
COVIDDX: AI-based Clinical Decision Support System for Learning
COVID-19 Disease Representations from Multimodal Patient Data
Veena Mayya
1,2 a
, Karthik K.
1 b
, Sowmya S. Kamath
1 c
, Krishnananda Karadka
3 d
and Jayakumar Jeganathan
4
1
Healthcare Analytics and Language Engineering (HALE) Lab, Dept. of Information Technology,
National Institute of Technology Karnataka, Surathkal, Mangalore 575025, India
2
Department of Information & Communication Technology, Manipal Institute of Technology,
Manipal Academy of Higher Education, Manipal, Karnataka, India
3
Penzigo Technology Solutions Pvt. Ltd., NITK-Science and Technology Entrepreneurs’ Park (STEP), NITK Surathkal, India
4
Dept. of Medicine, Kasturba Medical College, Manipal Academy of Higher Education (MAHE), Karnataka, India
Keywords:
Computational and Artificial Intelligence, Decision Support Systems, Automated Diagnosis, COVID-19.
Abstract:
The COVID-19 pandemic has affected the world on a global scale, infecting nearly 68 million people across
the world, with over 1.5 million fatalities as of December 2020. A cost-effective early-screening strategy is
crucial to prevent new outbreaks and to curtail the rapid spread. Chest X-ray images have been widely used
to diagnose various lung conditions such as pneumonia, emphysema, broken ribs and cancer. In this work,
we explore the utility of chest X-ray images and available expert-written diagnosis reports, for training neural
network models to learn disease representations for diagnosis of COVID-19. A manually curated dataset
consisting of 450 chest X-rays of COVID-19 patients and 2,000 non-COVID cases, along with their diagnosis
reports were collected from reputed online sources. Convolutional neural network models were trained on
this multimodal dataset, for prediction of COVID-19 induced pneumonia. A comprehensive clinical decision
support system powered by ensemble deep learning models (CADNN) is designed and deployed on the web
˚
.
The system also provides a relevance feedback mechanism through which it learns multimodal COVID-19
representations for supporting clinical decisions.
1 INTRODUCTION
COVID-19 has claimed the lives of more than one
million people worldwide and continues to pose a
severe threat to humanity (Max Roser and Hasell,
2020). Indicative clinical symptoms of COVID-19
include high fever, cough, sore throat, headache, fa-
tigue, muscle pain, and Dyspnea or shortness of
breath (SoB). Currently, the main testing proce-
dure employed for diagnosing COVID-19 is RT-PCR
(Real-time Reverse Transcription - Polymerase Chain
Reaction), which primarily detects the presence of
RNA in the test samples. Radiology tests like Com-
puted Tomography (CT) and X-rays have also been
used as additional diagnostic tools. Normally, CT
a
https://orcid.org/0000-0002-8091-5053
b
https://orcid.org/0000-0003-0846-2982
c
https://orcid.org/0000-0002-0888-7238
d
https://orcid.org/0000-0001-8385-3516
˚
CADNN COVID-19 Predictor, https://cadnn.penzigo.net
and X-rays show significant changes in the lung with
the onset of respiratory symptoms, while some stud-
ies have reported that discernible changes occur in an
infected person’s scans, starting at the first onset of
mild symptoms. In situations when RT-PCR kits are
limited in number, medical personnel have relied on
such radiography scans for confirming COVID-19 in-
fection. This opens up a significant research scope
for designing automated systems trained to process a
large number of radiography scans such as CT and
X-rays for testing for COVID-19 infection. More-
over, there exists a significant potential for reducing
the costs associated with mass testing to a large extent
and judiciously manage available RT-PCR kits. Cur-
rently, in the Indian healthcare system, RT-PCR test-
ing costs around |2,000-4,000, whereas X-ray scan
costs are in the range of |200-500 (BusinessToday,
2020). This price difference can be hugely beneficial
for patients and has a high cost-benefit tradeoff.
Mayya, V., K., K., Kamath, S., Karadka, K. and Jeganathan, J.
COVIDDX: AI-based Clinical Decision Support System for Learning COVID-19 Disease Representations from Multimodal Patient Data.
DOI: 10.5220/0010341906590666
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 5: HEALTHINF, pages 659-666
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
659

Clinical Decision Support Systems (CDSSs) pow-
ered by learnable AI-based models, computation tech-
niques such as image processing and big data analyt-
ics have proven to be effective in assisting health pro-
fessionals in a wide array of clinical tasks such as dis-
ease prediction (Tushaar et al., 2020), anomaly anal-
ysis, patient history modeling (Gangavarapu et al.,
2020) and so on. A CDSS for predicting the presence
or absence of COVID-19 infection using diagnostic
scans can be beneficial to healthcare professionals as
well as patients. A contactless X-ray scan workflow
could be achieved by using cameras for patient mon-
itoring purposes (Scheib, 2009; Forthmann and Pflei-
derer, 2019), after a few days of onset of COVID-19-
like symptoms, the patient is subjected to an X-ray.
For cases where the predicted risk is high, he or she
can then undergo a RT-PCR test to confirm the diag-
nosis. Based on the predicted risk score, the health
professionals may also decide to isolate the patient
and perform another X-ray in a couple more days to
ensure others’ safety. Such analysis can also con-
tribute to isolating asymptomatic COVID-19 patients,
who undergo chest X-ray for other reasons (e.g., pre-
operation evaluation, routine medical check-up, rib
fractures etc.).
Furthermore, there is a critical need for ensem-
ble/composite models that make use of laboratory ex-
amination results to help better screening detection
and diagnosis of COVID-19. In this work, we attempt
to build such a model that leverages the wealth of in-
formation contained in expert reports along with the
actual diagnostic scan data for learning disease rep-
resentations of COVID-19. A collated dataset con-
sisting of X-ray and the corresponding expert reports
of individual patients suffering from COVID-19 and
non-COVID cases were used for the experiments.
Also, a complete web-based framework is deployed
on the cloud, to provide a highly usable platform
for expert verified screening results, with which the
model is retrained based on the continuous feedback
provided by the expert radiologists. Through this re-
search, our focus is to enable a comprehensive clinical
decision support framework for fast and cost-effective
early screening of COVID-19, in a user-friendly and
unobtrusive manner.
The rest of this paper is organized as follows: Sec-
tion 2 presents a detailed discussion of the existing
research works in the area of interest. In Section 3, we
describe the methodology adopted for data collation
and the proposed approach for diagnosing COVID-19
using patients’ chest X-ray images and expert-written
diagnosis reports. Section 4 elaborates the exper-
iments performed and the observations regarding
the performance of the proposed models, followed by
conclusions and directions for future work.
2 RELATED WORK
Machine learning-based medical image/text analysis
and classification has seen extensive applications in
healthcare for enabling medical data management and
improved diagnosis. CDSSs that are built on the
premise of AI-based analysis of medical data for en-
abling decision making has been successfully used
by healthcare professional in various clinical settings.
Recently, significant research interest has focused on
the application of AI for diagnosing COVID-19.
Several works that make use of diagnostic scan
images like chest X-rays (Ozturk et al., 2020; Narin
et al., 2020; Hall et al., 2020; Ghoshal and Tucker,
2020; Hemdan et al., 2020) and computerized tomog-
raphy (CT) scan images (Mishra et al., 2020; Abbas
et al., 2020; Dansana et al., 2020; Mei et al., 2020)
have been proposed. Most existing works make use of
transfer learning using deep learning models that are
pretrained on ImageNet (Deng et al., 2009) data. We
observed several gaps after reviewing existing works
in this area. We found that the associated metadata of
patients has not been considered in most works. Also,
valuable expert-written diagnosis maintained as nat-
ural language text reports after checking a patient’s
chest radiography images have not been explored for
the task of disease prediction. Furthermore, there is
ample scope for the development of a complete, easy-
to-use diagnostic framework for the use of health-
care professionals. Using such tools, expert opinion
& other metadata about patients can also be obtained
to incorporate relevance feedback into the prediction
model, building accurate CDSSs.
In this work, we incorporate multiple deep learn-
ing models for classifying X-ray images as COVID-
19 positive or negative, wherein, the contributions of
image features and also the latent information con-
tained in the expert-written diagnosis text reports are
modeled for the diagnosis. To alleviate the manual
effort required to assess and generate diagnosis re-
ports when a large number of diagnosed cases arrive,
a content-based report generation model, to automat-
ically generate natural language diagnosis reports is
also designed, for reducing the cognitive burden of
radiologists and other medical personnel involved in
medical record management. The complete frame-
work is deployed on the cloud and is made avail-
able as a web application for managing patients meta-
data (from the day of admission till the discharge).
Functionalities like validity checks for X-ray images,
HEALTHINF 2021 - 14th International Conference on Health Informatics
660
evidence-based diagnosis support through highlight-
ing of important features learnt by the model, and au-
tomatic report generation for further processing are
incorporated in the proposed framework. A feedback
system is provided to verify the prediction and gener-
ated reports, which is later utilized for improving the
offline training process, for fine-tuning the prediction
performance of the CDSS.
3 MATERIALS & METHODS
3.1 Data Collation
Several open COVID-19 datasets are currently avail-
able (Cohen et al., 2020; Chung et al., 2020; Rah-
man, 2020) which are limited to only radiographical
imaging data. Other pertinent information such as pa-
tient history, findings from such images etc., have not
been made directly available. Thus, these datasets are
not well-suited for multimodal data modeling and for
multi-task learning. To address these lacunae, a mul-
timodal patient data amenable for multimodal clinical
tasks was collated from varied trusted sources.
For curating the dataset, a total of 150 confirmed
COVID-19 patient cases were collected from publicly
available sources
1,2,3
and also from a local hospital
with an active COVID-19 ward. Each X-ray in the
collated data is associated with metadata information
– demographics details like age, gender and findings
in the form of plain natural language text (reports) as
observed by expert radiologists. We have also col-
lected COVID-19 X-ray images only from the open
datasets mentioned earlier. In total, the dataset con-
tained 450 chest X-ray images of COVID-19 infected
patients, of which 150 images had associated meta-
data. In addition to this, about 2,000 normal cases
were taken from the Pneumonia Detection Challenge
(Radiological Society of North America, 2018). The
neural models were trained on collated data and fine-
tuned whenever a significant number of new cases
were uploaded through the online CDSS application.
3.2 Proposed Approaches
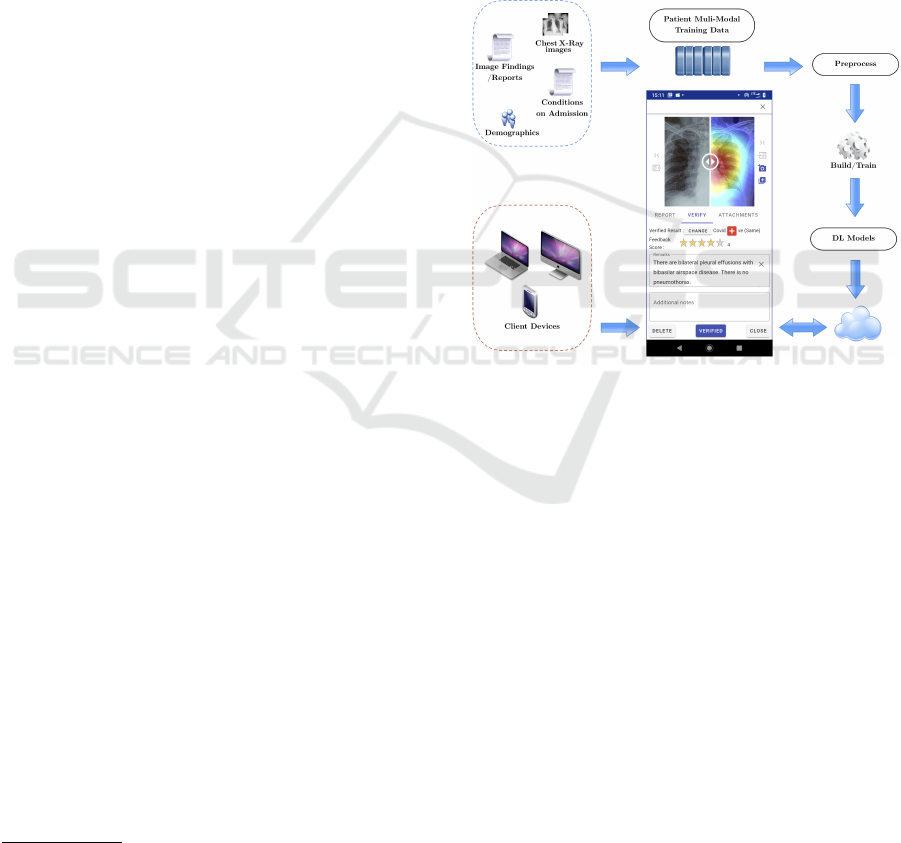
Fig. 1 illustrates the detailed workflow of the pro-
posed approach. The framework employs five deep
neural models as discussed in Section 3.2.1. As the
X-ray images were captured using different machines,
there exists a large variability, mainly in pixel in-
1
https://radiopaedia.org
2
https://www.sirm.org/COVID-19-database/
3
https://www.eurorad.org/
tensities and focus on lung regions. To reduce the
change in color intensities across the images, his-
togram matching (Gonzalez and Woods, 2008) was
applied to the dataset, for which, we considered an
X-ray image as a reference image (R
img
) and then
matched all the other X-ray pixel intensity histograms
with R
img
. To suppress the effect of rib shadows
on the classifier’s prediction, RIBDL model was used
(described in Section 3.2.1). Then, the lung regions
are segmented to reduce the effect of the surrounding
background on the model’s prediction. This enables
the classification models to learn the minute changes
in lung structure, which is often missed by human ex-
perts (due to the noise created by rib shadow).
Figure 1: High-Level design of the proposed approach.
Algorithm 1 details the process of segmentation.
Here, we initially segment the lung region using PIX-
GAN (Section 3.2.1), then the bounding box around
the lung cavity is cropped and resized to original size.
The sample output after each step is depicted in Fig.
2. Cropping only the region of interest (RoI) al-
lows the deep neural model to learn important fea-
tures from within the lung regions. Crop and rotate
facilities are also provided in the developed CADNN
online framework so that users can select only lung re-
gion while uploading new test images. The local con-
trast of RoI segmented input X-ray grayscale images
are further improved by applying Contrast Limited
Adaptive Histogram Equalization (CLAHE) (Reza,
2004). Image augmentation is performed by applying
rotation with different angles (10
˝
´120
˝
) with an in-
terval of 40
˝
to the training images, which resulted
in more than 3,000 COVID-19 and non-COVID-19 (a
total of 3,474 training case samples).
COVIDDX: AI-based Clinical Decision Support System for Learning COVID-19 Disease Representations from Multimodal Patient Data
661
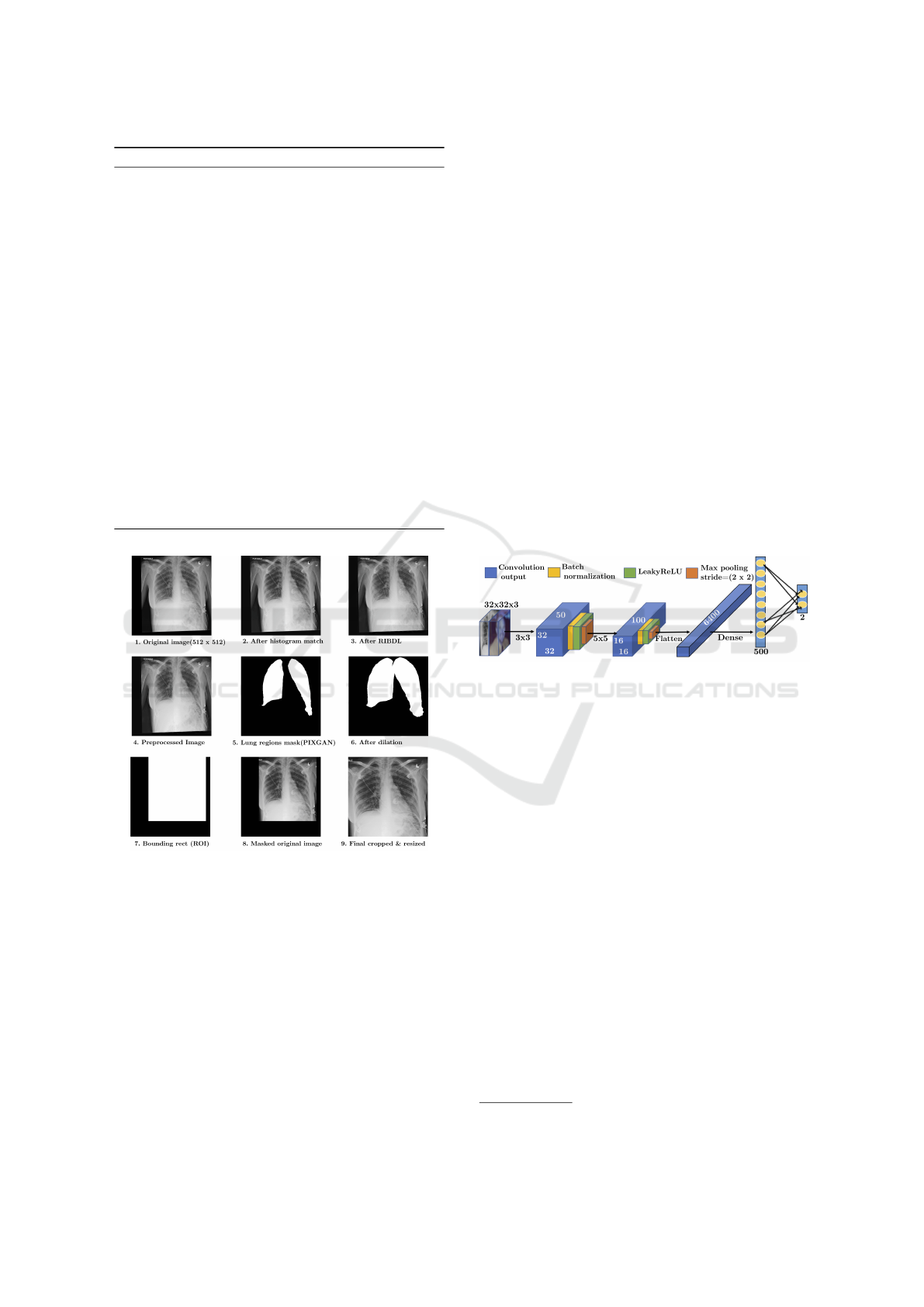
Algorithm 1: Chest X-ray prepossessing pipeline.
Input: Input chest X-ray images
Output: Preprocessed X-ray image
1: for each img P InputImages do
2: Perform histogram matching of img with
reference image.
3: Perform rib shadow removal using RIBDL.
4: Perform lung region segmentation using
PIXGAN by resizing img to (512, 512).
5: Find the contours of the generated MaskImg
6: Remove all small contours (with width ă 50
and height ă 50) in MaskImg
7: Dilate MaskImg with kernel size of (5,3)
until a single contour is formed.
8: Draw the bounding box over the single
contoured MaskImg Ź Set all other pixel
intensities to zero.
9: Remove all black regions from input image
and resize to required image shape.
10: Apply CLAHE.
11: end for
Figure 2: Chest X-ray preprocessing pipeline.
3.2.1 Deep Neural Models
The proposed framework is built on the predictive
framework powered by five neural models. Trans-
fer learning is employed to use pretrained weights
for the initial layers while some of the models were
trained from scratch. In the designed application,
users are provided with an interface to select and
upload X-ray images from those available on their
smartphones/system. However, there is a possibility
of them knowingly or unknowingly uploading natural
photographs. To accept only valid images, we used a
two-layered convolution network ValidateDL trained
on CIFAR10 dataset (Krizhevsky, 2012) (containing
60,000 32 ˆ 32 color natural images) and 5,000 X-
ray images to classify between natural images and X-
ray images. For every batch, randomly selected CI-
FAR10 images are converted to grayscale and copied
to all three channels. This is done to ensure that
grayscale natural images are also correctly classified
by the model.
The configuration of the network is shown in Fig-
ure3. The network is trained with stochastic gradient
descent (SGD) optimizer with learning rate of 0.01.
Batch size is set to 32 and trained for a maximum of
20 epochs. Early stopping was used to prevent over-
fitting problems. The rib cage forms a significant part
of the chest X-ray, and the rib shadow in the input
images is suppressed using a pretrained autoencoder
4
model, to ensure that the training of the classifica-
tion network focuses on relevant information within
the lungs region instead of the rib structure. The pre-
trained model is deployed during the validation phase
of the online CDSS, to generate the rib suppressed
image for all collated input images.
Figure 3: ValidateDL network architecture.
As the collated dataset includes the X-ray images
captured by different technicians using a variety of
X-ray machines, substantial variation is observed in
the area of focus. Some images included only lung
regions, while many others covered the entire abdom-
inal cavity. Also, several images from the collated
dataset included X-ray machine labels in the form
of characters/texts/numbers. To overcome variations
and to restrict the classifier for effectively learning
patterns from the lung region only, PIXGAN (Isola
et al., 2018) is trained from scratch to segment the
RoI. The number of convolution and deconvolution
layers were increased to handle the larger input im-
age size (512, 512) and a modified loss function (Son
et al., 2017) that uses a tuning parameter (λ) was in-
corporated. λ is used while summing up the discrim-
inator’s binary cross entropy loss (among predicted
and true labels) and generator’s binary cross entropy
loss (among generated and true lung masks). Thus,
the generator enables the discriminator to produce
outputs that are very similar to the real lung mask.
4
https://github.com/hmchuong/ML-BoneSuppression
HEALTHINF 2021 - 14th International Conference on Health Informatics
662
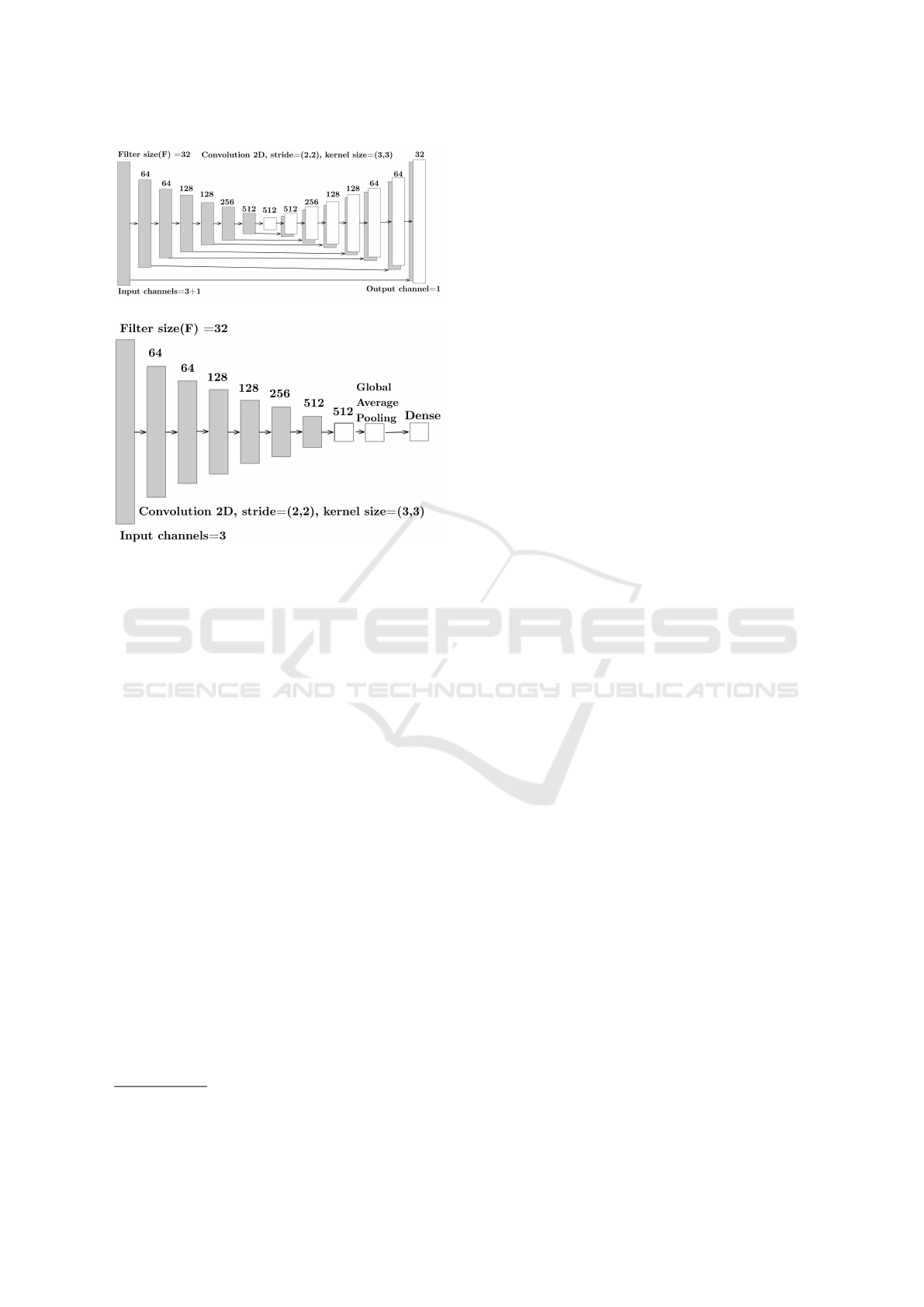
(a) Generator network configuration.
(b) Discriminator network configuration.
Figure 4: PIXGAN for lung region segmentation.
A total of 800 X-ray images and lung region
masks obtained from the Kaggle challenge
5
was used
to train the PIXGAN. The PIXGAN discriminator is
fed both X-ray (Image
xray
) and lung mask images
(Mask
lung
), which must determine whether Mask
lung
is a plausible transformation of Image
xray
, as local
style statistics are efficiently captured by PIXGAN.
The generator is built on U-Net (Ronneberger et al.,
2015) architecture and makes use of convolution and
deconvolution layers for learning to generate realis-
tic lungs mask from very few training X-ray images.
Fig. 4 depicts the configuration of PIXGAN model
which we used to segment the lung region for a given
input X-ray image. The generator is designed using
eight encoding and decoding units as shown in Fig.
4a, while the discriminator includes the complete en-
coding part of generator network with global average
pooling and final Dense layers (shown in Fig. 4b).
Once the lung region mask is generated, the original
images are cropped to include only the lung region, as
illustrated in Algorithm 1.
The preprocessed images are then trained using
deep residual network (ResNet) (He et al., 2015). We
used ResNet-18 for training the classifier to distin-
guish between COVID-19 and non-COVID-19 cases.
A content-based technique as described in Section
3.2.2 is utilized to obtain the findings in the input
5
https://www.kaggle.com/nikhilpandey360/chest-xray-
masks-and-labels
X-ray image in the form of natural language textual
report. The generated reports are pre-filled in the
CADNN framework so that the radiologists can verify
and do the changes if necessary. The collated expert
X-ray reports were classified into COVID and non-
COVID using the proposed explainable report predic-
tion deep learning model ERDX. This is mainly per-
formed to highlight the important terms in pre-filled
reports of CADNN framework. ERDX model archi-
tecture is discussed in Section 3.2.3.
3.2.2 Diagnostic Report Generation
For this task, we made use of ResNet18 for classi-
fying COVID and non-COVID cases. The last con-
volution layer output of ResNet18 provides a plausi-
ble disease representation of the input X-ray image.
A feature vector ( f eatures) is generated by summing
the last convolution layer output from the trained
ResNet18. A dictionary (D
f eatures
) of feature vec-
tors and reports indexed by image names is generated
for the collated X-ray images for which expert reports
were available. D
f eatures
is also updated with frontal
X-ray image features and textual findings obtained
from the IU dataset (Demner-Fushman et al., 2015).
In total, 820 COVID and non-COVID reports along
with corresponding frontal chest X-ray image features
were utilized for report generation and report classifi-
cation. For the given input test X-ray image, the im-
age features (Test
f eature
) are extracted during classi-
fication along with the predicted label. Cosine sim-
ilarity between Test
f eature
and features of D
f eatures
is
computed. The report is obtained using index(Isimax)
of D
f eatures
for which the maximum cosine similarity
exists between Test
f eature
and D
f eatures
rIsimaxs. The
generated reports are further processed by the X-ray
report classifier.
3.2.3 X-ray Report Classification
The expert reports consisting of physician observa-
tions contain a wealth of information regarding the
condition, symptoms and other details regarding the
patients’ status. This rich latent information can be
used to model patient representations, which can then
be leveraged to potentially screen COVID-19 infected
patients. Each report is subjected to preprocessing us-
ing standard natural language processing (NLP) tech-
niques. Any punctuation, digits and stop words are
removed from the patient’s X-ray reports. Out of vo-
cabulary(OOV) terms are handled by including spe-
cial OOV token, and the maximum allowed document
length is fixed to 100.
From the preprocessed text, embeddings are gen-
erated using the Word2Vec Continuous Bag-of-Words
COVIDDX: AI-based Clinical Decision Support System for Learning COVID-19 Disease Representations from Multimodal Patient Data
663

Figure 5: RDX X-ray report classification DL model.
(CBoW) model (Mikolov et al., 2013). The learning
rate was fixed to a default value of 0.025 (same as that
of Word2Vec model), the number of iterations was set
to 10 and embedding size used was 200. We used
Python Gensim library (
ˇ
Reh˚u
ˇ
rek and Sojka, 2010) to
generate the word embeddings using the preprocessed
X-ray reports. As the main purpose of NLP classifier
was to highlight important terms in the reports, we
designed a convolutional attention explainable neu-
ral network (RDX) to classify the X-ray reports. The
model consists of a 1D convolution layer followed by
an attention and a dense layer, which is depicted in
Figure 5. All the reports are padded up to a maxi-
mum length of the reports in a batch, 100 being the
maximum allowed length for a report. Given the test
report, which is generated for the input X-ray image
using the content-based approach, it is classified by
the RDX model. The important terms contributing
to the model’s prediction are highlighted using color
codes. This feature is also used to enable faster verifi-
cation features for experts through the functionalities
provided through the CADNN interface, and to up-
date/edit findings if necessary.
4 EXPERIMENTAL RESULTS
The proposed deep neural approaches were developed
using Pytorch (Paszke et al., 2019) and Tensorflow
(Abadi et al., 2016) deep learning python frameworks
and trained on Ubuntu 18.04 system with NVIDIA
Tesla M40 and Tesla V100-DGXS. All the test data
splits are made before image augmentation. Accu-
racy is used as an evaluation metric for verification
of classification results, which is calculated based on
true positives (T P), false positives (FP), true nega-
tives (T N) and false negatives (FN) cases predicted
by a particular neural model, and is given by Eq. (1).
Here, T P is the number of cases that are correctly
identified by the prediction model to be COVID-19
positives, which match with experts’ opinion, while
FN are incorrectly rejected cases. T N is the number
of correctly identified non-COVID-19 cases, and FP
gives the total incorrectly identified COVID-19 cases.
Accuracy “
T P ` T N
pT P ` FP ` FN ` T Nq
(1)
The ValidateDL model is evaluated on the CIFAR-
10 test set and 450 test X-ray images from RSNA
challenge data. The model was able to identify all
X-ray images correctly and achieved 97.8% accuracy.
Out of the 10,000 CIFAR-10 images, 221 images that
included only cloudy sky or runway images (which
appear similar to X-ray image structure in 32 x 32 di-
mension) were wrongly classified as X-ray images in-
stead of natural images. For training PIXGAN, batch
size of 32, λ value of 0.5 and Adam optimizer with
0.0002 learning rate is used. Training is performed
for a maximum of 100 epochs, and the PIXGAN was
evaluated on 50 test images out of 800 X-ray images.
The generator model that achieved the highest dice
coefficient (Zijdenbos et al., 1994) (obtained at 28
th
epoch on validation data) was used to extract lung
mask regions for the collated data.
Next, for testing the ResNet18 X-ray image clas-
sification model, 80% of input data for training, 10%
for validation and rest 10% was considered. The pre-
dicted and actual class for the X-ray images are sum-
marized in the confusion matrix shown in Fig. 6a.
An accuracy of 97% was achieved using the proposed
X-ray image classification model. The RDX model
was evaluated on the 20% test split, and accuracy of
96.74% was observed. The results are summarized in
the confusion matrix depicted in Fig. 6b.
(a) Chest X-ray dataset.
(b) Diagnostic report dataset.
Figure 6: Confusion matrix for different datasets.
In addition to experimental benchmarking with
standard datasets, a pilot study was conducted with
patient data collected from a COVID ward in a pri-
vate hospital. The experiments were performed on a
HEALTHINF 2021 - 14th International Conference on Health Informatics
664
collection of 95 COVID and 5 non-COVID (Bacte-
rial/Tubercular lung infection) X-ray images obtained
from patients admitted for screening/treatment at the
hospital. The results were very promising, as the
proposed CADNN framework performed very well
on this real-world data. Healthcare professionals in-
volved in the pilot study used the CADNN framework
for uploading patient data and observing the predic-
tions of the model. In these studies, the system iden-
tified all COVID-19 cases accurately, while only one
non-COVID case was wrongly predicted as COVID,
achieving an overall prediction accuracy of 99%.
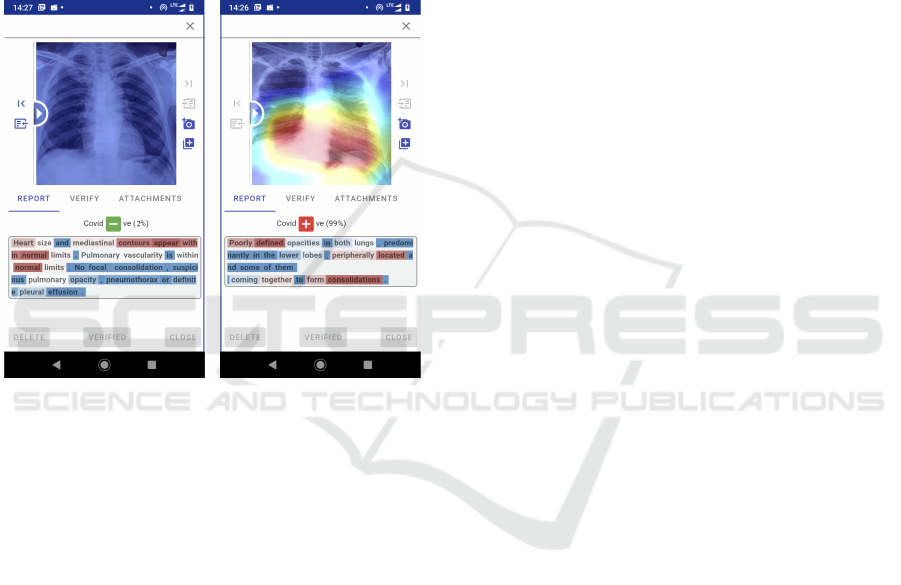
Figure 7: Proposed CDSS in action.
Qualitative Evaluation. For enabling evidence-
based diagnosis, we utilised Class Activation Map-
ping (Grad-CAM) (Selvaraju et al., 2017) to highlight
the regions of the input X-ray image that the classifi-
cation model considered relevant to perform the pre-
diction. The visualization is shown in the CADNN
framework to aid the clinical decision. The regions
in the image, where this gradient is predominant, are
shown in Fig. 7), along with the generated report that
shows the highlighted important terms. As can be ob-
served from the attentions, the model has successfully
learnt important features in the X-ray image, restrict-
ing itself mostly to within the lung region. The im-
portant radiography terms are identified (highlighted
with white and red colors) by RDX.
5 CONCLUSION & FUTURE
WORK
In this paper, a cost-effective, early-screening strat-
egy for COVID-19 diagnosis based on chest X-ray
images and expert-written diagnosis reports was pre-
sented. The proposed framework has been deployed
as a web-based CDSS called CADNN. The input im-
ages were subjected to extensive validation and pre-
processing steps to eliminate variance and ensure ef-
fective learning by the prediction model. Prepro-
cessed images were used to train a ResNet model
for COVID-19, Pneumonia or non-COVID-19 clas-
sification and the findings obtained from the images
were used to automatically generate the natural lan-
guage diagnosis reports, using content-based learn-
ing approach. The proposed CADNN also includes
feedback mechanisms so that the results could be ver-
ified by the experts, and feedback from experts can
be utilised during offline retraining of the models.
It allows users to upload additional documents like
CT scan images/DICOM sequences for additional in-
sights into the patient’s condition.
The proposed framework could be easily adapted
for diagnosis of other lung related diseases and pro-
vide a comprehensive CDSS support to medical pro-
fessionals. As part of future work, we aim to ex-
periment with CT-scan images for potential improve-
ments in performance, so that the final prediction is
generated based on an ensemble of three classifiers
that make use of X-ray, CT and radiography text re-
ports. We also intend to benchmark the performance
of the proposed method over existing solutions in
terms of both interpretability and accuracy.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the computa-
tional resources made available through the Google
Cloud COVID-19 Research Grant, awarded to the
third author in July 2020.
REFERENCES
Abadi, M., Barham, P., Chen, J., et al. (2016). Tensor-
flow: A system for large-scale machine learning. In
12th USENIX Symposium on Operating Systems De-
sign and Implementation (OSDI 16), pages 265–283.
Abbas, A., Abdelsamea, M., and Gaber, M. (2020). Classi-
fication of covid-19 in chest x-ray images using detrac
deep convolutional neural network. Applied Intelli-
gence, pages 1–11.
BusinessToday (2020). Coronavirus testing in India.
Chung, A., Wang, L., et al. (2020). Actualmed COVID-19
Chest X-ray Dataset Initiative .
Cohen, J. P., Morrison, P., et al. (2020). COVID-19 image
data collection. arXiv 2006.11988.
COVIDDX: AI-based Clinical Decision Support System for Learning COVID-19 Disease Representations from Multimodal Patient Data
665

Dansana, D., Kumar, R., et al. (2020). Early diagnosis of
covid-19-affected patients based on x-ray and com-
puted tomography images using deep learning algo-
rithm. Soft Computing.
Demner-Fushman, D., Kohli, M., et al. (2015). Preparing
a collection of radiology examinations for distribution
and retrieval. JAMIA, 23.
Deng, J., Dong, W., et al. (2009). ImageNet: A Large-Scale
Hierarchical Image Database. In CVPR09.
Forthmann, P. and Pfleiderer, G. (2019). Augmented dis-
play device for use in a medical imaging laboratory.
US2018197337 Google Patents.
Gangavarapu, T., Krishnan, G. S., Kamath, S., and Je-
ganathan, J. (2020). Farsight: Long-term disease
prediction using unstructured clinical nursing notes.
IEEE Transactions on Emerging Topics in Computing.
Ghoshal, B. and Tucker, A. (2020). Estimating un-
certainty and interpretability in deep learning for
coronavirus (COVID-19) detection. arXiv preprint
arXiv:2003.10769.
Gonzalez, R. C. and Woods, R. E. (2008). Digital image
processing. Prentice Hall, Upper Saddle River, N.J.
Hall, L. O., Paul, R., Goldgof, D. B., and Goldgof, G. M.
(2020). Finding covid-19 from chest x-rays using
deep learning on a small dataset. arXiv e-prints, page
arXiv:2004.02060.
He, K., Zhang, X., et al. (2015). Deep residual learning for
image recognition.
Hemdan, E.-D., Shouman, M., and Karar, M. E. (2020).
Covidx-net: A framework of deep learning classi-
fiers to diagnose covid-19 in x-ray images. arXiv
preprint:2003.11055.
Isola, P., Zhu, J.-Y., Zhou, T., and Efros, A. A. (2018).
Image-to-image translation with conditional adversar-
ial networks.
Krizhevsky, A. (2012). Convolutional deep belief networks
on cifar-10.
Max Roser, Hannah Ritchie, E. O.-O. and Hasell, J. (2020).
Coronavirus pandemic (COVID-19). Our World in
Data.
Mei, X., Lee, H.-C., et al. (2020). Artificial intelli-
gence–enabled rapid diagnosis of patients with covid-
19. Nature Medicine, 26:1–5.
Mikolov, T., Chen, K., Corrado, G., and Dean, J. (2013).
Efficient Estimation of Word Representations in Vec-
tor Space. arXiv e-prints, page arXiv:1301.3781.
Mishra, A., Das, S., et al. (2020). Identifying covid19
from chest ct images: A deep convolutional neural
networks based approach. Journal of Healthcare En-
gineering, 2020:1–7.
Narin, A., Kaya, C., and Pamuk, Z. (2020). Automatic de-
tection of coronavirus disease (COVID-19) using x-
ray images and deep convolutional neural networks.
arxiv preprint:2003.10849.
Ozturk, T., Talo, M., et al. (2020). Automated detection
of COVID-19 cases using deep neural networks with
x-ray images. Computers in Biology and Medicine.
Paszke, A., Gross, S., Massa, F., et al. (2019). Pytorch:
An imperative style, high-performance deep learning
library. In Advances in Neural Information Processing
Systems 32.
Radiological Society of North America (2018). Rsna pneu-
monia detection challenge. https://www.kaggle.com/
c/rsna-pneumonia-detection-challenge. Accessed:
2020-08-11.
Rahman, T. (2020). COVID-19 radiography database.
ˇ
Reh˚u
ˇ
rek, R. and Sojka, P. (2010). Software Framework for
Topic Modelling with Large Corpora. In LREC 2010
Workshop on New Challenges for NLP Frameworks.
ELRA.
Reza, A. (2004). Realization of the contrast limited adap-
tive histogram equalization (clahe) for real-time image
enhancement. VLSI Signal Processing, 38:35–44.
Ronneberger, O., Fischer, P., and Brox, T. (2015). U-
net: Convolutional networks for biomedical im-
age segmentation. In Medical Image Computing
and Computer-Assisted Intervention – MICCAI 2015,
pages 234–241. Springer.
Scheib, S. (2009). Dosimetric end-to-end verification
devices, systems, and methods. US 20150085993
Google Patents.
Selvaraju, R., Cogswell, M., et al. (2017). Grad-cam: Vi-
sual explanations from deep networks via gradient-
based localization. In IEEE international conference
on computer vision, pages 618–626.
Son, J., Park, S. J., and Jung, K.-H. (2017). Retinal Ves-
sel Segmentation in Fundoscopic Images with Gen-
erative Adversarial Networks. arXiv e-prints, page
arXiv:1706.09318.
Tushaar, G., Jayasimha, A., Krishnan, G. S., and Kamath,
S. (2020). Predicting icd-9 code groups with fuzzy
similarity based supervised multi-label classification
of unstructured clinical nursing notes. Knowledge-
Based Systems, 190:105321.
Zijdenbos, A., Dawant, B., Margolin, R., and Palmer, A. C.
(1994). Morphometric analysis of white matter lesions
in mr images: method and validation. IEEE transac-
tions on medical imaging, 13 4:716–24.
HEALTHINF 2021 - 14th International Conference on Health Informatics
666
