
Deep Learning Type Convolution Neural Network Architecture for
Multiclass Classification of Alzheimer’s Disease
Gopi Battineni
a
, Nalini Chintalapudi
b
, Francesco Amenta
c
and Enea Traini
Telemedicine and Tele Pharmacy Centre, School of Medicinal and Health Products Sciences,
University of Camerino, Camerino, 62032, Italy
Keywords: Alzheimer’s Disease (AD), OASIS-3, MRI Images, Deep Learning, CNN.
Abstract: Alzheimer’s disease (AD) is one of the common medical issues that the world is facing today. This disease
has a high prevalence of memory loss and cognitive decline primarily in the elderly. At present, there is no
specific treatment for this disease, but it is thought that identification of it at an early stage can help to manage
it in a better way. Several studies used machine learning (ML) approaches for AD diagnosis and classification.
In this study, we considered the Open Access Series of Imaging Studies-3 (OASIS-3) dataset with 2,168
Magnetic Resonance Imaging (MRI) images of patients with very mild to different stages of cognitive decline.
We applied deep learning-based convolution neural networks (CNN) which are well-known approaches for
diagnosis-based studies. The model training was done by 70% of images and applied 10-fold cross-validation
to validate the model. The developed architecture model has successfully classified the different stages of
dementia images and achieved 83.3% accuracy which is higher than other traditional classification techniques
like support vectors and logistic regression.
1
INTRODUCTION
Alzheimer's Disease (AD) is the most well-known
and largely diffused neurodegenerative disorder
occurring in the elderly. AD negatively affects
patients' everyday lives, causing an advanced decline
of cognitive capabilities such as memory, language,
behaviour, and critical thinking (Alzheimer’s Disease
International (ADI ) 2010). Changes in cognitive
impairment of AD patients start slowly and evolve
rapidly over the long run.
Similar to other body parts, brain can change as
people get older. Some people lost thinking and
incidental issues with recollecting certain things.
Excessive cognitive decline, and other significant
changes in the manner in which brain function is
impaired (Jaussent et al. 2012). The first symptoms of
AD are trouble recalling recently learned data
because Alzheimer's progressions regularly start in
the brain areas involved in learning and memory. As
Alzheimer's progresses progressively severe
symptoms like confusion, mood changes,
disorientation, unwarranted doubts about family and
companions, and trouble talking appear. Individuals
with cognitive decline or other potential indications
1
of AD may think that it’s difficult to remember they
have an issue.
AD is a type of dementia with several
implications on the cognitive domain, affecting
primarily thinking and memory. Specialists and
different parental figures screen the movement of AD
in patients by assessing the level of decrease in the
patients' psychological capacities that are often
classified into three stages: very mild (normal
cognitive), mild cognitive impairment (MCI), and
demented (Gaugler et al. 2016). Figure 1 presents
the magnetic resonance image (MRI) images of
different AD conditions. Although the MCI and
dementia patients both are experiencing a reduction
of cognitive abilities, dementia patients would suffer
from more pronounced difficulties with thinking or
hampered judgment.
a
b
c
https://orcid.org/0000-0003-0603-2356
https://orcid.org/0000-0003-0818-306X
https://orcid.org/0000-0002-0555-1034
Battineni, G., Chintalapudi, N., Amenta, F. and Traini, E.
Deep Learning Type Convolution Neural Network Architecture for Multiclass Classification of Alzheimer’s Disease.
DOI: 10.5220/0010378602090215
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 2: BIOIMAGING, pages 209-215
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
209
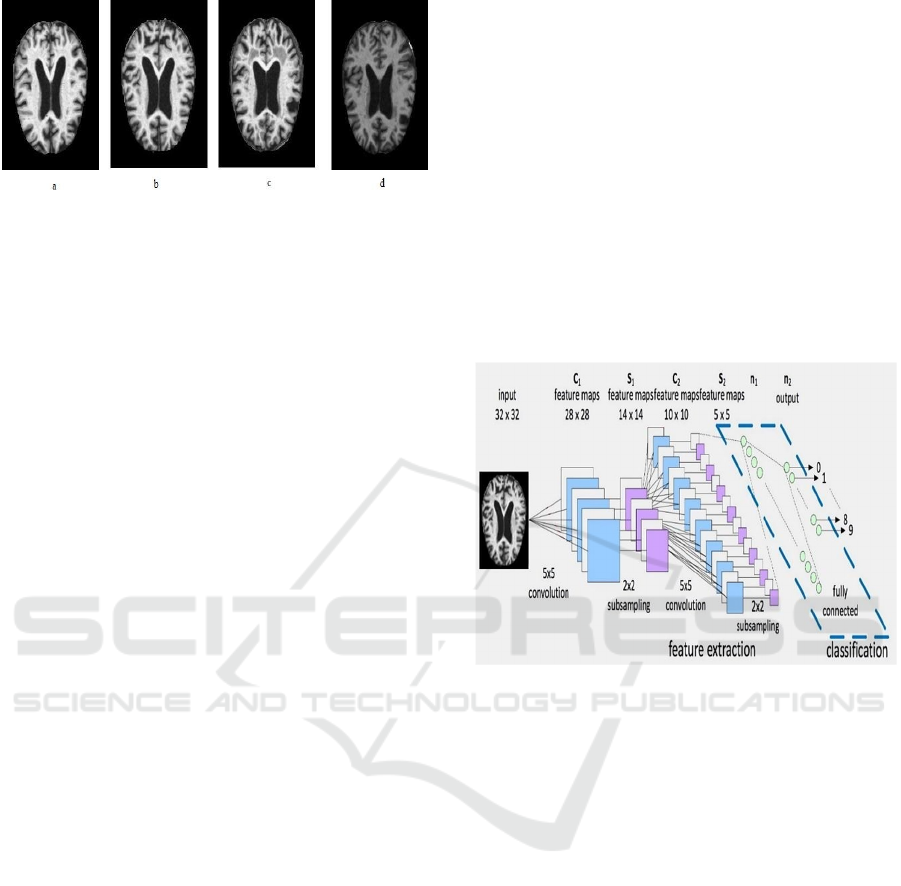
Figure 1: AD presented by MRI images (a) mild dementia;
(b) moderate demented; (c) nondemented; and (d) very
mild demented.
In clinical practice, the capacity to accurately
forecast the patient diagnosis can help by adding
appropriate medical decisions on treatment
approaches. Recently, machine learning (ML)
algorithms are largely applying to forecast and predict
diseases and helping in quick decision making
(Battineni, Sagaro, et al., 2020). Pattern-related
approaches like logistic regression (Johnson et al.,
2014), support vector machines (Battineni,
Chintalapudi, en Amenta 2019), and linear
discriminant analysis (Rathore et al. 2017) are
giving promising results in the prediction of AD
development and early AD detection.
Deep learning models were used unlabeled data
during preprocessing. These are well suited for
imbalanced datasets and achieve a knowledge base
(Mittal et al. 2019). At present these are largely
involved in all other problems that are not able to be
addressed by traditional artificial intelligence (AI)
techniques. Neural networks are the latest deep
learning algorithms that have discovered the
functionality of different situations. Convolutional
neural networks (CNN) are characterized
contributions to profits through a complex
composition of layers that presents building blocks
including nonlinear functions and transformations.
Medical experts feel that deep learning could be a
promising solution in AD identification and stage
detection (Khan et al., 2020). For instance,
(Basheera en Sai Ram, 2019) applied CNN
modeling for AD diagnosis based on T2 weighted
magnetic resonance imaging (MRI) and achieved
90.47% accuracy. A Siamese CNN can also help to
categorize the AD and studies reported 99.05% of
accuracy (Mehmood et al. 2020). It is also reported
that AD prediction from MCI using the CNN model
reported 79.9% of accuracy(Lin et al., 2018).
Therefore, it is assumed that an effective and
comprehensive deep learning model can help to
identify early AD prediction and ultimately provide
timely treatment to
the suffered patients. In this work, we proposed
convolutional neural networks (CNN) model of
deep learning type for detection of early-stage AD
and successfully classify the MRI images on four
different dementia stages presented in Figure 2.
Experiments were conducted on longitudinal
neuroimages of the OASIS-3 database that include
MR scans of T1-weighted, T2 weighted, ASL, SWI,
DTI sequences, FLAIR, time of flight, and resting-
state BOLD. The rest of the paper is structured
according to the following outline: Section 2 presents
the dataset and proposed model architecture; section
3 presents the experimental results, and section 4
makes a discussion which is followed by the
conclusion in section 5.
Figure 2: Brain image classification with the CNN model
framework.
2
METHODS
2.1 Dataset
The Open Access Series of Imaging Studies
(OASIS) contains MR scanning information that is
openly accessible to scientific communities. They
released OASIS-1 (cross-sectional) and OASIS-2
(longitudinal) MRI datasets among different subjects
and these datasets are widely used in many studies
(Sweeney et al. 2013; Palumbo et al. 2019). OASIS-
3 is the extension of previous datasets. It includes
1,098 patients aging from 42 to 95 years. Among
participants, 609 are associated with normal cognitive
decline (very mild), and 489 were associated with
different cognitive decline stages. OASIS-3 dataset
incorporated both functional and structural features of
more than 2,000 MRI images. The dataset outcome of
four categories of MR images has presented in Figure
3.
BIOIMAGING 2021 - 8th International Conference on Bioimaging
210
Figure 3: Dataset outcome of different dementia stages
(3*4 image matrix).
2.2 CNN Model Architecture
A convolutional neural network (ConvNet) is deep
learning type algorithms that take images as input,
assign features based on their importance (biases and
learnable weights) to different image objects, and
also be able to separate one from the other
(Krizhevsky, Sutskever, en Hinton 2017). When
compared with other classification models, ConvNet
possesses low complex pre-processing steps. In CNN,
each input image is gone through sequence
convolution layers namely pooling layers, filtering
layers (kernels), and fully connected layers (FCs).
To make the proposed model easier for
understanding, we created a dense layer block and
convolution block. The architecture of the CNN
model is inspired by the article (Pan et al. 2020). We
built the CNN model by using five convolutional
slabs covered with convolution layers, feature
engineering, max pooling, and classification. We
have used cross-entropy as a loss function and Adam
as an optimizer. SoftMax has been used to classify the
multiclass AD stages since it is associated with a
mutually exclusive relationship. The feature
representation (f
k
) works as an input to the SoftMax
layer and interprets output brain stages. A probability
score P (k) for each class as defined as
P
k
=
∑
;where fi feature representation, and
Cross entropy loss function as
(L)=
∑
𝑡𝑘. log 𝑝𝑘
; where t
k
ground truth of
MRimage then
=P
k
-t
k.
2.3 Experimental Setup
Figure 4 presents the most relevant procedures
followed to construct the feature data of brain images
and extraction of AD images developed in this paper.
After pre-processing steps, the given image dataset
has been divided into training and validation files
with standard (80:20) division.
The procedures indicated red line are MR images
that fed to the CNN model for training purposes.
The model extracts the input image features of
trained images under present parameters and supplies
them to the SoftMax classifier for testing. The
SoftMax function calculates the loss and model
accuracy. For avoiding high loss, network
parameters are adjusted by the back-propagation
algorithm. After applying several iterations (epochs)
the better-trained parameters have been achieved.
The model visualization metrics like loss and
receiver operating characteristic area under the curve
(ROC AUC) have been taken as the performance
parameter for AD classification since it has been
considered one of the key metrics in multi-image
classification techniques. The experimental setup
and AD detection and classification have been done
through TensorFlow and python language.
Deep Learning Type Convolution Neural Network Architecture for Multiclass Classification of Alzheimer’s Disease
211
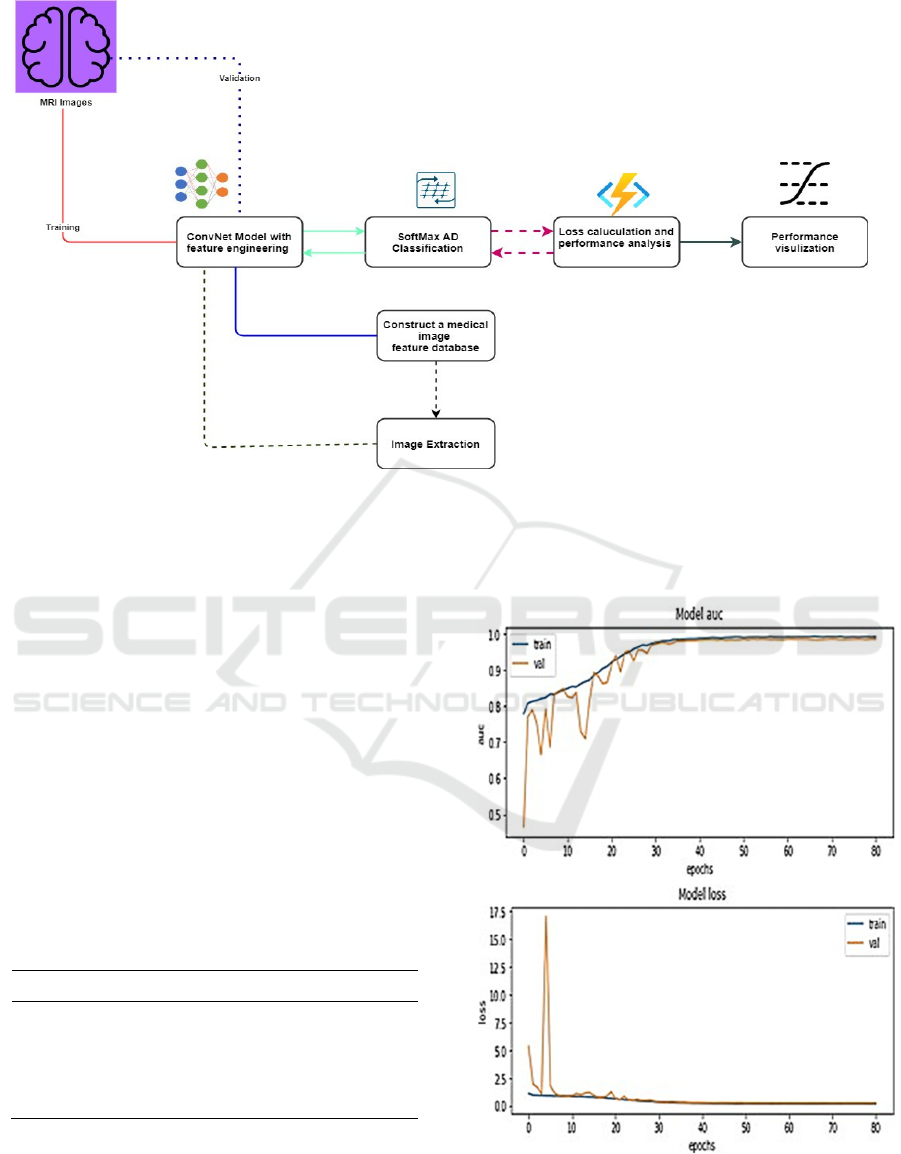
Figure 4: Experimental setup of the work.
3
RESULTS
To do efficient training on our CNN model, a back-
propagation algorithm is set to adjust the rate of
learning and stop the model automatically once it
reaches maximum accuracy. Since the learning rate is
one of the hyperparameters that decides model
accuracy and time to process the model. OASIS-3
dataset consisted of 2168 independent MRI
scanners. Among the given images, 1,734 are used
for training and 434 were used for validation
purposes. Because of the large image dataset, 10-
fold cross-validation has been used and we have
used each fold 70% as training, 10% as validation,
and 20% images are used testing. The distribution of
the dataset is presented in Table 1.
Table 1: Total image distribution.
Total Images: 2168
Type Percentage
Trained images 1517 (70%)
Testing images 434 (20%)
Validation images 217 (10%)
The model-fitting has to be done on a sample of
100 epochs and to prevent model overfitting we stop
the model early at the 80
th
iteration. The model took
a run time of 138 min to process the trained images.
Figure 5 presents a graphical representation of ROC.
AUC and loss metrics after each iteration on both
training and validation image data.
Figure 5: Model AUC and loss metric outcomes.
Though the model evaluation has been done on
the validation dataset, we also perform the
BIOIMAGING 2021 - 8th International Conference on Bioimaging
212
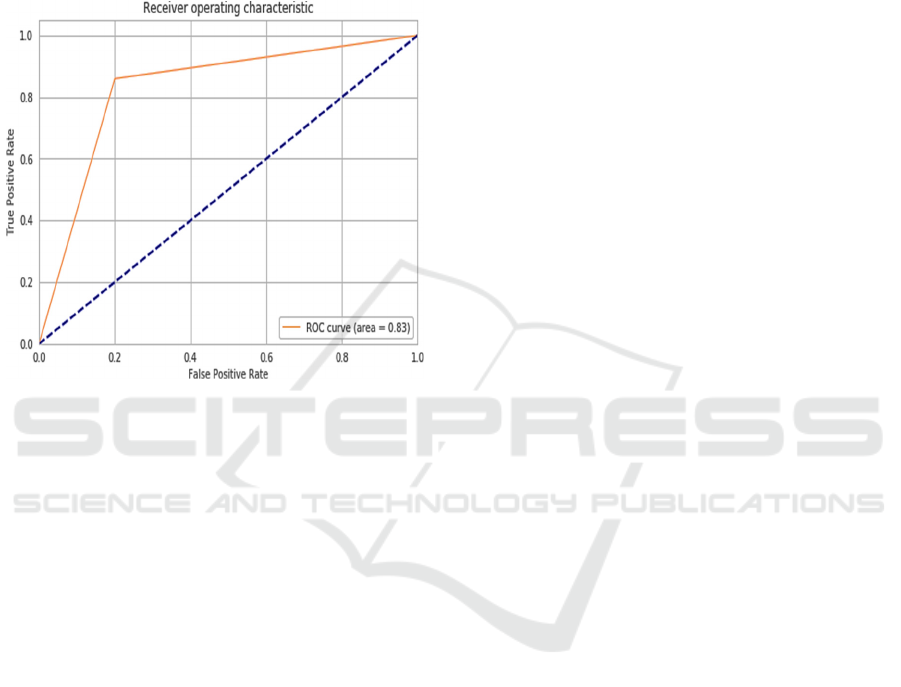
experiments on the testing dataset. The testing
dataset model AUC curve outcome has presented in
Figure 6 and the model achieved a ROC of 83.3%
which is considered as an optimal classifier for AD
image detection and this value is significantly higher
than traditional ML approaches (Battineni,
Chintalapudi, en Amenta 2019; A. Khan en Zubair
2020).
Figure 6: The ROC curve of test data.
4
DISCUSSION
In this work, we presented a novel deep learning
type CNN model for the classification of AD subjects.
As mentioned above, AD is the most common adult-
onset dementia and contributes about 60-70% of
worldwide dementia cases (A. Khan en Zubair 2020).
Unfortunately, there is no proper medication or cure
for AD, and advancements in AD cure have been
getting slow. Screening among people of AD risk
given electronic health records (EHR) in preclinical
stages may prompt early identification of AD
pathology and suggest better approaches for
complying with the AD beginning. Current
biomarkers of AD have required specimen collection
(like serum or liquid), MRI image data, or more
sophisticated markers that at the present can be
identified just in highly specialized centres
(Mantzavinos en Alexiou 2017; Hadjichrysanthou et
al. 2020).
On the other hand, the EHRs for example medical
records in clinical settings, or administrative health
information don't require extra time or effort for data
collection. Likewise, with the coming of
digitalization, the measures of such information have
drastically increased (Shao et al. 2019). Since it is
omnipresent, enormous, and cost-effective, the
digitized medical database might be a significant
asset for testing different AD predictive models.
Nonetheless, despite its enormous possible value,
somehow thought about the degrees to which the
enormous scope of EHR data can help in risk of AD
prediction (Shao et al. 2019; Mayer et al. 2015). The
possible prediction of future AD progression is
incredibly significant in clinical practice also, in
healthcare research. Advanced neuroimaging
techniques like MRI, positron emission tomography
(PET) is developed and presented to identify AD-
related molecular and structural biomarkers
(Hadjichrysanthou et al. 2020).
Computer scientists are recommending applying
sophisticated computing techniques like machine
learning and deep learning. It is reported that 99.1%
of accuracy has been achieved through the application
of ensemble learning models for late-life AD
detection among 150 patients (Battineni,
Chintalapudi, et al. 2020). AD prediction among 123
subjects with Pre-MCI and MCI was done by
clinically transmittable ML algorithms and results
reported the whole sample accuracy of 96.2% (Grassi
et al. 2018). However, most of the outcomes proposed
by these algorithms are based on demographic
magnetic resonance image (MRI) information.
Because of this, researchers believed that deep
learning algorithms are the best approaches if brain
images were included (Choi en Jin 2018). Most of the
works associated with Machine learning in the early
prediction of AD occurred with high success. For
instance, it is reported that 94.1% of accuracy by 3D
convolutional neural networks (CNN)
(Esmaeilzadeh et al. 2018).
This work presented a deep CNN with 10-fold
cross-validation and achieved more than 80%
accuracy. While applying computing methods for
diagnosis, a small portion of datasets are presented.
Therefore, our model maintained a random image
selection of train, test, and validation datasets. The
proposed model produced promising results in AD
image classification. The most notable outcome for
this study is the progressions among predictiveness of
AD diseases.
5
CONCLUSIONS
An autonomous AD detection classifier based deep
ConvNet framework is presented. We adopted the
latest release of the OASIS-3 dataset that contains
Deep Learning Type Convolution Neural Network Architecture for Multiclass Classification of Alzheimer’s Disease
213

different categories of AD datasets. For training,
more than 1,500 images model took a bit longer
process than expected, but it is faster than mankind
process. Deep ConvNets do not need any handcrafted
feature selection approach because of having
autonomous feature tuning. The main limitation of
the study is to adopt only a single classifier for the
brain MRI data classification and there are other
possibilities to do better improvements in the
proposed model architecture. Although attained
results of higher 80% accuracy while compared over
traditional ML classifiers, many advancements are
proposed to enhance the model quality.
CONFLICTS OF INTEREST
No author has produced any conflicts of interest.
REFERENCES
Alzheimer’s Disease International (ADI). 2010. “World
Alzheimer Report 2010: The Global Economic Impact
of Dementia”. Alzheimer’s Disease International (ADI).
https://doi.org/10.1111/j.0963-7214.2004.00293.x.
Basheera, Shaik, en M. Satya Sai Ram. 2019. “Convolution
neural network–based Alzheimer’s disease
classification using hybrid enhanced independent
component analysis based segmented gray matter of
T2 weighted magnetic resonance imaging with clinical
valuation”. Alzheimer’s and Dementia: Translational
Research and Clinical Interventions. https://doi.org/
10.1016/j.trci.2019.10.001.
Battineni, Gopi, Nalini Chintalapudi, en Francesco Amenta.
2019. “Machine learning in medicine: Performance
calculation of dementia prediction by support vector
machines (SVM)”. Informatics in Medicine
Unlocked. https://doi.org/10.1016/j.imu.2019.100200.
Battineni, Gopi, Nalini Chintalapudi, Francesco Amenta, en
Enea Traini. 2020. “A Comprehensive Machine-
Learning Model Applied to Magnetic Resonance
Imaging (MRI) to Predict Alzheimer’s Disease (AD) in
Older Subjects”. Journal of Clinical Medicine.
https://doi.org/10.3390/jcm9072146.
Battineni, Gopi, Getu Gamo Sagaro, Nalini Chinatalapudi,
en Francesco Amenta. 2020. “Applications of machine
learning predictive models in the chronic disease
diagnosis”. Journal of Personalized Medicine.
https://doi.org/10.3390/jpm10020021.
Choi, Hongyoon, en Kyong Hwan Jin. 2018. “Predicting
cognitive decline with deep learning of brain
metabolism and amyloid imaging”. Behavioural Brain
Research. https://doi.org/10.1016/j.bbr.2018.02.017.
Esmaeilzadeh, Soheil, Dimitrios Ioannis Belivanis, Kilian
M. Pohl, en Ehsan Adeli. 2018. “End-to-end
alzheimer’s disease diagnosis and biomarker
identification”. In Lecture Notes in Computer Science
(including subseries Lecture Notes in Artificial
Intelligence and Lecture Notes in Bioinformatics).
https://doi.org/10.1007/978-3-030- 00919-9_39.
Gaugler, Joseph, Bryan James, Tricia Johnson, Ken Scholz,
en Jennifer Weuve. 2016. “2016 Alzheimer’s disease
facts and figures”. Alzheimer’s and Dementia.
https://doi.org/10.1016/j.jalz.2016.03.001.
Grassi, Massimiliano, Giampaolo Perna, Daniela Caldirola,
Koen Schruers, Ranjan Duara, en David A.
Loewenstein. 2018. “A clinically-translatable machine
learning algorithm for the prediction of Alzheimer’s
disease conversion in individuals with mild and
premild cognitive impairment”. Journal of Alzheimer’s
Disease. https://doi.org/10.3233/JAD- 170547.
Hadjichrysanthou, Christoforos, Stephanie Evans, Sumali
Bajaj, Loizos C. Siakallis, Kevin McRae-Mckee, en
Frank De Wolf. 2020. “The dynamics of biomarkers
across the clinical spectrum of Alzheimer’s disease”.
Alzheimer’s Research and Therapy. https://doi.org/
10.1186/s13195-020-00636-z.
Jaussent, Isabelle, Jean Bouyer, Marie Laure Ancelin,
Claudine Berr, Alexandra Foubert-Samier, Karen
Ritchie, Maurice M. Ohayon, Alain Besset, en Yves
Dauvilliers. 2012. “Excessive sleepiness is predictive
of cognitive decline in the elderly”. Sleep.
https://doi.org/10.5665/sleep.2070.
Johnson, Piers, Luke Vandewater, William Wilson, Paul
Maruff, Greg Savage, Petra Graham, Lance S.
Macaulay, et al. 2014. “Genetic algorithm with logistic
regression for prediction of progression to Alzheimer’s
disease”. BMC Bioinformatics. https://doi.org/10.
1186/1471-2105-15-S16-S11.
Khan, Afreen, en Swaleha Zubair. 2020. “An Improved
Multi-Modal based Machine Learning Approach for
the Prognosis of Alzheimer’s disease”. Journal of
King Saud University - Computer and Information
Sciences. https://doi.org/10.1016/j.jksuci.2020.04.004.
Khan, Mehshan Ahmed, Muhammad Attique Khan,
Fawad Ahmed, Mamta Mittal, Lalit Mohan Goyal, D.
Jude Hemanth, en Suresh Chandra Satapathy. 2020.
“Gastrointestinal diseases segmentation and
classification based on duo-deep architectures”.
Pattern Recognition Letters. https://doi.org/10.1016/
j.patrec.2019.12.024.
Krizhevsky, Alex, Ilya Sutskever, en Geoffrey E. Hinton.
2017. “ImageNet classification with deep
convolutional neural networks”. Communications of
the ACM. https://doi.org/10.1145/3065386.
Lin, Weiming, Tong Tong, Qinquan Gao, Di Guo,
Xiaofeng Du, Yonggui Yang, Gang Guo, Min Xiao,
Min Du, en Xiaobo Qu. 2018. “Convolutional neural
networks-based MRI image analysis for the
Alzheimer’s disease prediction from mild cognitive
impairment”. Frontiers in Neuroscience. https://doi.
org/10.3389/fnins.2018.00777.
Mantzavinos, Vasileios, en Athanasios Alexiou. 2017.
“Biomarkers for Alzheimer’s Disease Diagnosis”.
BIOIMAGING 2021 - 8th International Conference on Bioimaging
214

Current Alzheimer Research. https://doi.org/10.2174/
15672050146661702031259 42.
Mayer, Miguel A., Laura I. Furlong, Pilar Torre, Ignasi
Planas, Francesc Cots, Elisabet Izquierdo, Jordi
Portabella, Javier Rovira, Alba Gutierrez-Sacristan, en
Ferran Sanz. 2015. “Reuse of EHRs to Support
Clinical Research in a Hospital of Reference”. In
Studies in Health Technology and Informatics.
https://doi.org/10.3233/978-1-61499-512-8-224.
Mehmood, Atif, Muazzam Maqsood, Muzaffar Bashir, en
Yang Shuyuan. 2020. “A deep siamese convolution
neural network for multi-class classification of
alzheimer disease”. Brain Sciences. https://doi.org/
10.3390/brainsci10020084.
Mittal, Mamta, Lalit Mohan Goyal, Sumit Kaur, Iqbaldeep
Kaur, Amit Verma, en D. Jude Hemanth. 2019. “Deep
learning based enhanced tumor segmentation approach
for MR brain images”. Applied Soft Computing
Journal. https://doi.org/10.1016/j.asoc.2 019.02.036.
Palumbo, L., P. Bosco, M. E. Fantacci, E. Ferrari, P. Oliva,
G. Spera, en A. Retico. 2019. “Evaluation of the intra-
and inter-method agreement of brain MRI
segmentation software packages: A comparison
between SPM12 and FreeSurfer v6.0”. Physica
Medica. https://doi.org/10.1016/j.ejmp.2019.07.016.
Pan, Dan, An Zeng, Longfei Jia, Yin Huang, Tory Frizzell,
en Xiaowei Song. 2020. “Early Detection of
Alzheimer’s Disease Using Magnetic Resonance
Imaging: A Novel Approach Combining
Convolutional Neural Networks and Ensemble
Learning”. Frontiers in Neuroscience. https://doi.org/
10.3389/fnins.2020.00259.
Rathore, Saima, Mohamad Habes, Muhammad Aksam
Iftikhar, Amanda Shacklett, en Christos Davatzikos.
2017. “A review on neuroimaging-based classification
studies and associated feature extraction methods for
Alzheimer’s disease and its prodromal stages”.
NeuroImage. https://doi.org/10.1016/j.neuroimage.
2017.03.057.
Shao, Yijun, Qing T. Zeng, Kathryn K. Chen, Andrew
Shutes-David, Stephen M. Thielke, en Debby W.
Tsuang. 2019. “Detection of probable dementia cases
in undiagnosed patients using structured and
unstructured electronic health records”. BMC Medical
Informatics and Decision Making. https://doi.org/
10.1186/s12911-019-0846-4.
Sweeney, Elizabeth M., Russell T. Shinohara, Navid Shiee,
Farrah J. Mateen, Avni A. Chudgar, Jennifer L.
Cuzzocreo, Peter A. Calabresi, Dzung L. Pham, Daniel
S. Reich, en Ciprian M. Crainiceanu. 2013. “OASIS is
Automated Statistical Inference for Segmentation, with
applications to multiple sclerosis lesion segmentation in
MRI”. NeuroImage: Clinical. https://doi.org/10.1016/
j.nicl.2013.03.002.
Deep Learning Type Convolution Neural Network Architecture for Multiclass Classification of Alzheimer’s Disease
215
