
Dynamic Flow Behaviour of a Blood Analogue Fluid in
Microchannels for Microcirculation Studies
I. Gonçalves
1,2
, J. Varelas
1
, G. Coutinho
1
, A. S. Moita
1,3
, D. Pinho
2,4,5
, R. Lima
2,6
, J. M. Miranda
6
,
E. J. Veja
7
, J. M. Montanaro
7
and A. L. N. Moreira
1
1
IN+ - Center for Innovation, Technology and Policy Research, Instituto Superior Técnico, Universidade de Lisboa,
Av. Rovisco Pais, 1049-001 Lisboa, Portugal
2
Metrics, Mechanical Engineering Department, University of Minho, Campus de Azurém, 4800-058, Guimarães, Portugal
3
CINAMIL – Military Academy Research Center, Department of Exact Sciences and Engineering,
Portuguese Military Academy, R. Gomes Freire, 203, 1169-203 Lisbon, Portugal
4
Center for MicroElectromechanical Systems (CMEMS
‐
UMinho), University of Minho, Campus de Azurém,
Guimarães, 4800-058 Portugal
5
INL, International Iberian Nanotechnology Laboratory, Av. Mestre José Veiga, 4715-330 Braga, Portugal
6
CEFT, Faculdade de Engenharia da Universidade do Porto (FEUP), R. Dr. Roberto Frias, 4200-465 Porto, Portugal
7
Dept. de Ingeniería Mecánica, Energética y de los Materiales and Instituto de Computación Científica Avanzada
(ICCAEx), Universidad de Extremadura, 06006 Badajoz, Spain
aluismoreira}@tecnico.ulisboa.pt, {ejvega, jmm}@unex.es, diana.pinho@inl.int, jmiranda@fe.up.pt,
moita.asoh@exercito.pt
Keywords: Blood Analogue Fluid, Fluid Characterization, Surfactant Concentration, Microcirculation, Microfluidics.
Abstract: This study proposes a simple, stable and low cost 2-phase blood analogue fluid, which can mimic multiphase
phenomena of real flow in microcirculation. This analogue fluid is mainly composed of Brij L4 surfactant
suspended in pure water. The analogue fluid is compared with real blood, both in terms of thermophysical
properties as well as in terms of its dynamic fluid flow behaviour, for different concentrations of the surfactant.
The results on the particle size distribution confirm the reproducibility of the fluid preparation, as well as of
its stability. The analogue fluid density is close to that of water, thus approaching the blood density. As for
the rheology, the blood analogue fluid depicts a shear thinning behaviour, matching that of blood, except for
very high Brij L4 concentrations. Fluid flow experiments show that the blood analogue can generate cell-free
layers (CFL), with thickness close to that of real blood, which corroborates that the proposed analogue is able
to mimic blood flow phenomena in microvessels. Increasing the surfactant concentration promotes the
augmentation of the CFL’s, but also endorses agglomeration and clogging. Flow separation occurs also at the
highest surfactant concentrations, which makes more difficult for the particles to follow the flow, so that flow
field evaluation becomes more problematic.
1 INTRODUCTION
Blood flow phenomena in microcirculation has been
studied both in vivo (Tateishi et al., 1994; Kim et al.,
2006; Namgung et al., 2014) and in vitro (Abkarian
et al., 2008; Tripathi et al., 2015; Bento et al., 2018;
Catarino et al., 2019). Despite of the significant
advances in this field, reported in the last decade,
understanding of blood flow phenomena at both
physiological and pathological conditions is still not
yet completely understood nor described.
In biomicrofluidics experiments, it is a common
practice to use in vitro blood to investigate blood flow
phenomena observed in real microvessels, such as the
plasma layer or cell-free layer (CFL) and the
bifurcation law effect (Completo et al., 2014, Pinho
et al., 2017; Catarino et al., 2019). However, handling
real blood fluids is not straightforward due to several
difficulties such as sanitary, bureaucratic and
technical problems (Sousa et al., 2011; Campo-
Deano et al., 2013). These issues have constrained the
use of blood in long term flow experiments. Hence, it
Gonçalves, I., Varelas, J., Coutinho, G., Moita, A., Pinho, D., Lima, R., Miranda, J., Veja, E., Montanaro, J. and Moreira, A.
Dynamic Flow Behaviour of a Blood Analogue Fluid in Microchannels for Microcirculation Studies.
DOI: 10.5220/0010343901750181
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 1: BIODEVICES, pages 175-181
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
175

is crucial to develop a simple and stable blood
analogue with flow properties close to real blood.
One of the first blood analogue fluids, used to
perform flow experiments, were Newtonian fluids
composed of mixtures of water and glycerol (Nguyen
et al., 2004; Yousif et al., 2011; Deplano et al., 2014).
Later, these fluids were improved to mimic the non-
Newtonian behaviour of human blood (Sousa et al.,
2011; Campo-Deano et al., 2013). However, all those
were homogeneous and were not able to mimic
multiphase phenomena of real blood in
microcirculation. Recently, several research studies
report the development of blood analogue fluids
containing solid suspended microparticles, which can
mimic the multiphase effects of blood (Calejo et al.,
2016; Pinho et al., 2017, 2019). Nevertheless, these
analogues have major drawbacks such as strong
aggregation tendency and consequent blockage of the
microchannels. Thus, it is important to develop
aggregation-free particulate blood analogues and test
them in representative experiments.
In this context, and following our previous work
(Moita et al., 2019), the present study proposes a
simple, stable particulate blood analogue, which can
mimic multiphase phenomena of real blood in
microcirculation. The proposed fluid is composed of
Brij L4 surfactant suspended in pure water. The
analogue fluid is characterized and compared with
real blood. Early results addressed deformability
behaviour in microchannels with a sudden
contraction (Moita et al., 2019) as well as the effect
of the constriction to generate cell-free layer at the
constriction downstream (Lima et al., 2020). In this
work, additional information is provided on the effect
of the surfactant in the fluid viscosity and surface
tension and the behaviour of the biomimetic fluid in
a bifurcation.
2 MATERIALS AND METHODS
2.1 Preparation and Characterization
of the Analogue Fluid
The surfactant Brij L4 has tendency to form stable
spherical clusters. Hence, in this work, 0.5wt% to
10wt% of Brij L4 surfactant was used to produce the
proposed blood analogue fluid. Briefly, the surfactant
was mixed with pure water. After the
accomplishment of a homogeneous mixture, the fluid
was forced to flow through precolumn filters having
a membrane with an average pore size of 20 µm. This
precolumn filter allows the generation of smaller and
more homogenized surfactant droplets.
The biomimetic fluid was characterized in terms
of density , surface tension
lv
and viscosity .
Furthermore, the wettability of the prepared solutions
with Polydimethylsiloxan PDMS (the material of the
microchannels), was also evaluated based on the
static contact angle.
Density was evaluated using a picnometer. The
measured value, 996 kgm
-3
is close to that of water,
as expected. The viscosity was measured using a
rheometer (Bohlin CVO, Malvern, Worcestershire,
UK) using a coneplate geometry, with a diameter of
55 mm and an angle of 1° with a gap size of 0.03 mm.
The steady shear viscosity curves were obtained
over a wide range of shear rates, ranging between 10
s
−1
and 10000 s
−1
.
Surface tension was measured on an optical
tensiometer (THETA, from Attention), using the
pendant droplet method. The final surface tension
value evaluated for each solution was averaged from
15 measurements. All the measurements depict
standard mean errors lower than 0.35.
Finally, the wettability of the solutions with the
material that was used to fabricate the microchannels
– PDMS, was quantified with the static contact angle
e
,
measured with the optical tensiometer THETA,
from Attention, using the sessile drop method. Images
with a resolution of 640×480 pixels are post-
processed by a drop detection algorithm based on
Young-Laplace equation (One Attention software).
The accuracy of these algorithms is argued to be of
the order of 0.1º (Cheng, 2008). For the current
optical configuration, the spatial resolution is 15.6
m/pixel.
Detailed description of the techniques used in the
characterization of the thermophysical properties of
the fluid can be found in Pereira et al. (2014) and in
Moita et al. (2016, 2018).
2.2 Characterization of the Particles
Size Distribution using Laser
Confocal Fluorescence Microscopy
The size distribution of the particles suspended in the
solutions was evaluated using a Laser Scanning
Confocal Microscope (SP8 from Leica). The images
were obtained with a 10X objective lens and recorded
with a resolution of 512×512 pixels. Rhodamine B
(Sigma Aldrich) was added to the solutions, with a
concentration of 3.968x10
-6
g/mL, which does not
alter the thermophysical properties of the analogue
fluid (Moita et al., 2019). For this dye, an excitation
laser with a wavelength of 552 nm was used, fixing
the laser power to 10.50 mW (3% of its maximum
power). The gain of the microscope photomultiplier
BIODEVICES 2021 - 14th International Conference on Biomedical Electronics and Devices
176
was fixed at 550 V. These values were chosen after a
sensitivity analysis on the contrast of the image
(before the post-processing) and on the Signal to
Noise Ratio (SNR). An in-house code, developed in
MATLAB was then used to process the 1024×1024
pixels images, which were taken with a scanning
frequency of 400 Hz.
2.3 Characterization of the Analogue
Fluid Flow in the Microchannels
To study the dynamic behaviour of the analogue fluid
flow and its ability to generate the CFL’s, flow
experiments were performed in two microchannels,
one with an abrupt contraction and one with a
bifurcation. The microchannels were fabricated in
PDMS using soft lithography, following the
fabrication method described in Faustino et al.
(2016). The depth of the microfluidic device was
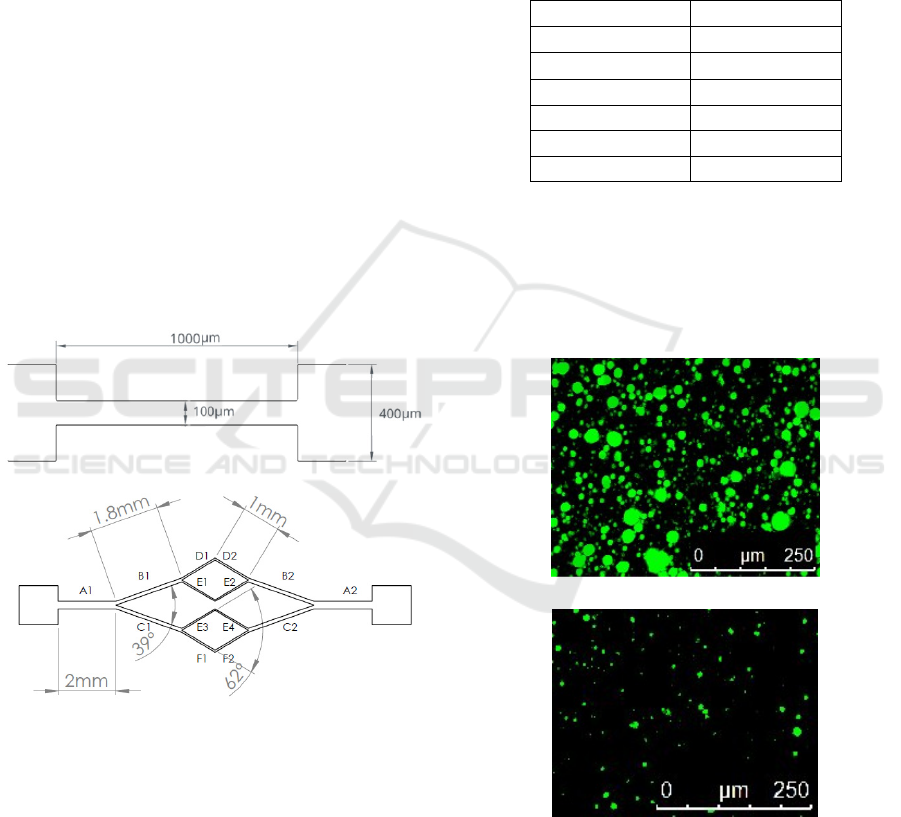
around 30 µm. Figure 1 shows a schematic drawing
of the microfluidic devices used in this study,
including their main dimensions. Table 1 depicts the
main dimensions of the bifurcated channel
represented in Figure 1b.
a)
b)
Figure 1: Schematic with the geometry and main
dimensions of the microchannel used to study the analogue
fluid flow behaviour and CFL’s generation. a)
microchannel with an abrupt constriction, b) microchannel
with a bifurcation.
The flow was driven at constant pressure using a
syringe pump (KD Scientific, USA). Images were
taken using a high-speed camera (Phantom v7.1;
Vision Research, USA), with a resolution of 640×640
pixels and with a frame rate of 4000 fps. The lens used
had a 20X magnification and the spatial resolution for
this optical configuration was 1.185 pixel/m. The
images were processed using the software ImageJ
(1.46r, NIH, USA). Regarding, the CFL
measurements, the recorded image sequences were
evaluated using the function “Z project” from the
ImageJ software.
Table 1: Main dimensions of the bifurcated microchannel
represented in Figure 1b). All the microchannels have a
height of 50mm.
Section
Width (m)
A
1
=A
2
200
B
1
=B
2
118.1
C
1
=C
2
84.8
D
1
=D
2
58.19
E
1
=E
2
=E
3
=E
4
46.55
F
1
=F
2
23.29
3 RESULTS AND DISCUSSION
3.1 Particle Size Distribution of the
Analogue Fluid
a)
b)
Figure 2: Images of the analogue fluid prepared with the
surfactant, obtained by Laser Scanning Fluorescent
Confocal Microscopy (objective of 20x magnification and
0.75x numerical aperture) after filtering the solution with a
20 m precolumn filter. a) without filtering, b) after passing
the filter.
Dynamic Flow Behaviour of a Blood Analogue Fluid in Microchannels for Microcirculation Studies
177
As reported in Moita et al. (2019), the initial scope
for the development of this analogue fluid was to
mimic biological fluids. Hence, images taken at the
earliest stages of this research with the confocal
microscope, as reported in Moita et al. (2019) show
that the devised solutions present an heterogeneous
precipitated of deformable particles, depicting a wide
range of particles sizes (between 2.5 mm and 40 mm).
Deformability experiments however, showed a very
good ability of these particles to mimic red blood
cells. To make the fluid more homogeneous in terms
of particles number and size distribution, Lima et al.
(2020) propose the use of a precolumn filter.
Following such procedure, the fluid becomes more
homogeneous, as shown in the images taken with the
Laser Scanning Confocal Microscope, depicted in
Figure 2.
a)
b)
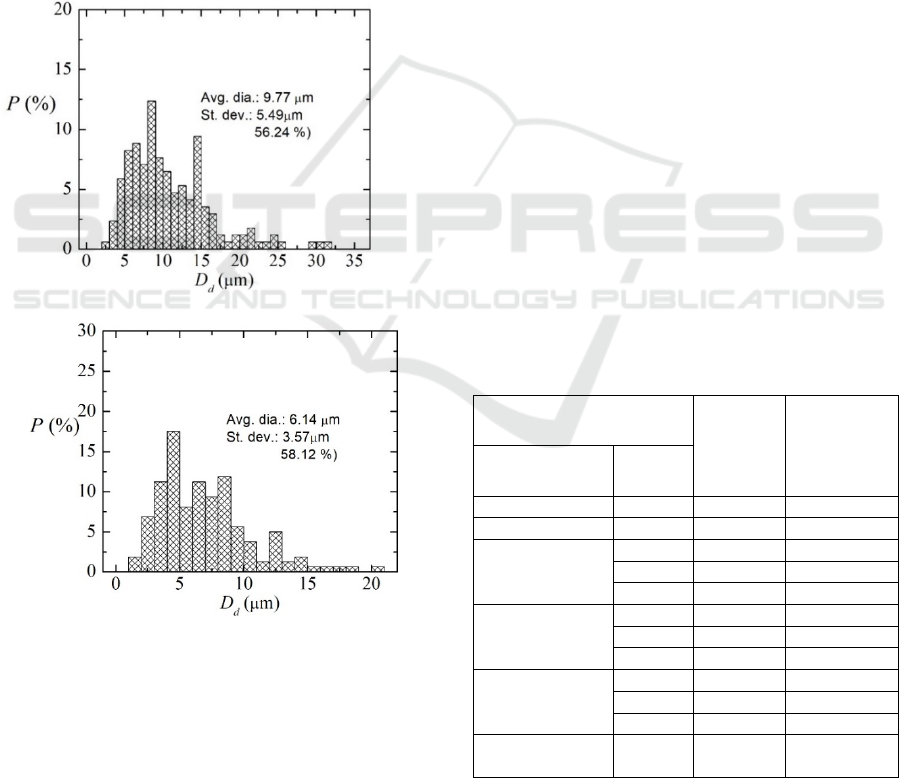
Figure 3: Probability distribution for the surfactant droplet
diameter (Dd) generated a) without filtering, b) after
passing the filter.
The homogeneity of the fluid after filtering is
quantitatively confirmed by the particles size
distribution obtained by image processing, as shown
in Figure 3. The figure also shows that the average
diameter of the surfactant droplets was reduced from
9.77 µm to 6.14 µm (size closer to that of red blood
cells) because of the precolumn filter. Additionally,
whereas the unfiltered fluid presented droplets larger
than 30 µm, a post-filtering analysis revealed a
maximum diameter of approximately 20 µm. The
standard deviation (presented both in absolute values
and in percentage) is also mildly reduced. Hence,
summing up, results show that the precolumn filter
used in this study enables the generation of smaller
and less polydisperse surfactant microdroplets.
3.2 Effect of the Surfactant
Concentration in the
Thermophysical Properties of the
Analogue Fluid
After controlling the size and distribution of the
particles on the analogue fluid, it is relevant to infer
on the adequate concentration of surfactant to use to
match the properties of the analogue fluid with those
of blood. In this context, this work addressed the use
of different Brij L4 concentrations, ranging between
0.5wt% and 2wt%. Being water-based solutions, their
density, evaluated with a pycnometer, was always
close to that of water (approximately 996 kgm
-3
),
regardless of the concentration of Brij L4. This is an
expected result given the high density of water, when
compared to that of the surfactant and given the still
relatively low mass concentrations of surfactant used.
Table 2: Effect of the concentration of Brij L4 in the surface
tension of the resulting analogue fluids and in the static
contact angle obtained with a PDMS surface.
Brij L4 Surface
tension
lv
(mNm
-1
)
Static
contact
angle
e
(º)
[PDMS]
Concentration Filter
(
m)
Water - 72.90 68.92
Brij L4 0.5wt% 20 27.21 65.47
Brij L4 5wt 1% 10 31.74 60.29
20 31.61 59.69
10+20 31.62 59.00
Brij L4 5wt 2% 10 31.74 58.45
20 31.74 51.00
10+20 31.69 48.66
Brij L4 5wt 5% 10 31.80 47.85
20 31.87 47.28
10+20 31.82 42.05
Brij L4 5wt
10%
20 26.20 59.81
A different trend is nevertheless observed for the
surface tension. Hence, given that the surfactants are
BIODEVICES 2021 - 14th International Conference on Biomedical Electronics and Devices
178
usually used to alter the surface tension of other
liquids, the surface tension of the Brij L4 solutions is
significantly reduced, when the surfactant is added,
even for the lowest concentration of Brij L4
(0.5wt%). However, following this decrease in the
surface tension value, observed for the analogue fluid
with the lowest surfactant concentration, when
compared to water, a stable plateau value is then
obtained, as the concentration of surfactant is
increased, up to the maximum value tested here.
Consistently, the contact angle measured for the
analogue solutions was also kept within a constant
value, for increasing concentration values. Such
trends can be observed in Table 2.
Finally, it is vital to infer on the effect of adding
the surfactant to the rheology of the resulting
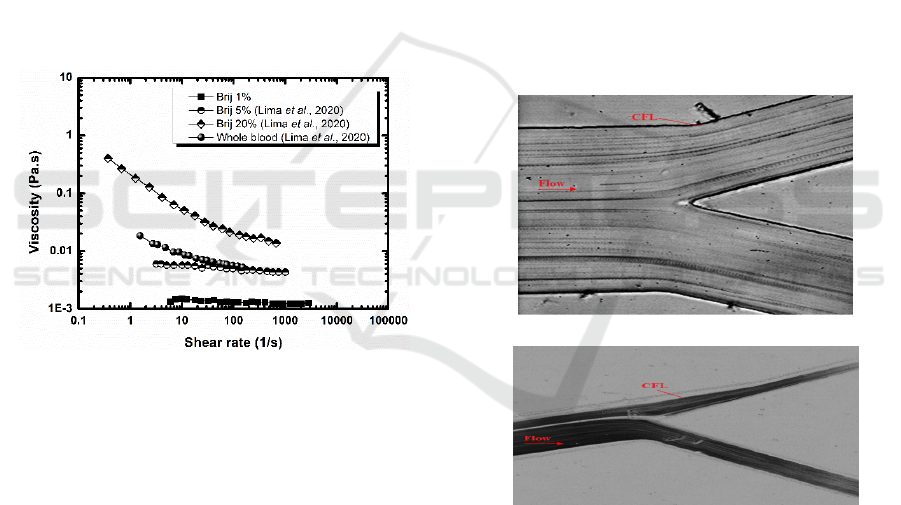
analogue fluid. Rheology curves obtained at 1% were
compared with those measured at 5% and at 10% (in
weight), as reported in Lima et al. (2020) as well as
with the viscosity curve for human blood (Lima et al.,
2020). These curves are depicted in Figure 4.
Figure 4: Steady shear viscosity curves for the analogue
fluid with a concentration of 1%, 5% and 10% of Brij L4,
and human whole blood.
Figure 4 depicts an increase in the analogue fluid
viscosity as one increments the concentration of Brij
L4. However, while the viscosity increases almost
linearly with the shear rates for low surfactant
concentrations (up to 5wt%), a strong shear thinning
behaviour is observed for the analogues containing
very high Brij L4 concentrations (larger than 10wt%).
Despite of the known shear-thinning behaviour of
blood (endorsed by the behaviour of the red blood
cells), the viscosity curves of the analogue fluids with
higher surfactant concentrations do not match the
curve of the whole blood, which is closer to that of
the analogue fluids with lower surfactant
concentrations (lower than 5%).
Given this trend, only these concentrations were
considered in the characterization of the flow
behaviour of the analogue fluid, as discussed in the
following sub-section.
3.3 Fluid Flow of the Analogue Fluid in
Microchannels
The tendency of the red blood cells to migrate to the
center of the microchannels or microvessels
originates the formation of a cell depleted layer
around the walls, known as the cell-free layer (CFL).
This is a well-known phenomenon that occurs in
microfluidic devices and microvessels with
dimensions lower than 300 µm. Hence, in this study
flow visualizations allowed observation of the CFL
thickness for the proposed blood analogue and in
vitro blood flowing through a microchannel with an
abrupt contraction. Figure 5 shows treated images for
the tested fluids for a flow rate of 15 µL/min. The
results show that, at the downstream region of the
microchannel contraction, there is a high propensity
for CFL formation both for the analogue fluid and for
blood.
a)
b)
Figure 5:
Flow and CFL visualization of: a) proposed blood
analogue, b) in vitro blood.
In addition, the measurements of the CFL
thickness for both fluids are in good agreement which
indicates that the proposed blood analogue fluid is
able to mimic blood flow phenomena happening in
microvessels and in microfluidic devices, such as
CFL and cross flow filtration. The CFL is also
generated at the bifurcated microchannel, as observed
in Figure 6. Analogue fluids with higher surfactant
concentration tend to generate a thicker CFL.
Dynamic Flow Behaviour of a Blood Analogue Fluid in Microchannels for Microcirculation Studies
179

However, aggregation and clogging occur more
often, and separation is also easier to occur, given the
higher viscosity which decelerates the fluid and
dissipates the momentum in the boundary layer.
a)
b)
Figure 6:
Flow and CFL visualization for the analogue
fluid, for different concentrations of Brij L4. a) and CFL
measurements of the a) 1% Brij L4, b) 2% Brij L4 (in
weight).
Analysing the particles velocity, and considering
the average velocity, as shown in Table 3, one can
observe an acceleration of the particles after the
bifurcation, which can be related to mass
conservation principles: as the cross section of the
bifurcation channels is smaller than that of the main
channel, the flow will accelerate, by mass flow
conservation. These results were obtained for the 1%
Brij L4 solution and for a flow rate of 1ml/min, but
similar trends were inferred for other surfactant
concentrations/flow rates, apart from the
aforementioned differences. The results also show
that the velocity of the particles near the center of the
channel (the red trajectory in Figure 6a) have higher
velocities than those closer to the wall. This means
that the particles are following the flow, which has
characteristically its maximum velocity at the center
of the channel, while the particles more apart from the
center are more affected by the wall effects.
Table 3: Characterization of the velocity of the particles
identified in the red and yellow trajectories in Figure 6. The
flow is the analogue solution with 1% Brij L4 (in weight)
after passing the 20 mm filter. The flow rate used is
1mL/min.
Location Average particle
velocity (m/s)
Particle 1 (red trajectory in
Figure 6) – before bifurcation
3.14
Particle 1 (red trajectory in
Figure 6) – after bifurcation
5.08
Average vel. particle 1 4.39
Particle 2 (yellow trajectory
in Figure 6) – before
bifurcation
2.23
Particle 2 (yellow trajectory
in Figure 6) –after bifurcation
3.36
Average vel. particle 2 2.90
4 CONCLUSIONS
The present work proposes a low-cost and stable
blood analogue fluid, for microcirculation studies.
This analogue fluid is based on water solutions with
surfactant Brij L4. Following our previous work, this
study infers on the effect of the surfactant
concentration in the particle distribution and in the
thermophysical properties of the resulting fluid.
Furthermore, the dynamic flow and CFL generation
are also evaluated. The thermophysical properties of
the analogue fluid are observed to be close to those of
real blood. This includes the shear thinning
behaviour, which is only deviated for very high
concentrations (>10%). Larger surfactant
concentrations (of the order of 1-2% promote the
generation of the CFL, but also endorses
agglomeration and clogging.
ACKNOWLEDGEMENTS
This work was supported by Fundação para a Ciência
e a Tecnologia (FCT) under the context grants
UIDB/04077/2020, UIDB/04436/2020 and
UIDB/00532/2020 and of project JICAM/0003/2017,
in Projecto 3599 - Promover a Produção Científica, o
Desenvolvimento Tecnológico. Authors also
acknowledge FCT for supporting I. Gonçalves with a
research fellowship through project LISBOA-01-
0145-FEDER-030171/ PTDC/EME-
SIS/30171/2017.
BIODEVICES 2021 - 14th International Conference on Biomedical Electronics and Devices
180

REFERENCES
Abkarian, M., Faivre, M., Horton, R., Smistrup, K., Best-
Popescu, C. A., Stone, H. A., 2008. Cellular-scale
hydrodynamics, Biomedical Materials, 3:034011.
Calejo, J.; Pinho, D.; Galindo-Rosales, F.J.; Lima, R.;
Campo-Deaño, L., 2016. Particulate Blood Analogues
Reproducing the Erythrocytes Cell-Free Layer in a
Microfluidic Device Containing a Hyperbolic
Contraction. Micromachines, 7:4.
Campo-Deaño, L., Dullens, R. P. A., Aarts, D. G. A. L.,
Pinho, F.T., Oliveira, M. S. N., 2013. Viscoelasticity of
blood and viscoelastic blood analogues for use in
polydymethylsiloxane in vitro models of the circulatory
system. Biomicrofluidics, 7:034102.
Catarino, S. O., Rodrigues, R. O., Pinho, D., Miranda, J. M.,
Minas, G., Lima, R., 2019. Blood Cells Separation and
Sorting Techniques of Passive Microfluidic Devices:
From Fabrication to Applications. Micromachines.
10:593.
Cheng, P., 2008. Automation of Axisymmetric Drop Shape
Analysis using Digital Imaging Processing, PhD thesis,
University of Toronto, Canada.
Completo, C., Geraldes, V., Semiao, V., 2014. Rheological
and dynamical characterization of blood analogue
flows in a slit. Int. J. Heat Fluid Flow. 46:17-28.
Deplano, V., Knapp, Y., Bailly, L., Bertrand, E., 2014.
Flow of a blood analogue fluid in a compliant
abdominal aortic aneurysm model: Experimental
modelling. J. Biomech, 47:1262–1269.
Faustino, V., Catarino, S. O., Lima, R., Minas, G., 2016.
Biomedical microfluidic devices by using low-cost
fabrication techniques: A review, Journal of
Biomechanics, 49 (11):2280–2292.
Kim, S., Kong, R. L., Popel, A. S., Intaglietta, M., Johnson,
P. C., 2006. A computer-based method for
determination of the cell-free layer width in
microcirculation. Microcirculation 13(3):199-207.
Lima, R., Vega, E. J., Moita, A. S., Miranda, J. M., Pinho,
D., Moreira, A.L.N., 2020. Fast, flexible and low-cost
multiphase blood analogue for biomedical and energy
applications. Exp. Fluids, 61:231 (11 pages).
Moita, A. S., Laurência, C., Ramos, J.A., Prazeres, D. M.
F., Moreira, A. L. N., 2016. Dynamics of droplets of
biological fluids on smooth superhydrophobic surfaces
under electrostatic actuation, J. Bionic Eng., 13:220-
234.Moita, A.S., Caldeira, C., Golçalves, I., Lima, R.,
Veja, E. J., Moreira, A. L. N., 2020. Analogue fluids for
cell deformability studies in microfluidic devices.
Biomedical Engineering Systems and Technologies.
Series: Communications in Computer and Information
Science, Springer International Publishing AG, part of
Springer Nature Switzerland, A. Roque et al. (Eds.):
BIOSTEC 2019, CCIS 1211, pp. 1–12, 2020.
Namgung, B, Liang, L. H., Kim, S., 2014. Physiological
significance of cell-free layer and experimental
determination of its width in microcirculatory vessels.
In: Lima et al. editors. Visualization and simulation of
complex flows in biomedical engineering. Dordrecht:
Springer; p. 75–87.
Nguyen, T.T., Biadillah, Y., Mongrain, R., Brunette, J.,
Tardif, J.-C., Bertrand, O.F., 2004. A Method for
Matching the Refractive Index and Kinematic Viscosity
of a Blood Analog for Flow Visualization in Hydraulic
Cardiovascular Models. J. Biomech. Eng., 126, 529–
535.
Pereira P., Moita, A. S., Monteiro, G., Prazeres, D. M. F.,
2014. Characterization of English weed leaves and
biomimetic replicas. Journal of Bionic Engineering,
11(3):346-359.
Pinho, D., Campo-Deaño, L., Lima, R., Pinho, F.T., 2017.
In vitro particulate analogue fluids for experimental
studies of rheological and hemorheological behavior of
glucose-rich RBC suspensions. Biomicrofluidics,
11:054105.
Pinho, D., Muñoz-Sánchez, B. N., Anes, C. F., Vega, E. J.,
Lima, R., 2019. Flexible PDMS microparticles to
mimic RBCs in blood particulate analogue fluids.
Mechanics Research Communications. 100:103399.
Sousa, P.C., Pinho, F.T., Oliveira, M. S. N., Alves, M. A.,
2011. Extensional flow of blood analogue solutions in
microfluidic devices. Biomicrofluidics, 5:14108.
Tateishi, N, Suzuki, Y, Soutani, M, Maeda, N., 1994. Flow
dynamics of erythrocytes in microvessels of isolated
rabbit mesentery: cell-free layer and flow resistance. J.
Biomech. 27:1119–1125.
Tripathi, S, Bala, Varun, Kumar, Y. V., Prabhakar, A.,
Joshi, S. S., Agrawal, A., 2015. Passive blood plasma
separation at the microscale: a review of design
principles and microdevices. J Micromech Microeng.
25:8.
Yousif, M. Y., Holdsworth, D. W., Poepping, T. L., 2011.
A blood-mimicking fluid for particle image
velocimetry with silicone vascular models. Exp. Fluids,
50:769–774.
Dynamic Flow Behaviour of a Blood Analogue Fluid in Microchannels for Microcirculation Studies
181
