
Separating Local and Propagated Contributors to the Behnke-fried
Microelectrode Recordings
P. Jurczynski
1
, S. Le Cam
1
, B. Rossion
1,2
and R. Ranta
1
1
Universit
´
e de Lorraine, CNRS, CRAN, F-54000 Nancy, France
2
Universit
´
e de Lorraine, CHRU-Nancy, Service de Neurologie, F-54000, France
Keywords:
Microelectrode, Local Field Potential, Reference Estimation, Zero-reference Montage.
Abstract:
In electrophysiological measurements, a recording electrode is located in an electric field and has a certain
electric potential value. Each measured signal is a potential difference between two electrodes, a measuring
electrode and a so-called reference electrode. This reference electrode is not located at infinity and outside of
any electric field, thus its electric potential is found in the measured signals. In order to isolate the local activity
of the recorded structure, it is necessary to understand the relationships between the different contributors at
the electrode level and separate these different activities. In this paper, we focus on the particular setup of
Behnke-Fried micro-electrodes. We propose to adapt a previous re-referencing method for separating local and
distant propagated activities taking into account the non-stationarity of the signals and the particular geometry
of these microelectrodes. We demonstrate, on realistically simulated signals, that the new re-referencing
procedure improves the preprocessing of these signals and might help deeper interpretation.
1 INTRODUCTION
Microelectrode recordings make it possible to analyze
the activity of the very close neuronal population in
which the electrode is implanted, within a radius of
a few hundred micrometers depending on the type of
implanted microelectrode. Such measurements have
been widely used to analyze the activity of individual
neurons, the action potentials (AP) of close neurons
being one of the main contributors to the recorded
signal. A second main contributor to the signal are
the inhibitory and excitatory post-synaptic currents,
which mainly originate from the local population, but
can also be picked from more distant structures up
to several millimeters if their activities are synchro-
nized enough (Buzs
´
aki et al., 2012; Kajikawa and
Schroeder, 2011). The analysis of these dominant
contributors and of their relationships hold precious
information to interpret the activity of the implanted
structure (Einevoll et al., 2013), their separations be-
ing a prerequisite to such analysis.
This separation, which is already a challenging
signal processing task by itself due to overlaps be-
tween their (frequency) contents (Zanos et al., 2011),
is made even more complicated by the presence of
the reference potential. Indeed, the measured elec-
trical activity always corresponds to a potential dif-
ference - one electrode measures the potential rela-
tive to another electrode, considered as the reference.
The relationship between the potentials “seen” by the
reference electrode and by the measuring electrodes
depend on the particular recording setup. For ex-
ample, in the context of macroscopic EEG record-
ings, the reference is often one of the scalp electrodes,
fundamentally similar to the other (measuring) scalp
electrodes. In the intracerebral recordings context
(SEEG), the reference is either considered to be at
zero potential, or at least sufficiently far away from
the other electrodes so that the reference potential can
be considered independent (or at least uncorrelated)
with respect to the potential of interest (Libenson,
2012; Yao, 2001; Hu et al., 2007).
By the fact that all signals are potential differences
between measuring and reference electrodes, the po-
tential of the latter is implicitly present in all signals to
be analyzed. This is also the case for microelectrodes
recordings, and in particular for Behnke-Fried elec-
trodes, which are the focus of this work. These elec-
trodes (manufactured by AdTech and one of the few
microelectrode devices certified for human brain inva-
sive recordings), are mixed macro-micro electrodes,
with nine micro-wires deployed in the brain at the tip
of a SEEG macro electrode, one of them being chosen
as the reference electrode (see figure 1).
Jurczynski, P., Cam, S., Rossion, B. and Ranta, R.
Separating Local and Propagated Contributors to the Behnke-fried Microelectrode Recordings.
DOI: 10.5220/0010349303430350
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 4: BIOSIGNALS, pages 343-350
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
343
Figure 1: Behnke-Fried electrode. Image from AdTech
website.
In all cases, the reference signal can be seen as
“perturbation” which does not hold information on
the local activity of interest generated around the mea-
suring electrodes, and several methods have been de-
veloped for its cancelling. One of the simplest tech-
niques is the bipolar montage, which subtracts two
signals one from the other, achieving for one hand at
reference cancelling and, for the other hand yielding
an estimate of the local activity originated between
and around the two involved electrodes. While in
theory possible for Behnke-Fried recordings, the in-
terpretation of such a montage is difficult, because
it is impossible to know the microelectrodes respec-
tive positions exactly. Indeed, the microelectrodes are
at the and of flexible microwires, not visible on CT
scan as in the case of SEEG macroelectrodes (Hof-
manis et al., 2011). The same reasoning holds for
Laplacian / CSD estimates of local sources (Mitzdorf,
1985; Hjorth, 1975). Moreover, because the positions
are not known, a forward-inverse model based solu-
tion like REST (Yao, 2001; Salido-Ruiz et al., 2019)
is not possible neither.
Two other solutions are proposed in the literature:
the average reference (i.e estimating the reference po-
tential by averaging all measured signals and sub-
tracting it from them) and a weighted average refer-
ence estimation. The first contribution belonging to
this last family of solutions was the PCA-based blind
source separation proposed in (Hu et al., 2008), fur-
ther developed and justified for the elimination of the
SEEG reference by (Ranta et al., 2010). This solution,
called “zero-reference” (ZR) by the authors, has been
shown to be equivalent to the minimum power distor-
tionless response beamformer (MPDR), well known
in array signal processing, and which also maximises
the signal-to-noise ratio (SNR) (Madhu et al., 2012).
The first objective of this paper is to demonstrate
that this zero-reference method can make an impor-
tant contribution to the separation of components in
the context of Behnke Fried electrode recordings. The
solution depends on the quality of the estimation of
some statistical measures from the data (covariance
matrix), which are difficult to estimate in the context
of non-stationary signals. To cope with this problem,
we also propose in this paper an adaptative version of
this zero-reference algorithm.
The paper is organized as follows: first we intro-
duce the existing re-referencing methods in the con-
text. We next present the zero-reference method and
its update to make it adaptive over time. Finally, these
common activity estimation methods (average, ZR,
adaptive ZR) will be evaluated on simulated and real
data. In particular, it will be shown that this treat-
ment enhance the robustness of AP detection, which
is very often an important prerequisite to spike sort-
ing algorithms. Also its importance for further spike
to LFP relationship will be illustrated in the context
of Fast Periodic Visual Stimulation (FPVS) simulated
data. Finally, to the extent that propagated distant ac-
tivities can also be seen as external perturbations, re-
referencing becomes an artifact reduction technique.
An example of such artifact reduction and of its ef-
fects on spike analysis is presented on real microelec-
trode signals.
2 COMMON ACTIVITY
ESTIMATION
2.1 Model
In any point inside the brain, the extracellular poten-
tials are a mixture due to close and far current sources
of different natures: synchronized synaptic potentials
seen far from their originating point (like in the EEG
case), local synaptic activities due to the local pop-
ulation, more or less synchronized, and extracellular
action potentials, due to very close neurons. Issued
from different biophysical phenomena, these activi-
ties have different time-frequency characteristics, al-
though partially overlapping (Zanos et al., 2011).
The way we see them depends on the measuring
setup. We will not enter here in the detailed mod-
elling of the electrodes physics (see e.g (Nelson et al.,
2008)), but we will consider an approximate model
of the electrodes that interest us (Behnke-Fried). As
mentioned, they consist of nine microwires deployed
at the tip of an intracerebral macroelectrode. One of
the nine microwires is used as a reference electrode.
But this electrode has the specificity to be stripped
from its insulation on several millimeters at its ex-
tremity, while the 8 others are not. The former is then
exposed to the brain electrical field on a larger sur-
face (≈ 1mm
2
) compared to the latter, with a much
smaller recording surface (≈ 3000µm
2
), then a sur-
face ratio of the order of two to three magnitude.
Consequently, the nature of the activity captured on
the reference electrode is different with respect to the
much more local potentials acquired by the 8 remain-
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
344

ing microwires. Indeed, due to averaging effect over
a larger surface (Hagen et al., 2016), the activities due
to very local structure such as individual neurons (AP)
and group of neurons (local LFP) are drastically di-
minished. On the other hand, the reference electrode
itself is not at zero potential, but picks up an average
activity of the surrounding brain structures with suf-
ficient synchronization, such as suggested in (Lind
´
en
et al., 2010). Although the other 8 measuring micro-
electrodes are under the influence of the same prop-
agated electrical field originating from distant brain
sources, their impedance is higher thus the amplitude
of the propagated potential is not the same as the one
captured by the reference electrode. To sum up, every
available signal x
i
(i = 1..8) can be written as:
x
i
= x
local
+ αx
distant
− x
distant
(1)
with x
local
a mixture of extracellular action potentials
and local synaptic activity and x
distant
the propagated
synaptic activity of far sources captured by the refer-
ence electrode (x
distant
= r, α standing for the differ-
ent gain that the distant activity has on the measuring
electrodes with respect to the reference electrode). As
the actual amplitude of the propagated x
distant
is un-
known, equation (1) can also be written as:
x
i
= x
local
− r (2)
or, in matrix form,
x =
−1
A
.
.
.
−1
s
r
(3)
where x ∈ R
(M×1)
is the vector of measured signals
(M = 8 in our case), A is some mixing matrix ac-
counting for possible correlations between the local
activities, s are a basis for the local signal space (local
neural sources) and r the reference potential equal to
a scaled version of the propagated activities x
distant
.
In other words, it should be possible to use refer-
ence cancelling techniques for separating between lo-
cal and propagated components of the recorded sig-
nals.
2.2 State of Art
As noted in the Introduction, several techniques al-
ready exist to eliminate common reference activity,
depending on the measuring setup. Biophysically
founded methods such as REST or Laplacian/CSD
(common in EEG but not only), are not adapted for
the Behnke-Fried electrodes because the position are
unknown. One of the simplest remaining solutions is
the average reference estimation, which approximates
r as the average of x
i
.
More elaborated methods were developed for the
SEEG setup, for which the basic hypothesis is that
the reference potential is uncorrelated with the po-
tentials of the measuring electrodes. This hypothesis
holds also for the application we study here. Indeed,
the so-called local activities x
local
are a sum of action
potentials and local synaptic potentials. If it is very
unlikely to have correlated spike trains between two
electrodes, it could be in principle possible to have
correlated synaptic activities. But in all cases, we can
rather safely make the hypothesis that these local ac-
tivities are not correlated with the activity of distant
synchronized populations x
distant
and thus to the ref-
erence potential r.
The zero-reference method was introduced in a se-
ries of papers starting with (Ranta et al., 2010), which
propose to improve previous methods developed by
e.g (Hu et al., 2007). It is based on a semi-blind
source separation / beamforming technique. The ba-
sic assumption is that the potential of the common ref-
erence electrode is decorrelated from the potentials of
the measuring electrodes.
Under the decorrelation hypothesis, one can show
that the best estimate (Madhu et al., 2012) (in a min-
imum squares error sense) of the reference potential
(which has to be added to x for re-referencing) is:
ˆr = w
T
x , with w =
R
−1
xx
1
T
R
−1
xx
1
1 (4)
where R
xx
is the covariance matrix of the measured
signals x and 1 is a vector of ones.
2.3 Adaptive RLS Method
The zero-reference method seen above is implicitly
based on the assumption that the signals are station-
ary and thus the correlation matrix does not vary over
time. However, a stationary model is not accurate
when processing brain signals. In order to improve
the method, we propose to make it adaptive by esti-
mating the covariance matrix at every time step, us-
ing the well known exponentially weighted Recur-
sive Least Squares (RLS) signal processing algorithm
(Eleftheriou and Falconer, 1986).
This method makes it possible to calculate itera-
tively the inverse of the covariance matrix R
xx
(see
eq. 4) at time t + 1 thanks to the inverse covariance
matrix at time t:
R
xx
(t + 1)
−1
= λ
−1
(R
xx
(t)
−1
− G(t + 1)R
xx
(t)
−1
)
(5)
with
G(t + 1) =
λ
−1
R
xx
(t)
−1
x(t + 1)x
T
(t + 1)
1 + λ
−1
x
T
(t + 1)R
xx
(t)
−1
x(t + 1)
(6)
Separating Local and Propagated Contributors to the Behnke-fried Microelectrode Recordings
345

where x(t) is the M × 1 vector of the measured signals
at instant t, R
xx
(t) is the covariance matrix at instant t,
λ is the forgetting factor and G(t +1) an update factor
including the well-known Kalman gain.
The adaptive estimation of the common potential
r present on the M = 8 electrodes (3) will then be per-
formed at each time step by replacing R
xx
in (4) by its
adaptive estimate given by (5) and (6).
3 RESULTS
3.1 Simulations
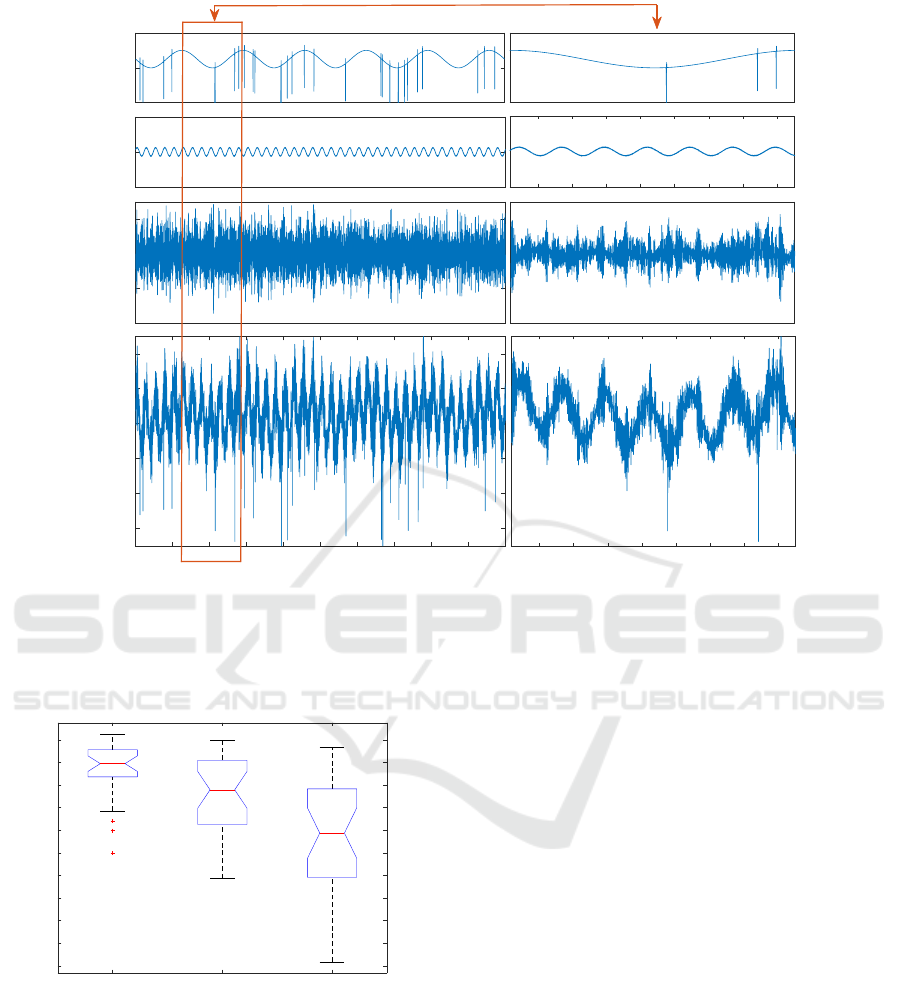
We use simulated data to compare the respective
performances of the average montage, the adaptive
and the non-adaptive zero referencing methods. 8
non stationary (two concatenated stationary epochs)
correlated signals are simulated by reproducing re-
alistic frequencies as observed in real electrophysi-
ology (typical theta and gamma physiological brain
rhythms), representing the local potential activities as
seen by the 8 microelectrodes (Figure 2, second row).
On one signal, a specific LFP with frequency 1.2Hz is
added, simulating the synaptic response to a specific
stimuli (e.g. a visual stimuli (Rossion et al., 2020)),
on which a synchronized train of simulated action po-
tentials (Tran et al., 2020) are superimposed following
a Poisson distribution with a 1.2Hz varying firing rate
(Figure 2, first row). On 4 of the 7 remaining signals,
action potentials are also randomly added, while the
2 others are left free of spikes. A ninth signal is simu-
lated, having a power spectral density reproducing the
one observed on raw micro recordings, representing
the propagated distant LFP (x
distant
in (1)) as picked
up by the reference electrode. Although mainly ly-
ing in the lower frequency band (below 100Hz), this
signal contains significant frequency components up
to 350Hz, thus partly recovering the frequency com-
ponents of the action potentials (Figure 2, third row).
This synthetic reference potential is simulated uncor-
related with the first 8 signals representing local mea-
suring microelectrode potentials (x
local
in (1)), to be in
line with the prerequisite of the zero-reference mon-
tage. The former are substracted to the latter to build
the simulated microelectrode recordings x (Figure 2,
last row). Finally, random Gaussian noise is added on
the 8 channels, with a signal to noise ratio SNR=4.
3.2 Reference Signal Estimation
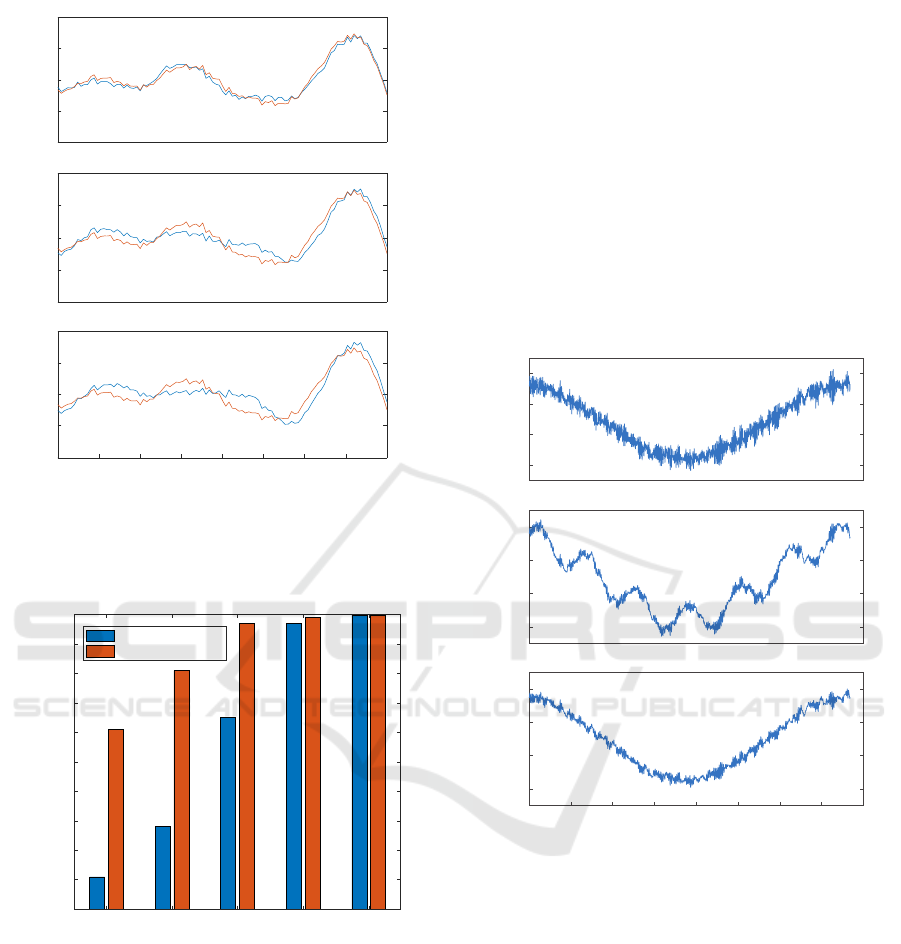
In order to compare the adaptive method with the
non-adaptive one and to the average montage, 50 re-
alizations of simulated sets of signals as described
above have been carried out. The performance crite-
rion is the correlation of the simulated reference with
the estimated reference, calculated for each methods.
From the boxplots of figure 3, we can see that the
correlation score are significantly higher when using
the adaptive method compared to the two other ap-
proaches, the average montage showing the worst per-
formance. The figure 4 illustrates the ability of the
adaptive method in estimating the reference signal, vi-
sually superior to the ones given by the non-adaptive
as well as the average methods.
3.3 Re-referencing for Spike Detection
The detection of spikes is a pre-requisite to the analy-
sis of the spiking activity of neurons located around
the electrode. This challenging task, if not carried
out with enough care, can lead to misinterpretation
regarding the role of the implanted structure. Remov-
ing the reference that has frequencies straddling the
spikes should improve their detection, as well as their
classification. In this paper, we focus on the influence
of the re-referencing on the accuracy of the detection
task. We consider here the classical method of de-
tection consisting in a bandpass filter between 300 Hz
and 6000 Hz that removes low frequencies commonly
attributed to the LFP. Then, a threshold calculation
depending on the MAD (Median absolute deviation)
is used to detect the action potential peaks on the sig-
nal (Quian Quiroga et al., 2004).
As described above, trains of simulated spikes
have been added on 5 of the 8 microelectrode chan-
nels. Several simulations were performed, with dif-
ferent spike amplitudes and thus different spike to
LFP ratios (seen as SNR). These amplitudes have
been fixed to reach peaks-to-noise ratio, defined as
the maximum amplitude of the spike divided by the
standard-deviation of the raw microelectrode signal,
in the range 3 to 7.
We compare the result of the detection on the raw
data and on the re-referenced data with the adap-
tive zero-referencing method (for this task, no sig-
nificant difference are reported between the adaptive
and no-adaptive method). Bar graphs of Figure 5 pro-
vide a summary of the detection results, namely the
percentage of spikes detected with and without re-
referencing, for different amplitudes of the spike to
be detected (different SNRs). It should be noted that
the number of false positives is less than 1% of the
spikes detected in all the simulations, so it will be ne-
glected in this study. The percentage of spikes de-
tected is consistently higher on the zero-referenced
signal than on the raw signal, whatever the amplitude
of the peaks. Clearly, the smaller the amplitude of the
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
346
-100
0
100
LFP local
+Spikes
-100
0
100
LFP population
-200
-100
0
100
propaged LFP
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5
Time (s)
-300
-200
-100
0
100
200
simulated channel
0.7 0.8 0.9 1 1.1 1.2 1.3 1.4
0.7 0.8 0.9 1 1.1 1.2 1.3 1.4
Time (s)
Figure 2: Example of simulated channel and its components (with a zoom in the right panel). First row : 1.2Hz response to the
stimuli superimposed with a synchronized spike train (one can see bursts of spikes locked to the same phase of the response).
Second row : other local LFP as seen by the microelectrode (correlated with the other channels). The sum of the first two
rows yields the local activity x
local
in (1). Third line : propagated LFP as seen by the reference electrode (r = x
distant
). The
last row is the resulting simulated microelectrode recording x obtained as a combination of the first three rows.
1 2 3
adaptive method No-adaptive method average method
0.94
0.945
0.95
0.955
0.96
0.965
0.97
0.975
0.98
0.985
0.99
Correlation rate
Figure 3: Correlation coefficients between the simulated
reference and the estimated ones using the adaptive RLS
method (1), the classical ZR method (2) and the average
reference (3), over 50 simulations.
peaks is in relation to the background LFP, the greater
the influence of the re-referencing for spike detection
will be. When the peak-to-noise ratio fall to 3, only
about 10% of the spikes are detected on the raw sig-
nal, while more than 60% of them are recovered on
the re-referenced signal. This emphasizes the impor-
tance of extracting the local activity from the propa-
gated (reference) signal for accurate spike detection,
in particular for detecting spikes with low amplitudes,
i.e. those originating from neurons that are not in the
very close vicinity of the electrode. The impact of
the re-referencing on the accuracy of spike sorting al-
gorithm might also be significant, by eliminating the
overlapping components of the reference signal in the
high frequency band over 300Hz and delivering a bet-
ter estimation of the spike shapes. This will be evalu-
ated in future works.
3.4 Spikes and LFP Synchronisation
The starting point for this section is the study of face
recognition mechanisms in humans with fast periodic
visual stimulation (FPVS). In one of the key FPVS
paradigms, natural variable photographs of faces are
presented at a frequency of 1.2 Hz among images
showing various living and non-living objects at 6
Hz (Rossion et al., 2015). This stimulation leads a
generic neural response in the visual cortex at 6Hz,
and a response at 1.2 Hz from populations of neurons
responding selectivity (i.e. differentially) to faces.
Separating Local and Propagated Contributors to the Behnke-fried Microelectrode Recordings
347
(1)
-20
-10
0
10
20
(2)
-20
-10
0
10
20
Amplitude (µV)
(3)
Times (s)
-20
-10
0
10
20
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8
Figure 4: Simulated reference (orange) and estimated ref-
erence (blue) using the adaptive RLS method (1), the ZR
method (2) and average (3).
3 4 5 6 7
x*SNR
0
10
20
30
40
50
60
70
80
90
100
Number of spikes detected/ Number of spikes simulated
(%)
raw signals
dereferenced signals
Figure 5: Percent of spike detected on raw signal (blue) and
re-referenced signal (orange).
When recording from a face-selective cortical region
such as the lateral middle fusiform gyrus, it is thus
likely that local neural activity is expressed 1.2 Hz
while distant-neural activity at 6 Hz is mainly cap-
tured by the reference electrode.
Eliminating the reference signal from the micro-
electrode recordings provides a more local vision of
the neural activity (synaptic LFP+spikes) surround-
ing each microelectrode, therefore not corrupted any-
more by remote neural activity unlikely to be related
to the local spiking activity. This procedure will help
in evaluating the synchrony between the local LFP
and the spiking activity, or a consistent response of
the LFP with respect to a given stimuli. The aim of
this section is to illustrate that the activity of the prop-
agated LFP (seen here as the reference) contaminates
the local LFP as seen by a given electrode, and can
mask or make it difficult to identify such relations.
To do this, we consider the microelectrode signal
on which a 1.2Hz face-selectivity response has been
simulated. From stimulus onset, windows of 200 mil-
liseconds succeeding each trigger can be selected and
averaged, and an average LFP pattern (evoked poten-
tial), synchronized with the stimuli, should emerge.
It is immediately noticeable in Figure 6 that a
(1)
10
20
30
40
(2)
10
20
30
40
Amplitude(µV)
(3)
10
20
30
40
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8
Times (s)
Figure 6: Local simulated LFP (1), raw simulated LFP (2)
and re-referenced LFP (3) (same time window as the right
panel in figure 2).
parasitic frequency appears when such stimuli trig-
gered averaging is applied on the raw signal. This 6Hz
activity originating from the propagated LFP (ref-
erence), is a multiple of the 1.2Hz stimulation fre-
quency, disturbing the identification of the local pat-
tern. Once the reference is removed, this frequency no
longer appears and the resulting signal is very close to
the expected one.
3.5 Observations on Real Data
In this section, we evaluate the re-referencing results
on a 2 minutes long window taken from a Behnke-
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
348
Fried micro-electrodes recordings, containing 8 chan-
nels sampled at 32kHz, and recorded in the anterior
human hippocampus of an epileptic patient. The ef-
fect of the re-referencing was evaluated in terms of ar-
tifact reduction, without altering the information con-
tained in the signals (spikes / LFP).
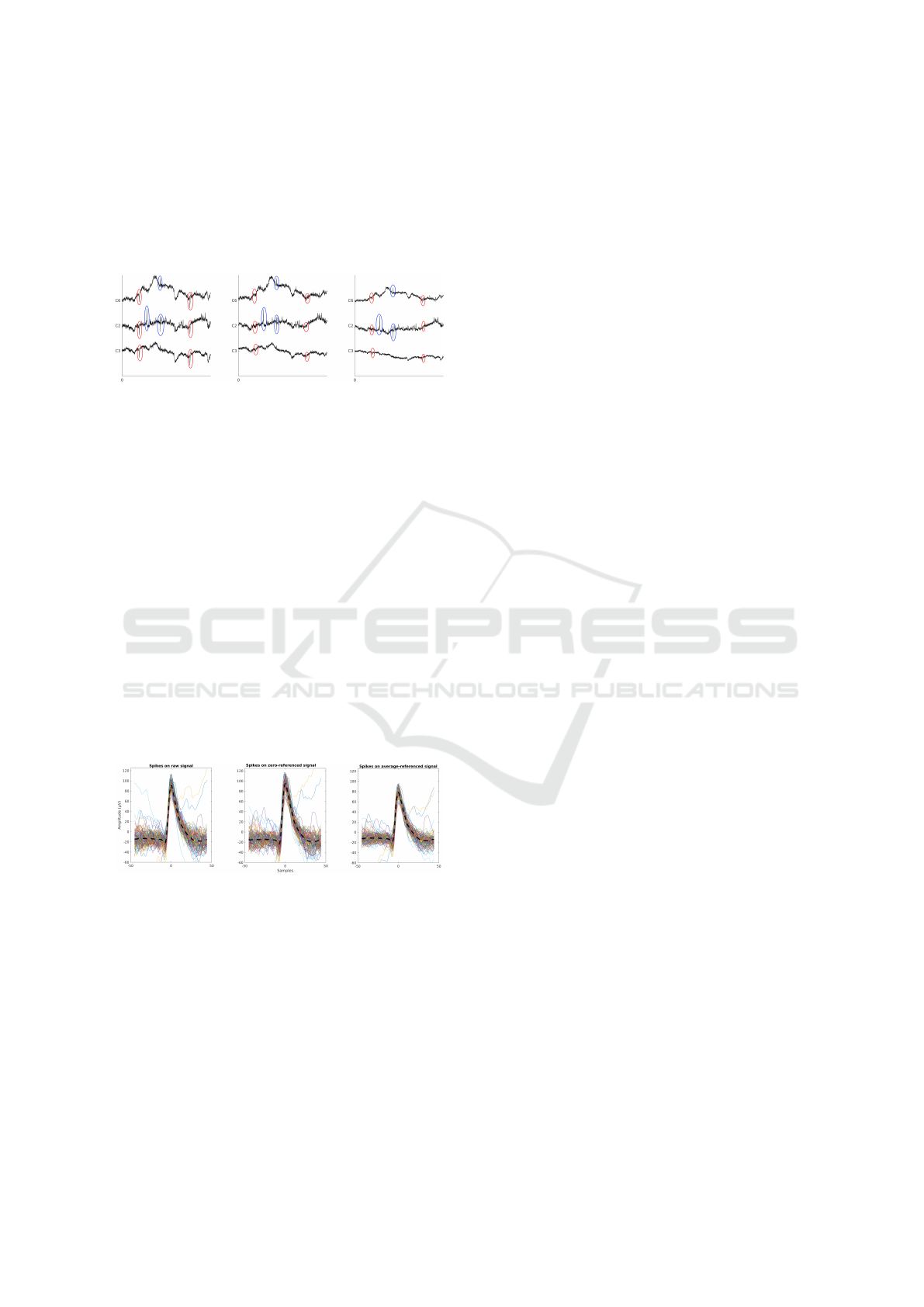
Figure 7: Example of re-referencing. Left: raw (com-
mon reference) signals, Middle: zero-referenced signals,
Right: average-referenced signals. Artifacts are circled in
red, spikes in blue.
Figure 7 presents 130ms of recording on 3 chan-
nels. Artifacts are circled in red (they are considered
artifacts because they are present on all the channels
and have a distinct shape from a spike). Referencing
by zero reference and average reference eliminates
these artifacts, without removing the neural spikes
(circled in blue). However, it can be noted that the
average distorts the low frequency activity of the sig-
nal (right-most panel, figure 7), which is not the case
with the zero-reference. Moreover, the spike shapes
(obtained here after applying a home-made Bayesian
spike sorting / despiking alogorithm (Le Cam et al.,
2019) inspired from (Zanos et al., 2011)) are pre-
served by the zero-referencing method while they are
distorted by the average referencing (Figure 8, right-
most respectively middle panels).
Figure 8: Superimposed spikes from a single unit (same
order as above).
Re-referencing has very little effect on spike sort-
ing except for the removal of artifacts common to sev-
eral channels. The objective of re-referencing is more
focused on the separation of low frequency compo-
nents in order to optimize the study of relationships.
4 CONCLUSION
The separation of components in the particular case
of Behnke-Fried microelectrodes is important for an-
alyzing the activity of the implanted region, in partic-
ular for understanding the relationships between the
different contributors to these recordings. We de-
velop in this paper an adaptative reference estimation
method which allows a separation of the local activity
close to the electrodes, consisting in spikes and local
LFP, from a propagated LFP activity originating from
distant structures.
The use of such re-referencing as a pre-processing
to microelectrode signals allows a better analysis of
the very local activity around each microelectrode,
consisting of local LFP as well as the spiking activity.
First, the detection of spikes is enhanced in particular
for low amplitude spikes, i.e. produced by mid-distant
neurons. It might then be possible with such methods
to broaden the vision of the micro-electrode by iden-
tifying the action potentials of neurons hidden by the
noise, or for which the shapes are impossible to dis-
tinguish (known as Multi-Units (Quian Quiroga et al.,
2004)).
We also illustrate that eliminating the propagated
LFP clarifies the analysis of the local LFP, e.g. it re-
veals possible particular patterns appearing in the ac-
tivity of the local structure with respect to a given
stimuli. We expect that such separation will allow
more accurate evaluation of the relationships between
the detected spikes and the local LFP, but also possi-
ble relations with propagated distant LFP activities.
Separating the reference from the rest of the signal
will also give the opportunity to study the inter-scale
relations between the SEEG and the micro-electrodes.
Indeed, due to its size, the potential as seen by the
reference electrode may be comparable to the one of
a macro SEEG electrode, and then might be consid-
ered as a macro sensor located within the implanted
region. As a perspective, it will then be possible to
separate the activity of the population from more dis-
tant structures by comparing the reference signal with
the SEEG signal of the nearest contact, then splitting
the LFP in three parts as the local LFP, the implanted
population LFP, and the propagated LFP activity of
distant structures.
This new separation method will be further evalu-
ated on real data in acquisition related to FPVS stim-
ulation (Rossion et al., 2015). This methodology is
a promising tool for disentangling the responses of
the targeted brain structure at different scales and will
help in clarifying its involvement in the Human face
recognition process.
Separating Local and Propagated Contributors to the Behnke-fried Microelectrode Recordings
349

ACKNOWLEDGEMENTS
Intracranial data was recorded at the Queen Eliza-
beth University Hospital Birmingham and provided
by Simon Hanslmayr, Bernhard Staresina, Maria
Wimber, George Parish, Marije Ter Wal, Frederic
Roux, Ramesh Chelvarajah, David Rollings and Vi-
jay Sawlani.
REFERENCES
Buzs
´
aki, G., Anastassiou, C. A., and Koch, C. (2012).
The origin of extracellular fields and currents - EEG,
ECoG, LFP and spikes. Nature Reviews, Neuro-
science, 13:407–420.
Einevoll, G. T., Kayser, C., Logothetis, N. K., and Panzeri,
S. (2013). Modelling and analysis of local field poten-
tials for studying the function of cortical circuits. Nat
Rev Neurosci, 14(11):770–785.
Eleftheriou, E. and Falconer, D. (1986). Tracking proper-
ties and steady-state performance of rls adaptive filter
algorithms. IEEE Transactions on Acoustics, Speech,
and Signal Processing, 34(5):1097–1110.
Hagen, E., Dahmen, D., Stavrinou, M. L., Lind
´
en, H., Tet-
zlaff, T., van Albada, S. J., Gr
¨
un, S., Diesmann, M.,
and Einevoll, G. T. (2016). Hybrid scheme for model-
ing local field potentials from point-neuron networks.
Cerebral Cortex, 26(12):4461–4496.
Hjorth, B. (1975). An on-line transformation of EEG
scalp potentials into orthogonal source derivations.
Electroencephalography and Clinical Neurophysiol-
ogy, 39(5):526 – 530.
Hofmanis, J., Caspary, O., Louis-Dorr, V., and Maillard, L.
(2011). Automatic depth electrode localization in in-
tracranial space. In 4th International Conference on
Bio-inspired Systems and Signal Processing, Biosig-
nals 2011, Rome, Italie.
Hu, S., Stead, M., and Worrel, G. (2007). Automatic iden-
tification and removal of scalp reference signal for
intracranial EEGs based on Independent Component
Analysis. IEEE Transactions on Biomedical Engi-
neering, 54(9):1560–1572.
Hu, S., Stead, M., and Worrell, G. (2008). Removal of
scalp reference signal and line noise for intracranial
EEGs. In Networking, Sensing and Control, 2008. IC-
NSC 2008. IEEE International Conference on, pages
1486–1491.
Kajikawa, Y. and Schroeder, C. E. (2011). How local is the
local field potential? Neuron, (72).
Le Cam, S., Tran, H., Ranta, R., and Louis-Dorr, V. (2019).
Simultaneous separation and sorting of extra-cellular
spikes. In 9th International IEEE/EMBS Conference
on Neural Engineering, NER 2019, San Francisco,
USA.
Libenson, M. (2012). Practical approach to electroen-
cephalography. Elsevier Health Sciences.
Lind
´
en, H., Pettersen, K. H., and Einevoll, G. T. (2010). In-
trinsic dendritic filtering gives low-pass power spec-
tra of local field potentials. Journal of computational
neuroscience, 29(3):423–444.
Madhu, N., Ranta, R., Maillard, L., and Koessler, L. (2012).
A unified treatment of the reference estimation prob-
lem in depth EEG recordings. Med. Biol. Eng. Com-
put., 50(10):1003–1015.
Mitzdorf, U. (1985). Current source-density method and
application in cat cerebral cortex: investigation of
evoked potentials and EEG phenomena. Physiologi-
cal reviews, 65(1):37–100.
Nelson, M. J., Pouget, P., Nilsen, E. A., Patten, C. D.,
and Schall, J. D. (2008). Review of signal distortion
through metal microelectrode recording circuits and
filters. Journal of Neuroscience Methods, 169:141–
157.
Quian Quiroga, R., Nadasdy, Z., and Ben-Shaul, Y.
(2004). Unsupervised spike detection and sorting with
wavelets and superparamagnetic clustering. Neural
computation, 16(8):1661–1687.
Ranta, R., Salido-Ruiz, R., and Louis-Dorr, V. (2010).
Reference estimation in EEG recordings. In 32nd
Ann. Int. Conf. of the IEEE-EMBS, Buenos Aires, Ar-
gentina.
Rossion, B., Retter, T., and Liu-Shuang, J. (2020). Under-
standing human individuation of unfamiliar faces with
oddball fast periodic visual stimulation and electroen-
cephalography. European Journal of Neuroscience
(review).
Rossion, B., Torfs, K., Jacques, C., and Liu-Shuang, J.
(2015). Fast periodic presentation of natural im-
ages reveals a robust face-selective electrophysiolog-
ical response in the human brain. Journal of Vision,
15(1):18–18.
Salido-Ruiz, R. A., Ranta, R., Korats, G., Le Cam, S.,
Koessler, L., and Louis-Dorr, V. (2019). A unified
weighted minimum norm solution for the reference
inverse problem in eeg. Computers in Biology and
Medicine, 115:103510.
Tran, H., Ranta, R., Le Cam, S., and Louis-Dorr, V. (2020).
Fast simulation of extracellular action potential signa-
tures based on a morphological filtering approxima-
tion. Journal of Computational Neuroscience, 48.
Yao, D. (2001). A method to standardize a reference of
scalp EEG recordings to a point at infinity. Physiolog-
ical Measurement, 22:693–711.
Zanos, T. P., Mineault, P. J., and Pack, C. C. (2011). Re-
moval of spurious correlations between spikes and lo-
cal field potentials. J Neurophysiology, 105:474–486.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
350
