
sary to perform it only once. On the other hand, the
trained feed-forward system (only Visio-based Deep
LSTMs pipeline followed by reconstructed PPG ex-
treme point(s) classifier, as described in the section
IV) is able to generate the output (blood pressure es-
timation) in a near real-time context (few seconds).
The so designed pipeline was ported to the STM32
architecture through the STM32-CUBE AI software
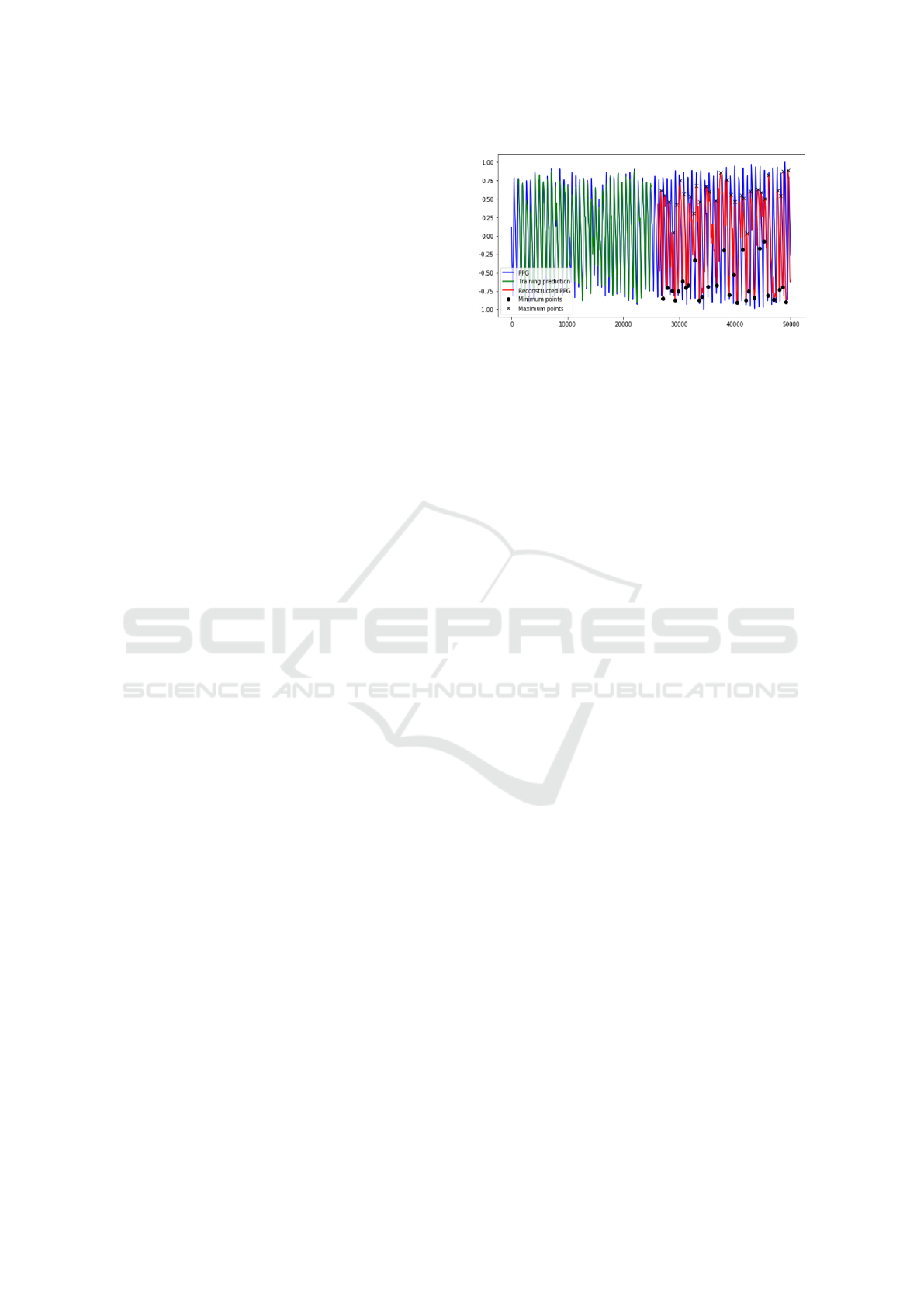
platform. In Figure 4, a graph reporting the esti-
mated extreme points by the proposed trained Deep
LSTM super-imposed with original source PPG sig-
nal is shown. Once we have collected the set of char-
acteristic extreme points of the waveforms of the PPG
signal, we are able to characterize the subject’s car-
diac activity with regard to the two phases of the car-
diac cycle i.e. systole and diastole, on which the level
depends on blood pressure. In Figure 5, we reported
a classical PPG waveform and the corresponding dy-
namic correlated to the cardiac cycle. By means of the
characteristic PPG visio-reconstructed extreme points
(m1, m2, m3, m4), we are able to estimate the heart
systole and diastole phases, therefore, the pressure
levels related to them. For each pair of PPG wave-
forms (PPG
j
, PPG
j+1
) we define the following indi-
cators:
ϕ = [m
j
1
,m
j
2
, m
j
3
,m
j
4
,dx
j
i
,dy
j
i
,mAI
J
]
∀ j = 1..N
PPG
− 1 ; i = 1,2, 3, 4
(7)
m
j
i
= (x
m
j
i
,y
m
j
i
)
∀ j = 1...N
PPG
− 1 ; i = 1,2, 3, 4
(8)
dx
j
i
= x
m
j+1
i
− x
m
j
i
∀ j = 1...N
PPG
− 1 ; i = 1,2, 3, 4
(9)
dy
j
i
= y
m
j+1
i
− y
m
j
i
∀ j = 1...N
PPG
− 1 ; i = 1,2, 3, 4
(10)
Figure 4: The reconstructed extreme points super-imposed
to the corresponding source PPG signal.
mAI
j
= ((y
m
j
3
− y
m
j
1
) − y
m
j
4
)/(y
m
j
3
− y
m
j
1
)
(11)
where mAI
J
is a modified version of the so-called
Augmentation Index usually computed for measur-
ing the arterial stiffness (Gonzalez et al., 2012) while
NPPG represents the number of PPG waveforms. The
other indicators reported in the Equations (8)-(10) al-
low us to characterize cardiac cycles (and therefore
the relative pressure levels) according to the PPG sig-
nal mapping reported in Figure 5. The elements of the
vector ϕ represents the input of a machine learning
framework (Fully Connected Multi-Layers Network
with binary output) designed to learn the correlation
between the so computed input elements and the cor-
responding value of the systolic and diastolic blood
pressure. The output of the machine learning frame-
work is a binary value which can be considered as a
discriminating flag to indicate if the subject presents
normal pressure values (0) or not (1). The set 120/80,
which indicates 120 mmHg for systolic pressure and
80 mmHg for diastolic pressure, has been considered
as normal blood pressure values. Under the supervi-
sion of a team of physiologists, we have defined all
pressure values less than or equal to 120/80 as ac-
ceptable blood pressure while higher values are con-
sidered anomalous and as such must be signaled and
monitored. It should be noted that the proposed sys-
tem can monitor and discriminate even different pres-
sure levels (with respect to the classic 120/80 mmHg)
requiring a different and adequate calibration. The
proposed pipeline has been tested and validated as de-
scribed in the following paragraph.
4 EXPERIMENTS
In order to train and validate the proposed system, un-
der the supervision of the physiologists who collabo-
rated with us in this work, we have collected a dataset
of subjects to perform systolic and diastolic pressure
measurements simultaneously with the acquisition of
IMPROVE 2021 - International Conference on Image Processing and Vision Engineering
78
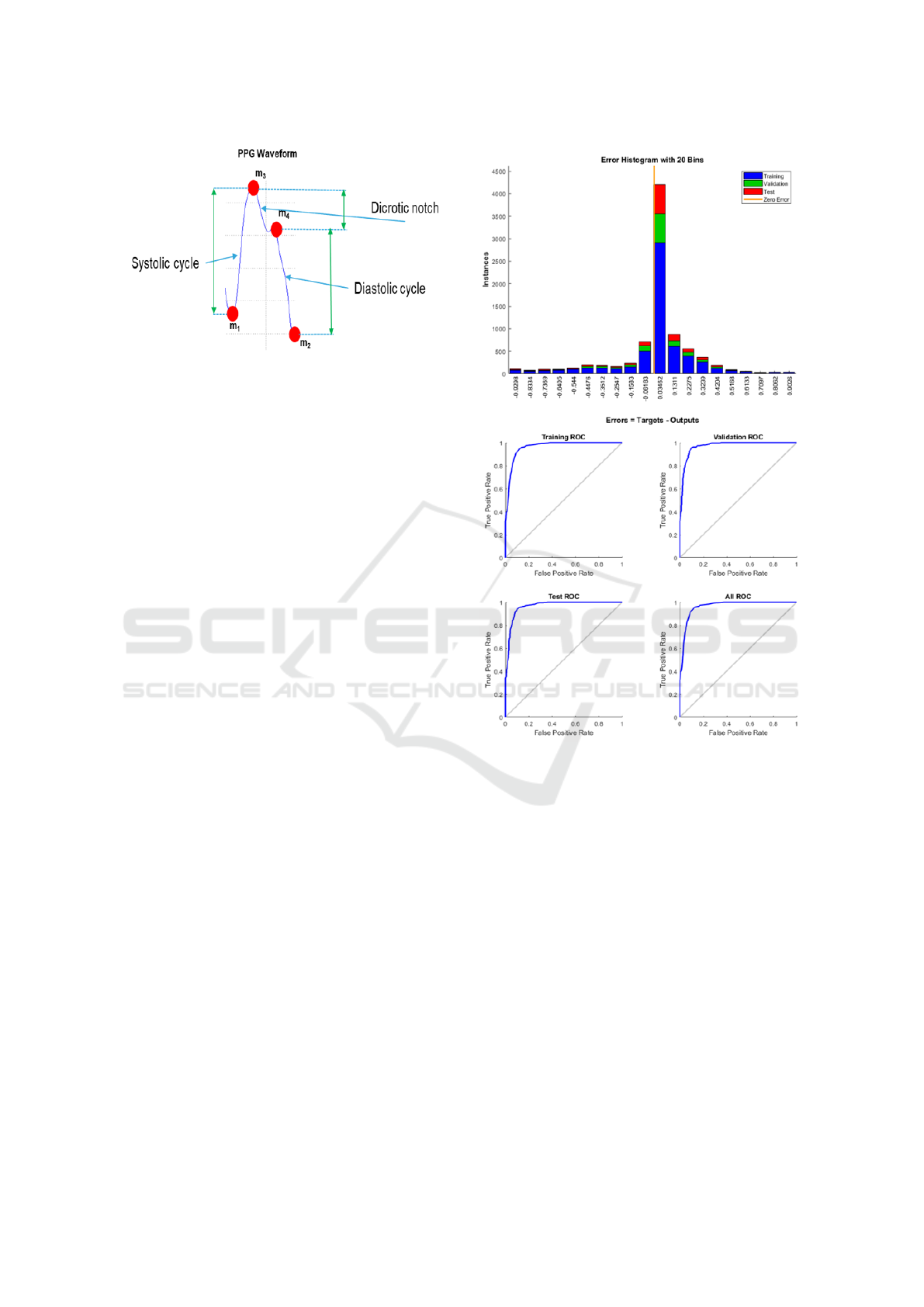
Figure 5: The reconstructed extreme points super-imposed
to the corresponding source PPG signal.
the PPG signal and contextually the video sequence
reporting the subject’s face. The blood pressure mea-
surements were performed using a classic certified
medical sphygmomanometer. All procedures were
carried out under the supervision of the physiologists
and after we have received the informed consent of
each patient and having acquired the consent from the
Ethical Committee CT1 (authorization n.113 / 2018 /
PO), which were conducted in accordance with the
Declaration of Helsinki. The dataset is composed of
56 both healthy and hypertensive subjects including
both males and females individuals. The minimum
age of the subjects in the dataset is 21 years while the
maximum age is 70 years. The collected minimum
pressure value is around 110/75 while the maximum
pressure value is 140/85. For each subject, systolic
and diastolic pressure was acquired as well as a few
minutes (5 min) acquisition of PPG signal and video
signal (subject face), as above described. The acquisi-
tions of the PPG signal, needed for the preliminar cal-
ibration and training of the whole pipeline, were per-
formed at a sampling frequency of 1 kHz and by us-
ing the system described in (Rundo et al., 2018a). We
designed a Fully Connected Network (FCN) trained
with the Scaled Conjugate Gradient backpropagation
(SCG) algorithm described in (Møller, 1993) with a
unique hidden layer of 500 neurons. The designed
FCN learns as input the visio-reconstructed PPG ex-
treme points while the output is a binary flag con-
firming if the corresponding blood pressure is normal
(0) or not (1). In Figure 6, we reported the learn-
ing error dynamic of the proposed machine learning
framework as well as the ROC curves both in train-
ing and validation. The dataset has been divided as
follow: 70% of the data has been used for the train-
ing activity while the remaining 30% for testing and
validation. In order to robustly validate the proposed
method, we calibrated the proposed pipeline for all
subjects of the training and validation dataset and
then we created tests consisting of multiple frames
Figure 6: (a) Learning error dynamic histogram of the Ma-
chine Learning classifier (b) ROC curves both in validation
and training dataset.
of the subjects that are sequentially and randomly
fed to the Machine Learning system (for each sub-
ject we initialize the machine learning system with
related weights computed during calibration). For
each patient, we reconstituted the characteristic ex-
treme points of the PPG signal which were then fed
to the machine learning framework for classifying the
corresponding blood pressure level. The total accu-
racy (in test set) of the proposed system in discrim-
inating normal-pressure subjects from subjects with
blood pressure higher than normal or hypertensive is
91.7%.
Deep Visio-PhotoPlethysmoGraphy Reconstruction Pipeline for Non-invasive Cuff-less Blood Pressure Estimation
79

5 CONCLUSIONS
The obtained results are very promising in the field
of medical-health applications for the early preven-
tion of cardiovascular pathologies. The main benefit
of the proposed system is the non-invasive and effec-
tive estimation of the subject’s blood pressure level
in few seconds. The experimental results allow us to
be confident about the applicability of this approach
in different applications in the medical field. Future
works will focus on collecting more data in order to
improve the effectiveness of the proposed approach
as well as to implement a robust pipelines for moni-
toring the response to certain oncological treatments
(such as chemotherapy and immunotherapy) as many
anti-neoplastic drugs are known to produce abnor-
mal increases in blood pressure which therefore re-
quires continuous monitoring and within acceptable
times (Banna et al., 2018; Rundo et al., 2019).
REFERENCES
Alty, S. R., Angarita-Jaimes, N., Millasseau, S. C., and
Chowienczyk, P. J. (2007). Predicting arterial stiffness
from the digital volume pulse waveform. IEEE Trans-
actions on Biomedical Engineering, 54(12):2268–
2275.
Banna, G. L., Camerini, A., Bronte, G., Anile, G., Ad-
deo, A., Rundo, F., Zanghi, G., Lal, R., and Li-
bra, M. (2018). Oral metronomic vinorelbine in
advanced non-small cell lung cancer patients unfit
for chemotherapy. Anticancer research, 38(6):3689–
3697.
Dastjerdi, A. E., Kachuee, M., and Shabany, M. (2017).
Non-invasive blood pressure estimation using phono-
cardiogram. In 2017 IEEE International Symposium
on Circuits and Systems (ISCAS), pages 1–4. IEEE.
Gonzalez, R., Manzo, A., Delgado, J., Gomis-Tena, J., and
Saiz, J. (2012). Photoplethysmographic augmentation
index using the signal fourth derivative. In 2012 Com-
puting in Cardiology, pages 821–824. IEEE.
Hochreiter, S. and Schmidhuber, J. (1997). Long short-term
memory. Neural computation, 9(8):1735–1780.
Huynh, T. H., Jafari, R., and Chung, W.-Y. (2018). Nonin-
vasive cuffless blood pressure estimation using pulse
transit time and impedance plethysmography. IEEE
Transactions on Biomedical Engineering, 66(4):967–
976.
Møller, M. F. (1993). A scaled conjugate gradient algo-
rithm for fast supervised learning. Neural networks,
6(4):525–533.
Monte-Moreno, E. (2011). Non-invasive estimate of blood
glucose and blood pressure from a photoplethysmo-
graph by means of machine learning techniques. Arti-
ficial intelligence in medicine, 53(2):127–138.
Oh, T.-H., Jaroensri, R., Kim, C., Elgharib, M., Du-
rand, F., Freeman, W. T., and Matusik, W. (2018).
Learning-based video motion magnification. In Pro-
ceedings of the European Conference on Computer Vi-
sion (ECCV), pages 633–648.
Rundo, F., Conoci, S., Fallica, P. G., and Petralia, S. (2017).
Processing of electrophysiological signals.
Rundo, F., Conoci, S., Ortis, A., and Battiato, S.
(2018a). An advanced bio-inspired photoplethysmog-
raphy (ppg) and ecg pattern recognition system for
medical assessment. Sensors, 18(2):405.
Rundo, F., Ortis, A., Battiato, S., and Conoci, S. (2018b).
Advanced bio-inspired system for noninvasive cuff-
less blood pressure estimation from physiological sig-
nal analysis. Computation, 6(3):46.
Rundo, F., Petralia, S., Fallica, G., and Conoci, S. (2018c).
A nonlinear pattern recognition pipeline for ppg/ecg
medical assessments. In Convegno Nazionale Sensori,
pages 473–480. Springer.
Rundo, F., Spampinato, C., Banna, G. L., and Conoci, S.
(2019). Advanced deep learning embedded motion
radiomics pipeline for predicting anti-pd-1/pd-l1 im-
munotherapy response in the treatment of bladder can-
cer: Preliminary results. Electronics, 8(10):1134.
Slapni
ˇ
car, G., Mlakar, N., and Lu
ˇ
strek, M. (2019). Blood
pressure estimation from photoplethysmogram using
a spectro-temporal deep neural network. Sensors,
19(15):3420.
Trenta, F., Conoci, S., Rundo, F., and Battiato, S. (2019).
Advanced motion-tracking system with multi-layers
deep learning framework for innovative car-driver
drowsiness monitoring. In 2019 14th IEEE Inter-
national Conference on Automatic Face & Gesture
Recognition (FG 2019), pages 1–5. IEEE.
Vinciguerra, V., Ambra, E., Maddiona, L., Romeo, M.,
Mazzillo, M., Rundo, F., Fallica, G., di Pompeo,
F., Chiarelli, A. M., Zappasodi, F., et al. (2018).
Ppg/ecg multisite combo system based on sipm tech-
nology. In Convegno Nazionale Sensori, pages 353–
360. Springer.
Wu, C.-Y., Hu, H.-Y., Chou, Y.-J., Huang, N., Chou,
Y.-C., and Li, C.-P. (2015). High blood pressure
and all-cause and cardiovascular disease mortalities in
community-dwelling older adults. Medicine, 94(47).
Wu, H.-Y., Rubinstein, M., Shih, E., Guttag, J., Durand, F.,
and Freeman, W. (2012). Eulerian video magnifica-
tion for revealing subtle changes in the world. ACM
transactions on graphics (TOG), 31(4):1–8.
IMPROVE 2021 - International Conference on Image Processing and Vision Engineering
80
