
On Feasibility of Fluorescence-based Bacteria Presence
Quantification: P.Aeruginosa
Alexander Caschera
1
and Gennadi Saiko
2a
1
Ryerson University, Toronto, Canada
2
Swift Medical Inc., 1 Richmond St W, Toronto, Canada
Keywords: Bacteria, Bacterial Growth, Fluorophores, Fluorescence Imaging.
Abstract: Introduction: Wound healing typically occurs in the presence of bacteria at levels ranging from contamination
to colonization to infection. The role of bacteria in wound healing depends on multiple factors, including
bacterial concentration, species present, and host response. Thus, the determination of bacterial load is of
great importance. However, existing clinical bacteria load assessment methods (biopsy or swabbing combined
with culture methods) are slow, labor- and time-consuming. Pseudomonas aeruginosa is a known pathogen
implicated in numerous healthcare-associated infections and can express fluorescent metabolites during
proliferation. In particular, the siderophore pyoverdine produces a fluorescent emission between 450-520 nm
when excited at 400nm and can be measured quantitatively via fluorescence spectroscopy. The current project
aims to investigate the possibility of quantifying bacterial presence using fluorescence measurements.
Methods: Cultures of P.aeruginosa (PA01) were grown at various temperatures (ambient temperature, 30,
37-43°C), inoculum starting condition (5*10
7
-5*10
8
CFUmL
-1
), and initial nutrient’s concentration (0.6, 1.5,
3.0 gL
-1
) in Tryptic Soy Broth media. Media optical density (OD, as a proxy of bacterial concentration) and
fluorescence (ex. 400nm, em. 420- 520nm) were measured hourly for 10 hours. Results: Cultures remained
metabolically active in the whole temperature range, producing pyoverdine fluorescence (emission max at
455nm). We have correlated optical density with a fluorescent signal to establish a dependence between
fluorescence and growth stage. Noticeable pyoverdine accumulation started approximately 3 hours after the
beginning of the log growth phase and experienced saturation at the beginning of the stationary phase. Three
distinct regimes (a sigmoid curve) were observed: linear dependence of fluorescence on OD for low
concentrations, more rapid nonlinear dependence, and saturation when approaching the stationary phase.
Conclusions: The sigmoid dependence of bacterial fluorescence on their concentration persisted through
variations in temperature and inoculum starting condition; thus, it may have the potential for determining
culture growth phase progression. These results, combined with classical knowledge on disease progression,
could also lead to an advanced infection diagnosis before current pathogenesis observation techniques.
1 INTRODUCTION
Wound healing occurs in the presence of bacteria
(e.g., Staphylococcus, Streptococcus, Pseudomonas
species, and Coliform bacteria, including aerobic and
anaerobic types), at levels ranging from
contamination to critical colonization to infection.
The role of bacteria within wounds depends on
multiple factors, including bacterial concentration,
species present in the wound, and host response.
There are several distinct levels of bacteria
presence in the wound: contamination, colonization,
a
https://orcid.org/0000-0002-5697-7609
and infection. These levels delineate from the number
of microorganisms present per gram of tissue, which
can be highly variable and can range from less than 1
to 10
8
or 10
10
colony-forming units (CFU).
The increased bacterial burden may be confined
to the superficial wound bed or present deep within
the wound or even surrounding tissue.
Contamination and colonization by low microbial
concentrations are considered normal and are not
believed to inhibit healing. However, critical
colonization and infection are associated with a
significant delay in wound healing.
Caschera, A. and Saiko, G.
On Feasibility of Fluorescence-based Bacteria Presence Quantification: P.Aeruginosa.
DOI: 10.5220/0010344001930200
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 2: BIOIMAGING, pages 193-200
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
193

With the ubiquity of microorganisms within the
natural environment, lacerations and skin tissue
lesions can lead to health-threatening issues if
infectious pathogens can take hold within a
compromising injury. With that in mind, the
importance of assessing the stage of infection and
identifying primary microorganisms involved can
assist with wound treatment. It can also help prevent
disease progression if pathogens are properly
characterized early on.
The gold standard collection method is to do a
tissue biopsy or needle aspirate of the wound's
leading edge after debridement. However, the
practical standard method for bacterial load
determination for skin infections is to culture (e.g.,
pour or spread plate) a microbiological swab of the
wound surface. Obviously, this method suffers from
multiple shortcomings. Among them, a) specimen
can be contaminated by normal skin or mucosa flora,
b) swabs frequently yield too small a specimen for
accurate microbiologic examination (Washington.
1996), and c) the duration of incubation for certain
cultures can be long. While most aerobic and
anaerobic bacteria will grow overnight, some
mycobacteria require as many as 6 to 8 weeks before
colonies are observed.
Other methods (e.g., Polymerase Chain Reaction
or PCR) have been proposed. However, they mostly
address the quantification step, while swabbing
remains the primary specimen collection technique.
Thus, other methods of bacterial load
quantification, preferably close to real-time, are
required. This could be accomplished by monitoring
the potential site of infection for unique metabolites
that can be quantified easily. Endogenous
fluorescence (FL) has great potential as a remote and
non-invasive modality. It can be performed remotely,
thus decreasing the risk of contamination.
Most clinically important strains (both gram-
positive and negative) clearly show a distinctive
double-peak of tryptophan fluorescence (emission
peaks at 340 nm, with two excitation maxima; at 230
nm and 280 nm) (Dartnell, 2013) with comparable
intensity between the strains studied. However, the
use of UVC light in vivo is quite problematic.
Fluorescence extending through the 400-500nm
emission range, from excitation of around 350 nm, is
also reported for many cells due to cellular
metabolites such as NAD(P)H. However, it is highly
variable, dependent on the microbial strain and
metabolic state, is not always detectable (Estes,
2003), and is much less intense than the tryptophan
signal (Dartnell, 2010).
In addition to that, it is known that most clinically
relevant bacteria (S. aureus, S. epidermidis, Candida,
S. marcescens, Viridans streptococci,
Corynebacterium diphtheriae, S. pyogenes,
Enterobacter, and Enterococcus) produces red FL
(from porphyrins (Kjeldstad, 1985)) when excited at
405nm. In contrast, P.aeruginosa produces a bluish-
green FL (from pyoverdin (Cody, 1987)) while
excited at 405nm. Considering the polymicrobial
nature of most chronic wounds, it is possible to use
this endogenous fluorescence to characterize bacterial
load.
Fluorescence has been previously used to
differentiate between different species. In particular,
the fluorescence of extracellular pyoverdines has
been used to distinguish between cultures of certain
strains of Pseudomonas (Shelly, 1980 a and b).
Leblanc et al. (Leblanc, 2002) successfully
distinguished between different species of bacteria
using principal component analysis (PCA) of the
autofluorescence of the aromatic amino acid,
nucleotide, and NADH components of the cell. Giana
et al. (Giana, 2003) successfully discriminated
between the clinically important Escherichia coli,
Enterococcus faecalis, and Staphylococcus aureus.
However, both porphyrins and pyoverdine are
mostly extracellular compounds produced by
bacteria. Moreover, their production and fluorescence
can be affected by numerous factors. Thus,
quantification of bacteria presence using porphyrin-
and pyoverdine-based fluorescence is not
straightforward.
The goal of the current project is to establish the
feasibility of fluorescence imaging of bacterial load
under clinical parameters and investigate the
possibility of quantifying bacterial presence using
fluorescence.
In this article, we will present the results of
bacteria fluorescence quantification on the cultures of
P.aeruginosa (PA01). The bacterium Pseudomonas
aeruginosa is an increasingly prevalent human
pathogen, responsible for 12% of hospital-acquired
urinary tract infections, 10% of bloodstream
infections, and 8% of surgical wound infections. In
the UK, 7.6% of acute hospital patients acquire
healthcare-associated infections, around a sixth of
which are caused by methicillin-resistant
Staphylococcus aureus and another sixth by
Clostridium difficile
(Smyth, 2008).
Pseudomonas aeruginosa is known to express
fluorescent metabolites during proliferation. These
metabolites include pyoverdine and pyocyanin and
are thought to play a role in the virulence of other
hemolytic pathogens as well. Of pyoverdine
BIOIMAGING 2021 - 8th International Conference on Bioimaging
194
specifically, 400 nm light is known to produce a
fluorescent emission between 450-500 nm. It can be
measured quantitatively based on concentration
within an appropriate growth media such as tryptic
soy broth. When correlated to optical density, this
fluorescent signature can be compared to the cell
quantity and growth stage. Additional factors, such as
temperature and initial starting concentration, also
play a role in cell growth and pyoverdine expression.
2 METHODS
2.1 Culture Preparation
Many of the materials and consumables used through
this study for the manipulation of microbial cultures,
including pipette tips, Petri dishes, and culture tubes,
were sourced from Sarstedt unless otherwise listed.
Tryptic Soy Broth (cat. 1054590500) was purchased
from Millipore-Sigma, and phosphate-buffered saline
tablets were purchased from Bio Basic (cat. PD0435)
and were used as indicated.
Bacterial strains used for this study were supplied
from ATCC and maintained at Ryerson University by
the Wolfaardt lab group. PAO1 and PA14 are
commonly used for studying the basic biology and
genetics of P. aeruginosa, and PA01 was chosen as
the representative strain for this study.
2.1.1 Inoculum Preparation
Several colonies of Pseudomonas aeruginosa (PA01)
were collected from a maintained 3 gL
-1
Tryptic Soy
Agar (TSA) culture plate using an inoculation loop
and deposited into a 50 mL conical tube containing
10 mL of 3 gL
-1
Tryptic Soy Broth (TSB) under
aseptic conditions. The tube was then sealed and
placed onto a shaking incubator set to 37 °C and left
to incubate to the late-exponential growth stage,
between 16-20 hours after initial cell deposition. The
following day, the overnight solution was rinsed by
centrifuging two 1 mL portions of overnight serum
collected into 2 mL microtubes, replacing the
supernatant solution with sterile phosphate-buffered
saline (PBS), and by resuspending the microbial
pellet within the solution. The process was repeated
twice to ensure proper rinsing of cells, and then both
rinsed aliquots were combined within a single 2 mL
microtube for sample inoculation.
Table 1: Inoculum Parameters.
Test microorganism
Pseudomonas aeruginosa
(PA01)
Growth media 3 gL
-1
Tryptic Soy Broth (TSB)
Incubation perio
d
16-20 hours (Overnight)
Incubation
tem
p
erature 37 °C
Rinse solution
1x Phosphate Buffered Saline
(PBS)
Centrifuge duration 4 min at 9000 x g
Expected inoculum
loa
d
10
5
-10
7
2.2 Culture Quantification
2.2.1 Microbial Reference Quantification
Following inoculum preparation, cells were
enumerated by serially diluting the prepared
inoculum (100 µL of the previous dilution was added
to 900 µL of PBS per step) and spot plating 0.1 µL of
the 10
5
, 10
6
, and 10
7
dilutions onto 3 g L
-1
TSA plates.
The plates were then left to incubate at ambient
temperature for 7 days prior to counting developed
colonies of PA01. The PA01 colonies grown during
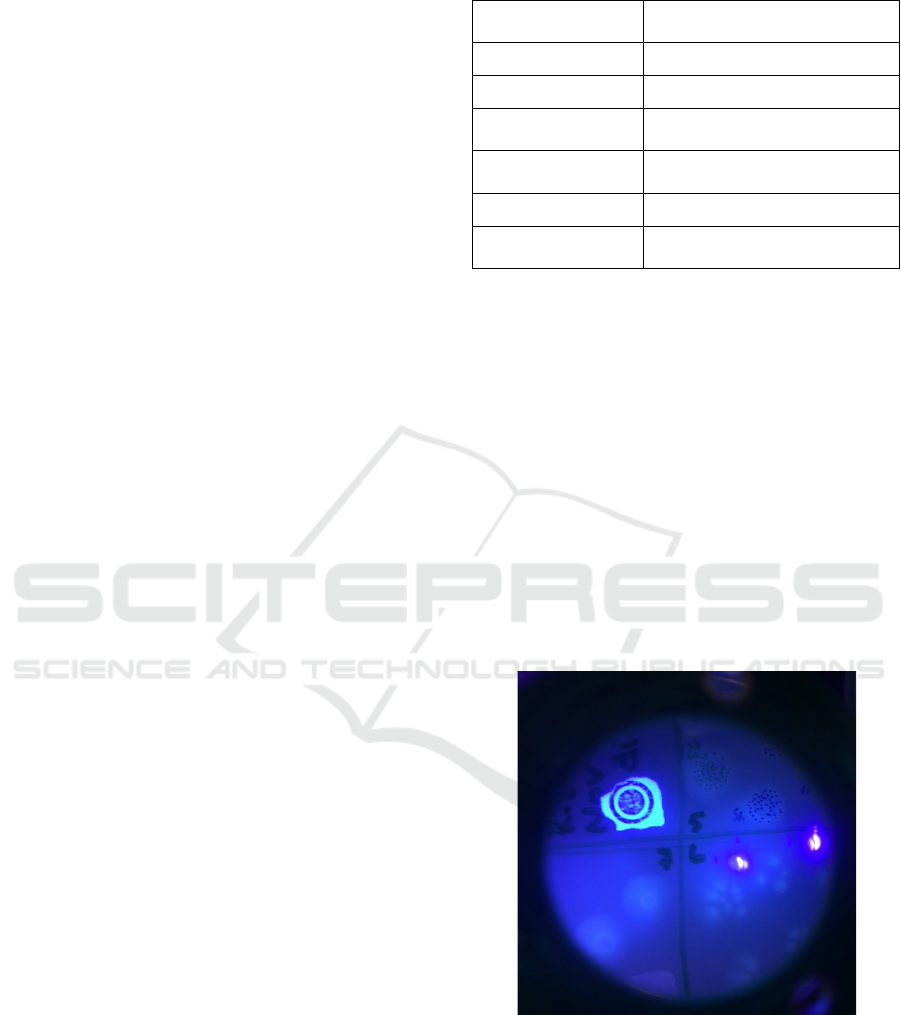
spot plating are depicted in Fig 1. The Petri dish was
imaged by a camera with 425nm long pass filter and
405nm excitation. The bluish-green fluorescence of
pyoverdine is clearly visible.
Figure 1: The PA01 colonies grown during spot plating.
The colonies are imaged by a camera with 425nm long pass
filter and 405nm excitation. Two bright spots are caused by
specular reflection of the excitation light.
On Feasibility of Fluorescence-based Bacteria Presence Quantification: P.Aeruginosa
195
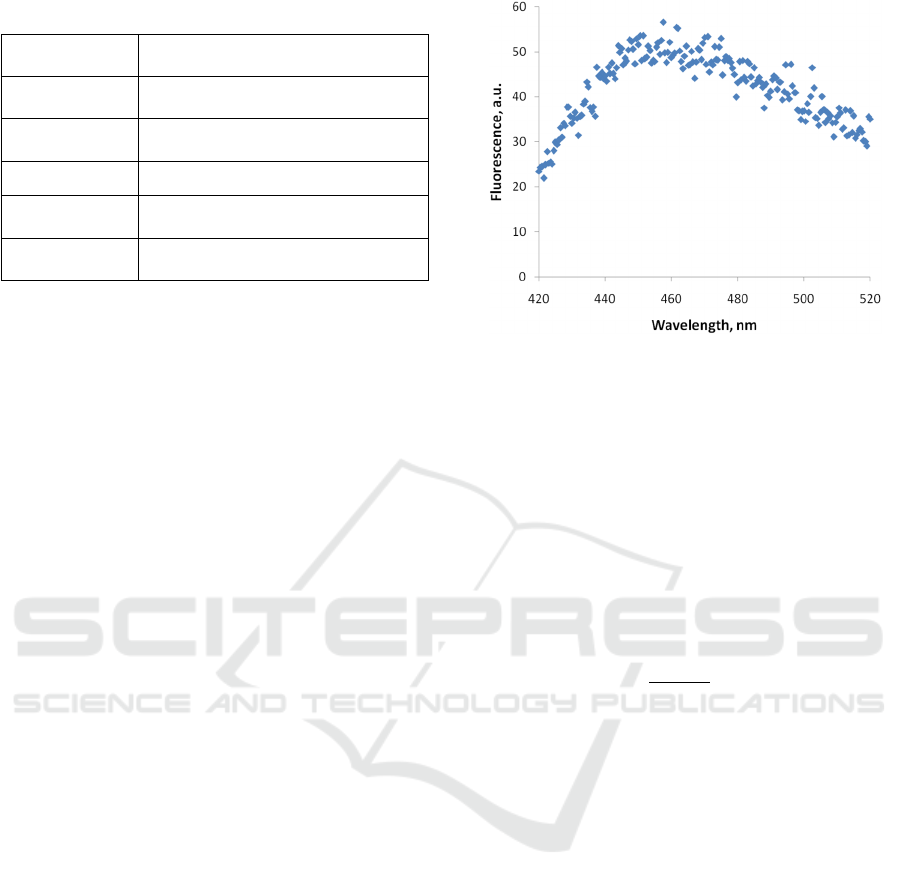
Table 2: Microbial Quantification.
Quantification
Metho
d
Spot plate technique
Quantification
p
erio
d
Immediatel
y
after rinsin
g
cells
Maximum
dilution facto
r
10
7
Growth media 3 gL
-1
Tryptic Soy Agar (TSA)
Incubation
tem
p
erature 25 °C
(
ambient Tem
p
erature
)
Incubation
p
erio
d
7 days
2.2.2 Microbial Rapid Quantification
To allow for the rapid quantification of PA01
bacterial cell concentration within liquid media,
optical density (OD) measurements were calibrated to
colony-forming unit counts obtained from microbial
reference quantification. Since plate counting
requires multiple days and significant resources to
determine the bacterial concentration at any point in
time, rapid quantification of bacteria concentration
can be achieved by measuring the absorbance or
scattering of light. For this purpose, standard OD
measurements at 600nm were performed using
parafilm-sealed macro-cuvettes (cat. BR759035,
Millipore-Sigma) with a 10 mm light pathlength,
within a BioPhotometer (S/N 6131 21925, Eppendorf
AG). The concentration of bacterial cells suspended
within 3 mL of 3 g L
-1
TSB was then calibrated using
multiple concentration points between 10
0
-10
2
CFU
and graphed to produce a standard calibration curve.
For spectroscopic blanking purposes, a sterile TSB
control was maintained at 4 °C for each trial
performed.
2.2.3 OD-Correlated Fluorescence
Spectroscopy
Fluorescence spectroscopy was performed using an
LS 50 B Luminescence Spectrometer (S/N 50801,
Perkin-Elmer Ltd.) on macro-cuvettes containing 3
mL TSB inoculated with PA01, as defined by the
microbial quantification methods above.
Fluorescence emission scans were achieved using
400nm (2.5 nm slit width) as the excitation
wavelength, while emission was recorded in 420-
520nm (2.5 nm slit width) range with 0.5nm
increments for 1 minute. OD measurements at 600 nm
were taken prior to each fluorescence microscopy
scan. The typical raw fluorescence spectrum is
depicted in Fig 2.
Figure 2: An example of a raw fluorescence spectrum of the
PA01 sample.
A spectral integral was used over the whole 420-
520nm range to quantify the fluorescence signal:
𝑓
𝑙= 𝑆
(
𝜆
)
𝑑𝜆
(1)
Here, S(λ) is a fluorescence signal measured at a
particular wavelength λ. To eliminate background
fluorescence and dependence on various starting
conditions instead of absolute values, the normalized
fluorescence ration was used,
𝐹𝐿 =
𝑓
𝑙−
𝑓
𝑙
𝑓
𝑙
(2)
where fl
c
is the fluorescence spectral integral for a
sterile TSB control sample.
2.3 Experimental Protocol
Initial trials were performed by inoculating macro-
cuvettes containing 3 mL of 3 g L
-1
TSB with 30 µL
(1/100 dilution) PA01 inoculum, prepared and
quantified using the above methods. Samples were
then incubated at a predetermined temperature
(ambient temperature, 30°C, 37-43 °C) within a
standing incubator for 8-10 hours. Prior to incubation
and after each following 1 h interval, OD-correlated
fluorescence spectroscopy was performed on the
prepared samples to determine the concentration of
PA01 cells within the growth medium and the
quantity of fluorescence emitted by each sample.
To investigate the effect of the initial bacterial
concentration, another set of trials were performed at
37 °C, where the starting concentration of PA01 was
altered by inoculating the macro-cuvettes with 150
(1/20), 75 (1/40), and 37.5 µL (1/80) of quantified
PA01 inoculum.
BIOIMAGING 2021 - 8th International Conference on Bioimaging
196
Finally, the impact of nutrient availability was
investigated by altering the initial concentration of
tryptic soy broth (0.6, 1.5, and 3.0 gL
-1
)) while
keeping the number of cells inoculated and
temperature the same (30 µL (1/100 dilution) PA01
inoculum and 37 °C, respectively).
3 RESULTS
Calibration procedure for PA01 shows the following
dependence between bacteria concentration N
(CFUmL
-1
) and optical density OD (R
2
=0.991):
𝑁=(5∗10
∗𝑂𝐷)
.
(3)
To estimate the carrying capacity K, we have
grown the culture in 3 g L
-1
TSB for 26 hours and
found that the optical density saturates at
approximately 0.462 value, which according to Eq.3,
corresponds to K=1.21*10
9
CFUmL
-1
.
We performed three types of experiments. All
results are presented in normalized fluorescence (FL)
vs. optical density (OD), a proxy for the bacterial
concentration.
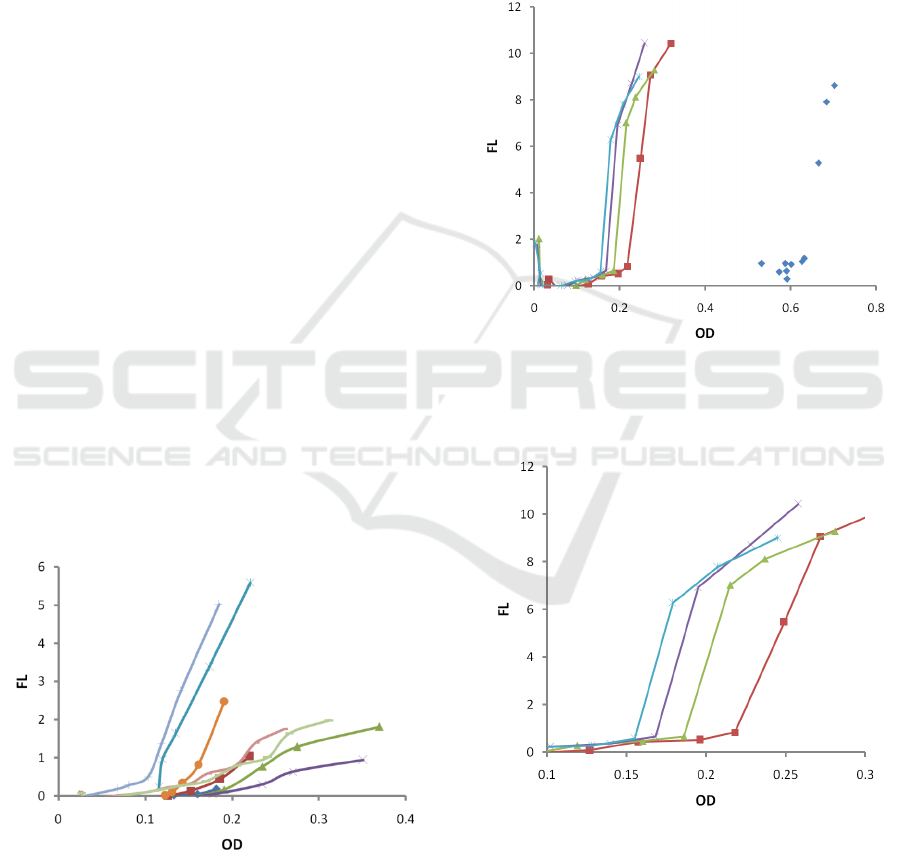
3.1 Dependence on Temperature
Firstly, we studied growth and fluorescence at several
temperatures (ambient temperature, 30C. 37C, 38C,
39C, 40C, 41C, 42C, 43C), while maintaining
inoculation and nutrient's initial concentrations the
same (30 µL (1/100 dilution) PA01 inoculum and 3.0
gL
-1
TSB, respectively). The results are depicted in
Fig 3.
Figure 3: Dependence of fluorescence (FL) on optical
density (OD) for various temperatures: ambient
temperature (blue rhombs ◊), 30C (red, squares □), 37C
(red, line), 38C (green, triangles Δ), 39C (green, line), 40C
(blue, star *), 41C (blue, |), 42C (purple, cross x), 43C
(brown, dot). All other parameters (inoculation and
nutrient’s concentration) were kept the same.
3.2 Dependence on Inoculums
Concentration
To investigate the effect of the initial bacterial
concentration, another set of trials were performed at
37 °C, where the starting concentration of PA01 was
altered by inoculating the macro-cuvettes with 150
(1/20), 75 (1/40), and 37.5 µL (1/80) of quantified
PA01 inoculum. The results are depicted in Fig 4. In
Fig 5, one can see the zoomed area with low OD.
Figure 4: Dependence of fluorescence (FL) on optical
density (OD) for various starting concentrations: original
stock (blue rhombs ◊), 1/20 (red, squares □), 1/40 (green,
triangles Δ), 1/60 (purple, cross x), 1/80 (blues, star *).
Figure 5: Dependence of fluorescence (FL) on optical
density (OD) for various starting concentrations: 1/20 (red,
squares □), 1/40 (green, triangles Δ), 1/60 (purple, cross
x), 1/80 (blues, star *).. Zoomed area with low OD.
On Feasibility of Fluorescence-based Bacteria Presence Quantification: P.Aeruginosa
197
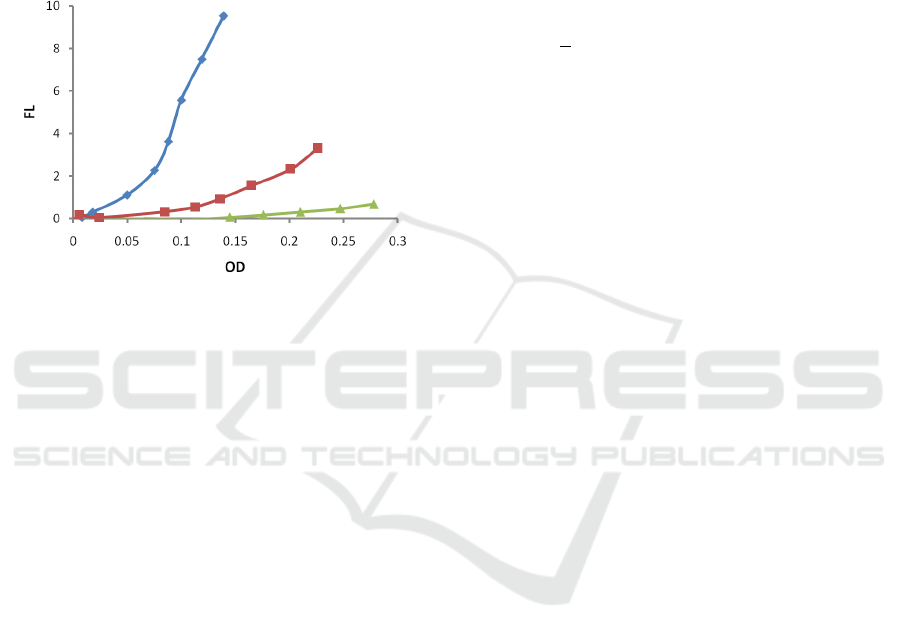
3.3 Dependence on Initial Nutrients
Concentration
Finally, the impact of the initial nutrient's
concentration was investigated by altering the initial
nutrient's concentration (0.6, 1.5, and 3.0 gL
-1
Tryptic
Soy Broth (TSB)) while keeping inoculation and
temperature the same (30 µL (1/100 dilution) PA01
inoculum and 37 °C, respectively). The results are
presented in Fig 4.
Figure 6: Dependence of fluorescence (FL) on optical
density (OD) for various starting nutrient's concentrations:
3gL
-1
(green, triangle, Δ), 1.5 gL
-1
(red, squares □), 0.6 gL
-
1
(blue, rhomb ◊)
4 DISCUSSION
Our data supports the view that P.aeruginosa is a
versatile and opportunistic microorganism. It remains
metabolically active even at temperatures
approaching 43 °C.
Our preliminary results support the nutrient-
dependent siderophore production model developed
in (Saiko, 2021). According to the developed
approach, siderophore production in a resource-
limiting environment has three distinct phases:
I. Slow siderophore production at low
bacteria concentrations where resources
are abundant (S> S
th
)
II. Rapid accumulation of siderophores
upon reaching a specific nutrient's
concentration S
th
. Linear dependence on
the bacteria concentration N.
III. Upon reaching resource limits, the
bacteria focus on growth solely, which
will result in saturation of compound
accumulation (while bacterial
concentration still growth)
The transition into rapid siderophore
accumulation regime (Phase I -> Phase II) occurs at
N
th
:
𝑁
=𝑁
+𝛾(𝑆
−𝑆
)
(4)
here N
0
and S
0
are the initial bacterial and resource
concentration, accordingly. S
th
is the resource
concentration below which bacteria start producing
siderophore rapidly. During that phase II, the
siderophore accumulation depends on the bacterial
concentration linearly:
𝐶=
𝜉
𝛾
(
𝑁−𝑁
)−𝜉(𝑆
−𝑆
)
)
(5)
Finally, when the bacteria population approaches
the carrying capacity, K (K=(N
0
+γS
0
)), the bacteria
divert all resources to replication only, thus reducing
siderophore production.
All these phases were observed in our
experiments.
We found that at the early stages of the growth,
where nutrients are abundant, the siderophore
production is relatively small.
We also found that the infliction point Nt
h
is
affected by the initial bacterial concentration N
0
(Fig
5) and the initial nutrient concentration S
0
(Fig 6).
There is a clear linear dependence of the infliction
point N
th
on the initial inoculums concentration N
0
.
The higher the initial concentration, the higher the
infliction point N
th
is (Fig 5). Also, we found a clear
linear dependence of the infliction point N
th
on the
initial nutrient's concentration S
0
. The higher the
initial concentration S
0
, the higher the infliction point
N
th
is (Fig 6).
In all our tests, upon reaching approximately K/2
bacteria concentration (OD=0.23), the siderophore
accumulation slope starts decreasing (see Fig 3 and
Fig 4). This finding agrees with the nutrient-
dependent siderophore production model (Saiko,
2021), and available experimental data from other
groups (Bren, 2013). Thus, our results support the
view that under starvation, bacteria will focus on
growth only (Bren, 2013) and stop diverting
resources to siderophore synthesis.
Despite promising results in culturing media, the
possibility of quantification of P.aeruginosa presence
based on pyoverdine fluorescence within wounds
requires further challenges to be solved. P.aeruginosa
is a particularly difficult model. Firstly, the
fluorescence of bacteria can be impacted by other
factors. Specifically, Pseudomonas fluorescence is
determined by two factors (Meyer, 1978): a) iron
bonded to pyoverdine quenches fluorescence, b)
pyoverdine production is affected by iron availability.
BIOIMAGING 2021 - 8th International Conference on Bioimaging
198

Thus, P.aerugenosa fluorescence can be diminished
near blood vessels (due to fluorescence quenching
and/or decreased pyoverdine production).
Secondly, it is known (Smith, 2006) that P.
aeruginosa isolated from acute infections differ
substantially in phenotype from those isolated from
chronic infections. It was found (Morgan, 2019) that
P.aeruginosa isolated from chronic human wounds
were frequently defective in virulence functions and
biofilm formation. In addition to that, P.aeruginosa
has an extensive "quorum sensing" (QS) system with
three autoinducers. These QS sub-systems act
hierarchically and regulate cell survival, biofilm
formation, and virulence (Gellatly, 2013).
Thirdly, P.aeruginosa can sequester iron in ways
other than pyoverdine production. It can (i) produce
another siderophore (pyochelin); (ii) utilize a wide
range of siderophores synthesized by other organisms
(Cornelis, 2002); (iii) acquire Fe(II) through the Feo
system (Cartron, 2006). P. aeruginosa can also utilize
heme-iron by expressing two different heme-uptake
systems, namely phu and has (Ochsner, 2000).
Finally, a weak fluorescence signal from bacteria
in vivo can be masked by strong autofluorescence
from nearby tissues. Thus, the proper selection of the
excitation wavelength and emission filter may be
required. Therefore, quantification of P.aeruginosa
presence through pyoverdine fluorescence in vivo
seems quite challenging at this stage.
There are certain limitations regarding the
extrapolation of our results in vivo. They were
obtained in a resource-limiting environment, which
may or may not be the case in vivo. Thus, future
studies on animal models are required.
In future work, we plan to investigate porphyrins
production by another clinically relevant bacteria,
S.aureus.
5 CONCLUSIONS
We found that a fluorescent emissive signature
between 420-520 nm for PA01-produced pyoverdine
can be observed when excited with light at 400 nm in
a wide range of conditions.
Our temperature-dependence studies demonstrate
the production of fluorescent siderophores at
temperatures between ambient and 43 °C. Results
also point towards a local maximum in fluorescence
expression for P.aeruginosa around 40- 41 °C,
although further experimentation would be required
if this is to be determined.
We found that the sigmoid dependence of
bacterial fluorescence on their concentration
persisted through variations in temperature and
inoculum starting condition. This preliminary data
supports the hypothesis that siderophore production
in P.aeruginosa is governed by nutrient-dependent
mechanisms.
Starting nutrient concentration data also indicates
a positive relation between nutrient exhaustion and
fluorescent metabolite expression. This result agrees
with previous findings (Bren, 2013) and indicates that
siderophore production may become inhibited in
situations with high-nutrient concentrations.
REFERENCES
Washington J.A., 1996, Principles of Diagnosis. In: Baron
S, editor. Medical Microbiology..: U of Texas Medical
Branch at Galveston; Galveston (TX), 4th edition.
Dartnell L.R., Roberts T.A., Moore G., et al., 2013,
Fluorescence Characterization of Clinically-Important
Bacteria. PLoS ONE 8(9): e75270.
doi:10.1371/journal.pone.0075270
Estes C., Duncan A., Wade B,, et al., 2003, Reagentless
detection of microorganisms by intrinsic fluorescence.
Biosens Bioelectron 18: 511-519. doi:10.1016/S0956-
5663(03)00008-3.
Dartnell L.R., Storrie-Lombardi M.C., Ward J.M., 2010,
Complete fluorescent fingerprints of extremophilic and
photosynthetic microbes. Intl J of Astrobiol 9: 245-257.
doi:10.1017/S1473550410000224.
Kjeldstad B., Christensen, T., Johnsson, A., 1985,
Porphyrin photosensitizationof bacteria, Adv. Exp.
Med. Biol. 193: 155–159.
Cody Y.S., Gross, D.C, 1987, Characterization of
pyoverdin (pss), the fluorescent siderophore produced
by Pseudomonas syringae pv. syringae, Appl. Environ.
Microbiol. 53(5): 928–934.
Shelly D.C., Quarles J.M., Warner I.M., 1980,
Identification of fluorescent Pseudomonas species. Clin
Chem 26: 1127-1132. PubMed: 6771056.
Shelly D.C., Warner I.M., Quarles J.M., 1980,
Multiparameter approach to the "fingerprinting" of
fluorescent Pseudomonads. Clin Chem 26:1419-1424
Leblanc L., Dufour E., 2002, Monitoring the identity of
bacteria using their intrinsic fluorescence. FEMS
Microbiol Lett 211: 147-153. doi:10.1111/j.1574-
6968.2002.tb11217.x.
Giana H., Silveira L., Zângaro R., et al, 2003, Rapid
Identification of Bacterial Species by Fluorescence
Spectroscopy and Classification Through; Principal
Components Analysis. J Fluoresc 13: 489-493. doi:
10.1023/B:JOFL.0000008059.74052.3c.
Smyth E.T., McIlvenny G., Enstone J.E., et al., 2008, Four
Country Healthcare Associated Infection Prevalence
Survey 2006: overview of the results. J Hosp Infect 69:
230-248. doi: 10.1016/j.jhin.2008.04.020.
On Feasibility of Fluorescence-based Bacteria Presence Quantification: P.Aeruginosa
199

Saiko G., Bacterial growth and siderophore production in
bacteria: an Analytical model, Accepted to Bioimaging
2021. SCITEPRESS
Bren A, Hart Y, Dekel E., Koster D., Alon U, 2013, The
last generation of bacterial growth in limiting nutrient.
BMC Systems Biology 7:27.
Meyer J.M., Abdallah M.A., 1978, The Fluorescent
Pigment of Pseudomonas fluorescens : Biosynthesis,
Purification and Physicochemical Properties, J of
General Microbiolog, 107: 319-328.
Smith E.E., Buckley D.G., Wu Z., et al., 2006, Genetic
adaptation by Pseudomonas aeruginosa to the airways
of cystic fibrosis patients. P Natl Acad Sci USA 103:
8487–8492.
Morgan S.J., Lippman S.I., Bautista G.E., et al., 2019,
Bacterial fitness in chronic wounds appears to be
mediated by the capacity for high-density growth, not
virulence or biofilm functions. PLoS Pathog 15 (3):
e1007511. doi:10.1371/journal.ppat.1007511
Gellatly S.L., Hancock, R.E.W., 2013, Pseudomonas
aeruginosa: new insights into pathogenesis and host
defenses, Pathogens and Disease, 67, 159–173
Cornelis, P., and Matthijs, S., 2002, Diversity of
siderophore-mediated iron uptake systems in
fluorescent pseudomonads: not only pyoverdines.
Environ. Microbiol. 4, 787–7898. doi: 10.1046/j.1462-
2920.2002.00369.x
Cartron, M.L., Maddocks, S., Gillingham, P., et al.,. 2006,
Feo-transport of ferrous iron into bacteria. Biometals
19, 143–157. doi: 10.1007/s10534-006-0003-2
Ochsner, U.A., Johnson, Z., Vasil, M.L., 2000, Genetics
and regulation of two distinct haem-uptake systems,
phu and has, in Pseudomonas aeruginosa. Microbiology
146, 185–198. doi: 10.1099/00221287-146-1-185.
BIOIMAGING 2021 - 8th International Conference on Bioimaging
200
