
3D Fetal Face Reconstruction from Ultrasound Imaging
Ant
`
onia Alomar
1 a
, Araceli Morales
1 b
, Kilian Vellv
´
e
2 c
, Antonio R. Porras
3,4,5 d
,
Fatima Crispi
2 e
, Marius George Linguraru
3,6 f
, Gemma Piella
1 g
and Federico Sukno
1 h
1
Department of Information and Communications Technologies, Universitat Pompeu Fabra, Barcelona, Spain
2
Fetal Medicine Research Center (BCNatal), Hospital Cl
´
ınic and Hospital Sant Joan de D
´
eu, Universitat de Barcelona,
Barcelona, Spain
3
Sheikh Zayed Institute for Pediatric Surgical Innovation, Children’s National Hospital, Washington, D.C., U.S.A.
4
Department of Biostatistics and Informatics, Colorado School of Public Health,
University of Colorado Anschutz Medical Campus, Aurora, CO, U.S.A.
5
Department of Pediatrics, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, U.S.A.
6
Departments of Radiology and Pediatrics, School of Medicine and Health Sciences, George Washington University,
Washington, D.C., U.S.A.
Keywords:
Craniofacial Morphology, 3D Morphable Model, Facial Dysmorphology, Fetal Reconstruction, Prenatal
Diagnosis.
Abstract:
The fetal face contains essential information in the evaluation of congenital malformations and the fetal brain
function, as its development is driven by genetic factors at early stages of embryogenesis. Three-dimensional
ultrasound (3DUS) can provide information about the facial morphology of the fetus, but its use for prenatal
diagnosis is challenging due to imaging noise, fetal movements, limited field-of-view, low soft-tissue contrast,
and occlusions. In this paper, we propose a fetal face reconstruction algorithm from 3DUS images based on
a novel statistical morphable model of newborn faces, the BabyFM. We test the feasibility of using newborn
statistics to accurately reconstruct fetal faces by fitting the regularized morphable model to the noisy 3DUS
images. The algorithm is capable of reconstructing the whole facial morphology of babies from one or several
ultrasound scans to handle adverse conditions (e.g. missing parts, noisy data), and it has the potential to aid
in-utero diagnosis for conditions that involve facial dysmorphology.
1 INTRODUCTION
Craniofacial malformations that occur because of ab-
normal development comprise over one third of all
congenital (i.e., birth) defects (Mossey and Catilla,
2001). These anomalies comprise a wide range of
heterogeneous conditions and often have a multifac-
torial origin, including genetic and environmental fac-
tors (S
,
orop Florea et al., 2018). These malforma-
tions can impact swallowing, breathing, hearing, vi-
sion, speech, and cognitive development (on Gov-
ernment Affairs, 2020; EvansAnne et al., 2018), and
a
https://orcid.org/0000-0003-3658-5832
b
https://orcid.org/0000-0003-4930-6142
c
https://orcid.org/0000-0002-2376-7664
d
https://orcid.org/0000-0001-5989-2953
e
https://orcid.org/0000-0002-7422-5240
f
https://orcid.org/0000-0001-6175-8665
g
https://orcid.org/0000-0001-5236-5819
h
https://orcid.org/0000-0002-2029-1576
they impose a large psychosocial, healthcare, and eco-
nomic burden.
Early diagnosis is often crucial for the effective
treatment of functional and developmental aspects
(Learned-Miller et al., 2006; Tu et al., 2018; Tu et al.,
2019). However, not all syndromes are easily identi-
fied, some of them having subtle physical manifesta-
tions; careful clinical assessment may be necessary to
distinguish an isolated abnormality from an atypical
or mildly manifested syndrome. Moreover, the iden-
tification of the specific syndrome is important for the
overall care of the patient (EvansAnne et al., 2018). In
this sense, the analysis of facial morphology can pro-
vide relevant information and serve as a pre-screening
tool, facilitating the early detection of developmen-
tal disorders (Menezes et al., 2016; Merz and Welter,
2005). Efforts are being made to shift from diagno-
sis at birth, or during the first years of life, to prenatal
diagnosis, which facilitates parents’ counselling and
careful planning of delivery and postnatal treatment
(Pooh and Kurjak, 2011). However, prenatal diag-
Alomar, A., Morales, A., Vellvé, K., Porras, A., Crispi, F., Linguraru, M., Piella, G. and Sukno, F.
3D Fetal Face Reconstruction from Ultrasound Imaging.
DOI: 10.5220/0010340306150624
In Proceedings of the 16th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2021) - Volume 4: VISAPP, pages
615-624
ISBN: 978-989-758-488-6
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
615

nosis of fetal syndromes is not easy, mainly because
of the wide range of morphological features involved
and the challenging nature of medical images.
Ultrasound is the primary imaging modality for
fetal assessment. It is a noisy image modality, but
it has the advantage of being widely available, cost-
effective, non-ionizing, and it allows real-time exam-
ination. Three-dimensional ultrasound (3DUS) facil-
itates the evaluation of anatomical structures from the
facial surface and can therefore aid diagnosis (An-
dresen et al., 2012; Werner et al., 2016; Merz and
Abramowicz, 2012). A detailed 3D model of the fetus
face could thus play a crucial role in prenatal diagno-
sis.
Little research has been done in 3D face recon-
struction from fetal images, mainly due to the limita-
tions of prenatal imaging itself. In (Dall’Asta et al.,
2017), it was presented a statistical shape model con-
structed from 20 3DUS scans that were manually seg-
mented and aligned, and statistically significant dif-
ferences in face shape were found between normal
and abnormal fetuses. There are some works on gen-
erating physical fetal models (although not always
face specific) from 3DUS, magnetic resonance imag-
ing, and computer tomography (Werner et al., 2010;
Werner et al., 2015; Menezes et al., 2016; Speranza
et al., 2017). However, they involve slice-by-slice
manual segmentation and post-processing with pro-
prietary software.
In this paper, we explore the feasibility of recon-
structing the facial morphology before birth by ana-
lyzing 3DUS images of the fetus from routine scan-
ning with the help of a recently proposed statisti-
cal model constructed from 3D scans of babies and
newborns: the Baby Face Model (BabyFM) (Morales
et al., 2020). Differently from previous works, we do
not build our model directly from the noisy fetal im-
ages, but employ statistics from newborns to constrain
the geometric reconstruction of the fetal face. In this
way, we circumvent the difficulties associated with
building accurate models from the noisy 3DUS im-
ages. Tests on a small set of fetal scans show promis-
ing results in both qualitative and quantitative terms,
even in adverse conditions (e.g. missing parts, noisy
data).
2 MATERIALS
2.1 3D Baby Face Morphable Model
A 3D morphable model (3DMM) is a tool for repre-
senting 3D shapes and textures. In the context of face
analysis, the idea is to learn a general 3D face model
that is able to encode the statistics of facial shape. A
crucial aspect to consider when using a 3DMM is that
the statistics encoded in the model must match those
of the target population, e.g., in terms of ethnicity,
gender and, especially important for this work, age.
The latter has been an important obstacle for the ap-
plication of pre-existing 3DMMs to fetal data, since
all available 3DMMs were built from adults and, al-
though sometimes they also included children, none
of them included babies. However, very recently,
Morales et al. (Morales et al., 2020) have published
the Baby Face Model (BabyFM), which constitutes
the first 3DMM built exclusively from babies, includ-
ing an important proportion of data from newborns.
The BabyFM was built from 45 3D scans of baby
faces (mean age 8.42 ±6.45 months). Several ethnici-
ties were included: Caucasian (47%), African Ameri-
can (24%), Hispanic (20%), and Asian (9%). Also,
the data were roughly gender-balanced: 56% male
and 44% female. The BabyFM covers the facial re-
gion that is delimeted by the chin the forehead and
the ears, all included. Additionally, the vertice indices
for 23 anatomical landamrks (Figure 1) are provided,
which are used to initialise the 3D facial reconstruc-
tion (see Section 3.1.1).
Figure 1: Anatomical landmarks. Illustration of the 23
anatomical landmarks considered in this project on the
mean baby morphable model face. Landmark abbrevia-
tions: enL/R = endocanthion Left/Right; n = nasion; exL/R
= exocanthion Left/Right; aL/R = alare Left/Right; acL/R
= alar crest Left/Right; prn = pronasale; sn = subnasale;
chL/R = cheilion Left/Right; cphL/R = crista philtrum
Left/Right; ls = labiale superius; li = labiale inferius; sl =
sublabiale; pg = pogonion; tL/R = tragion Left/Right; and
oiL/R = otobasion inferius Left/Right.
2.2 Test Database
To evaluate our methods, 10 3DUS scans from 4 fe-
tuses were collected, i.e., there were multiple 3DUS
images for each of fetus, corresponding to different
VISAPP 2021 - 16th International Conference on Computer Vision Theory and Applications
616

viewing directions. These fetuses had no relation to
any of the babies used for the construction of the
BabyFM. The 3DUS scans were obtained at Hospi-
tal Cl
´
ınic and Hospital Sant Joan de D
´
eu, Barcelona,
according to its Ethical Research Committee and the
current legislation. Images were acquired using a
General Electric Voluson E6 (General Electric, IL,
USA) US machine with a low-frequency probe (4-8
MHz).
Three-dimensional meshes were extracted using
just a threshold segmentation. The meshes contained
not only the face but also other parts of the fetus’
body, placenta, and noise. Using the Landmark soft-
ware 3.6
1
, we positioned a subset from the 23 tar-
geted landmarks in the BabyFM (see Figure 1) on
each fetal scan, according to their visibility. The iden-
tification of these anatomical landmarks in the fetal
scan is challenging because of the occlusions (e.g.,
the baby may be positioned with the hand on the face)
and the noisy nature of the data, and therefore not all
of them could be positioned for each fetus.
Additionally to the 3DUS scans, for each of the
babies we had three 2D postnatal photographs taken
from different viewpoints by the parents with their
mobile phones. These images were used to obtain a
2D-3D reconstruction of the baby face to which we
could quantitatively compare with the reconstruction
obtained from the US scans (see Section 3.2). This
simple setup was aimed to avoid having to scan new-
born babies with special equipment.
3 METHODS
Our data processing pipeline consists of two main
stages (Figure 2): 3DUS fitting and multiple image
fitting.
3.1 3D Ultrasound Fitting
First, the 3D reconstruction of the fetal face is ob-
tained from the 3DUS images by fitting the BabyFM
to it, i.e., finding the shape parameters in the 3DMM
that best reproduce the face observed in the US. At
this stage, the BabyFM works as a statistical regular-
izer allowing a better robustness to noise and other
artifacts.
3.1.1 Landmark-based Fitting
A first estimation of the 3D fetal face is obtained
considering only the landmarks positioned in the US
1
https://landmark2.software.informer.com/download/
mesh. For this, an iterative procedure consisting of (1)
landmark alignment and (2) shape parameter calcula-
tion is performed. In the landmark alignment stage,
Procrustes analysis is used to find a similarity trans-
formation to fit the mean face shape of the BabyFM to
the US landmarks. Then, the shape parameters α that
best define the fetal face in the US scan are estimated
by first solving the normal equation:
α = (Φ
T
r
Φ
r
)
−1
Φ
T
r
(x − ¯x), (1)
where Φ
r
is the reduced shape basis matrix (i.e., the
rows of the eigenvector matrix that correspond to the
landmarks), and then regularizing to ensure obtain-
ing plausible faces. The shape parameters are as-
sumed to follow a multivariate Gaussian distribution.
Therefore, we constrain the shape parameter vectors
to lie within a hyper-ellipsoid in the parameter space,
the size of which is determined by the variances (the
eigenvalues) of the data.
The two-stage landmark-based fitting is iterated
20 times to ensure convergence. Finally, the mean
approximation error (
¯
E) between the landmarks of the
fitted morphable face model and those of the US mesh
is calculated as follows:
¯
E =
1
m
m
∑
j=1
||l
us, j
− T (l
model, j
)||,
(2)
where l
j
∈ R
3
is the j-th landmark, T is the transfor-
mation that maps the BabyFM mean to the US, and
m is the number of anatomical landmarks that were
positioned in the US mesh.
3.1.2 Iterative Closest Point with Statistical
Constraints
The fetal face reconstruction obtained from the
landmark-based fitting is refined using an iterative
closest point (ICP) algorithm. In every iteration, the
ICP algorithm fits the face reconstruction to the 3DUS
mesh and then recovers the model’s shape parameter
α, analogously to the landmark-based fitting (i.e., by
Eq. 1 followed by regularization) but now using the
whole surface, i.e., all rows of the shape basis Φ in-
stead of just Φ
r
. To increase the robustness to input
artifacts, the point-matching was applied under geo-
metric and uniqueness constraints (to minimize the
impact of outliers and ensure one-to-one mapping).
The ICP with statistical constraints was repeated by
alternating between the correspondence mapping and
the model’s parameter update followed by statistical
regularization, until the error difference between con-
secutive iterations was below a predefined threshold.
In this work, this convergence threshold was set to
10
−2
mm.
3D Fetal Face Reconstruction from Ultrasound Imaging
617

Figure 2: Proposed pipeline: 3DUS fitting to obtain the fetal face and multiple image fitting to obtain the baby face after birth.
3.2 Multiple Image Fitting
In order to quantitatively validate the fetal shapes esti-
mated from the 3DUS scans, we reconstruct the new-
born 3D face from a set of three 2D images (frontal,
left, and right pose) taken shortly after birth. The
BabyFM is used here to estimate the facial 3D ge-
ometry of the newborn. Once the 3D geometry is ob-
tained, facial texture can also be added to obtain a
photo-realistic 3D reconstruction.
We address the 3D-from-2D reconstruction prob-
lem using sparse geometric features (edges and land-
marks). Our approach is based on the algorithm pro-
posed in (Bas et al., 2016), but using multiple images
rather than only a single image. We start by position-
ing the anatomical landmarks in the different images
using a 2D landmarker and obtaining the edges by ap-
plying the Canny edge detector.
Then, an initialization of the 3D face is obtained
using only the landmarks. The landmark fitting is then
refined in an iterative closest point manner by finding
the closest image edge to each model contour vertex.
The model edge vertices can then be considered as
landmarks with known 2D position, for which opti-
mal pose and shape estimates can be easily computed
under the assumption of a scaled orthographic projec-
tion. In particular, we obtain the optimal pose and
shape parameter by minimizing an objective function
that include landmark, edge, and prior terms:
E(α, R, t, s) = w
1
E
lmk
(α, R, t, s)+
+ w
2
E
Edge
(α, R, t, s) + w
3
E
Prior
(α), (3)
where α is the shape parameters vector and R, t, and s
the pose parameters (rotation, translation, and scale)
assuming a scaled orthographic projection. The pa-
rameters w
1
, w
2
and w
3
correspond to the weights
given to each error term, the sum of the three weights
should be equal to one. The used values to perform
the reconstructions were 0.25, 0.25, 0.50 respectively.
The landmark term penalizes differences between the
actual landmarks positions on the images and the ones
obtained by projecting the 3D model landmarks. The
edge term compares the edges detected on the image
with those induced by the model due to occluding
boundaries. The prior term acts as a regularizer of
the shape parameters based on the statistics encoded
in the BabyFM.
4 RESULTS
4.1 US Fitting
We applied our reconstruction pipeline to each of the
fetuses scans. Figure 3 shows the US images ob-
tained from the Voluson system (GE Healthcare), the
US meshes obtained after the threshold segmentation,
and the 3D reconstruction that we obtained. As can
be observed in Figure 3, the input data is quite chal-
lenging. For example, in most of the 3DUS images,
the ears are not present or are extremely noisy. Never-
theless, our method is able to estimate an approximate
ear shape by exploiting the geometric correlations en-
VISAPP 2021 - 16th International Conference on Computer Vision Theory and Applications
618
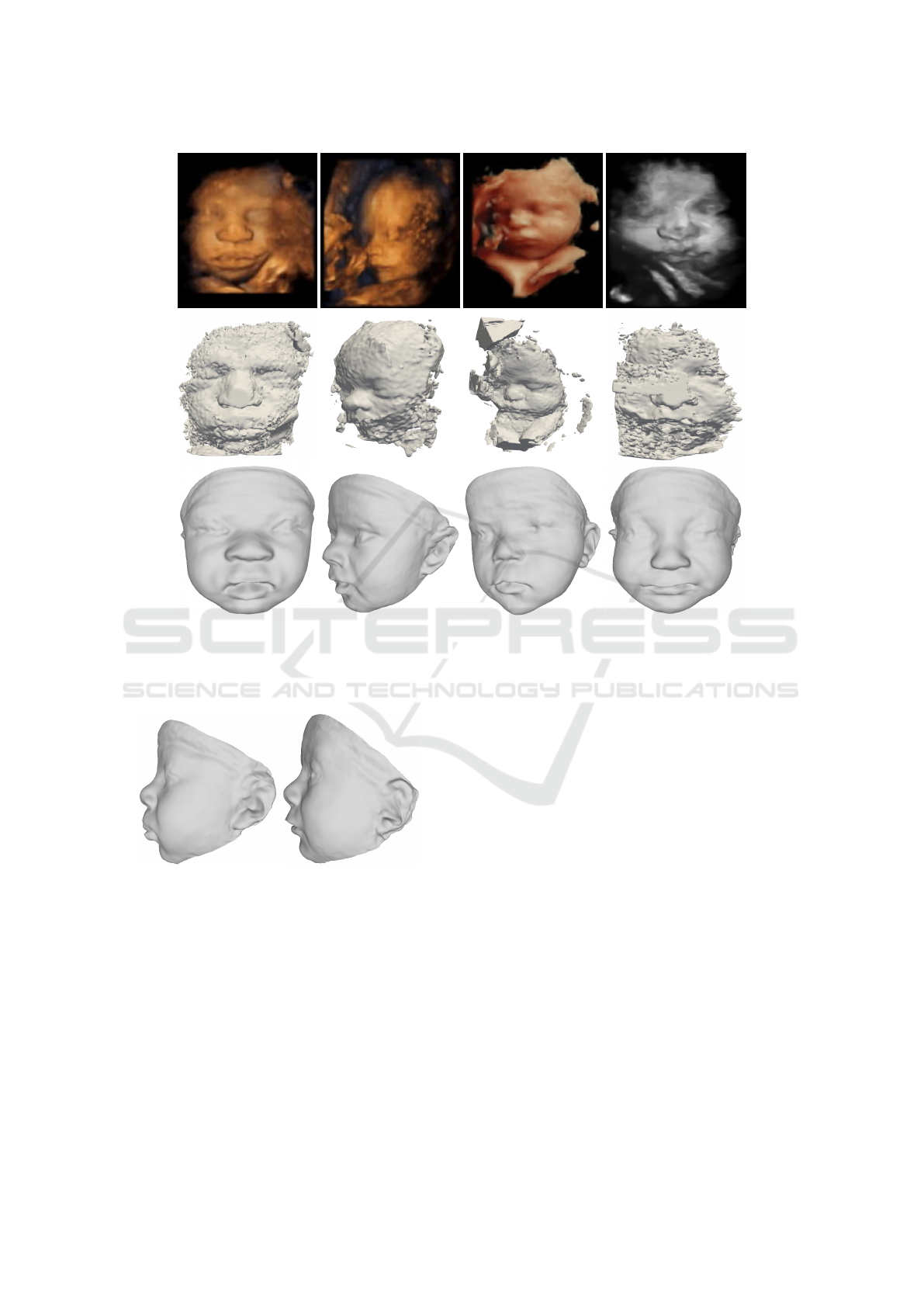
Figure 3: US images (top), US meshes (middle), and their corresponding reconstructions (bottom). From left to right: case 1,
case 2, case 3, and case 4.
coded in the BabyFM. Figures 4 displays the profile
view of cases 1 and 2 to show the ears.
Figure 4: Example of reconstructions (left: case 1, right:
case 2) in profile view.
4.1.1 Single US Fitting vs Multiple US Fitting
As we have multiple 3DUS scans from different views
for each individual, we checked whether the recon-
structions obtained independently for the same fetus
were similar. Also, we investigated whether the si-
multaneous use of multiple views improves the ob-
tained results. For this, the algorithm was adapted to
merge the correspondences of each 3DUS view and
then calculate a unique vector of shape parameters for
the multiple scans.
Figure 5-6 show examples of reconstructions ob-
tained independently for the same individual (cases 1
and 2, respectively) from two different 3DUS views.
In Figure 5, we can appreciate that the fetus has plump
lips (African ethnicity) and the model is not able to
full reconstruct them accurately, but it attempts to
compensate this fact by opening the mouth. The used
BabyFM was built including only a 24% of African
American babies, which might explain why it is not
able to perfectly reconstruct the lips. Nevertheless,
the nose, eyes, and pose are well estimated, and the re-
constructions are convincingly similar to the original
renderings. More importantly, there are no substan-
tial differences between the reconstructions obtained
form the two 3DUS scans of this same fetus; the sec-
ond one has wider cheeks, but the nose, mouth, and
eyes are similar. In Figure 6 (which corresponds to
a case of fetal growth restriction (Peleg et al., 1998;
Albu et al., 2014)), the obtained reconstructions cap-
ture the skinny face. On the other hand, the second
US has part of the mouth and chin occluded by an
arm, which makes the reconstruction more challeng-
ing.
Next, we reconstruct the face considering simulta-
neously the multiple US scans for each fetus. This can
3D Fetal Face Reconstruction from Ultrasound Imaging
619
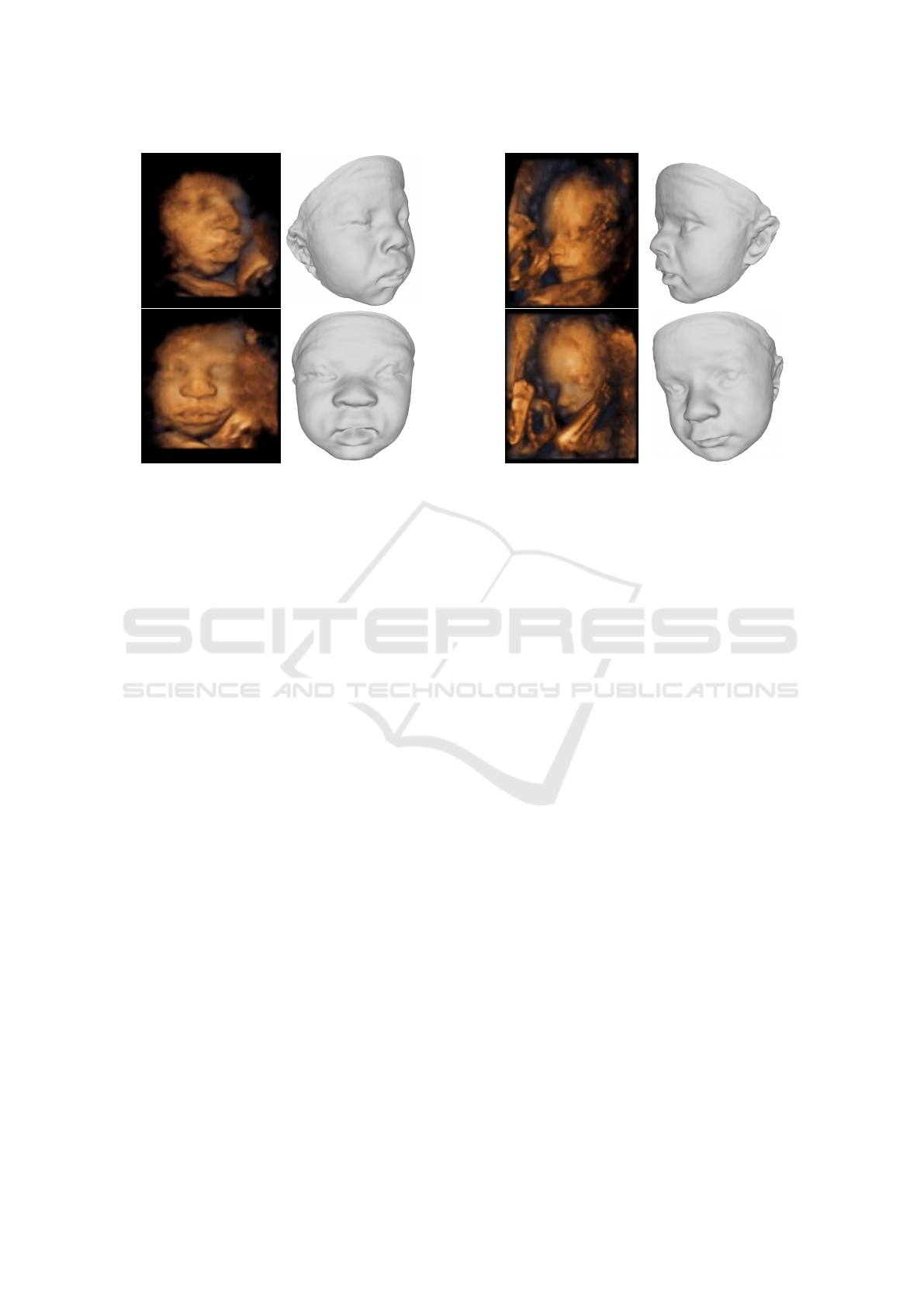
Figure 5: Reconstructions from different views for case 1.
Figure 6: Reconstructions from different views for case 2.
be especially useful to obtain an accurate complete
reconstruction even when each scan provides only a
partial view of the facial anatomy. Figure 7 illustrates
this by reconstructing the face for case 3 from 4 dif-
ferent views.
4.2 Post-natal Fitting from Multiple
Images
Since we wish to use the post-natal reconstructions
to validate the geometries reconstructed from the
3DUS scans, it is important to assess the accuracy
that we can expect in the post-natal reconstruction.
To do so, we reconstructed the baby faces of an ad-
ditional dataset, which contained three 2D images
(frontal, right and left pose), as well as the 3D scan
for each of the three babies from the Children’s Na-
tional Hospital, Washington D.C. and one newborn
from the Hospital Cl
´
ınic and Hospital Sant Joan de
D
´
eu, Barcelona. To evaluate the quality of the recon-
structed 3D face, we compute the geometric error for
each reconstruction, by first applying a transforma-
tion to align it with the ground-truth, which in this
case is the 3D scan, and then computing its point-to-
point distance.
The color error maps of the three babies in Figure
8 show that the 2D-3D fitting performed obtained ac-
ceptable results, as the mean error of the reconstruc-
tions is around 4 mm. In the three cases, the large
errors in the forehead are caused by the lack of land-
marks in this region and the black hat that the babies
wear, as it interferes with the image edge detection
resulting in wrong correspondences. However, no-
tice that the error in the rest of the face, which cor-
responds to the main facial features, is considerably
smaller. This, together with the fact of using uncali-
brated input images, suggests that the accuracy of the
reconstructions is satisfactory.
Once we have obtained the 3D reconstruction
from multiple 2D images, we add texture to it to have
more photo-realistic results to show to the parents. As
expected, the textured meshes improve the visual re-
semblance with the images (bottom row of Figure 8).
4.3 Pre- and Post-natal Comparison
The reconstructions obtained by fitting the BabyFM
to the 3DUS scan and to the set of 2D images after
birth are compared quantitatively, using color error
maps, as a proxy to evaluate the performance of the
fetal face reconstruction. The color error maps are
obtained computing the geometric error between the
prenatal and the postnatal reconstructions.
We compared the reconstructions from the 3DUS
fitting and the multiple image fitting for case 3, for
which we had both the postnatal 2D images and the
US scans, even if the difference between them was 16
weeks. Figure 9 shows the 2D frontal image of the
baby, the baby face reconstruction using the multiple
image fitting, the US fetal face obtained, and the color
map error between the prenatal and the postnatal re-
construction. Although no age correction was applied
to the 3DUS reconstruction, we can appreciate suffi-
cient resemblance to support the statement these are
two instances of the same baby, taken a few months
apart from each other.
In quantitative terms, the overall mean reconstruc-
tion error is 1.73 mm. Main facial features such as
eyes, nose, and mouth present the smallest reconstruc-
VISAPP 2021 - 16th International Conference on Computer Vision Theory and Applications
620
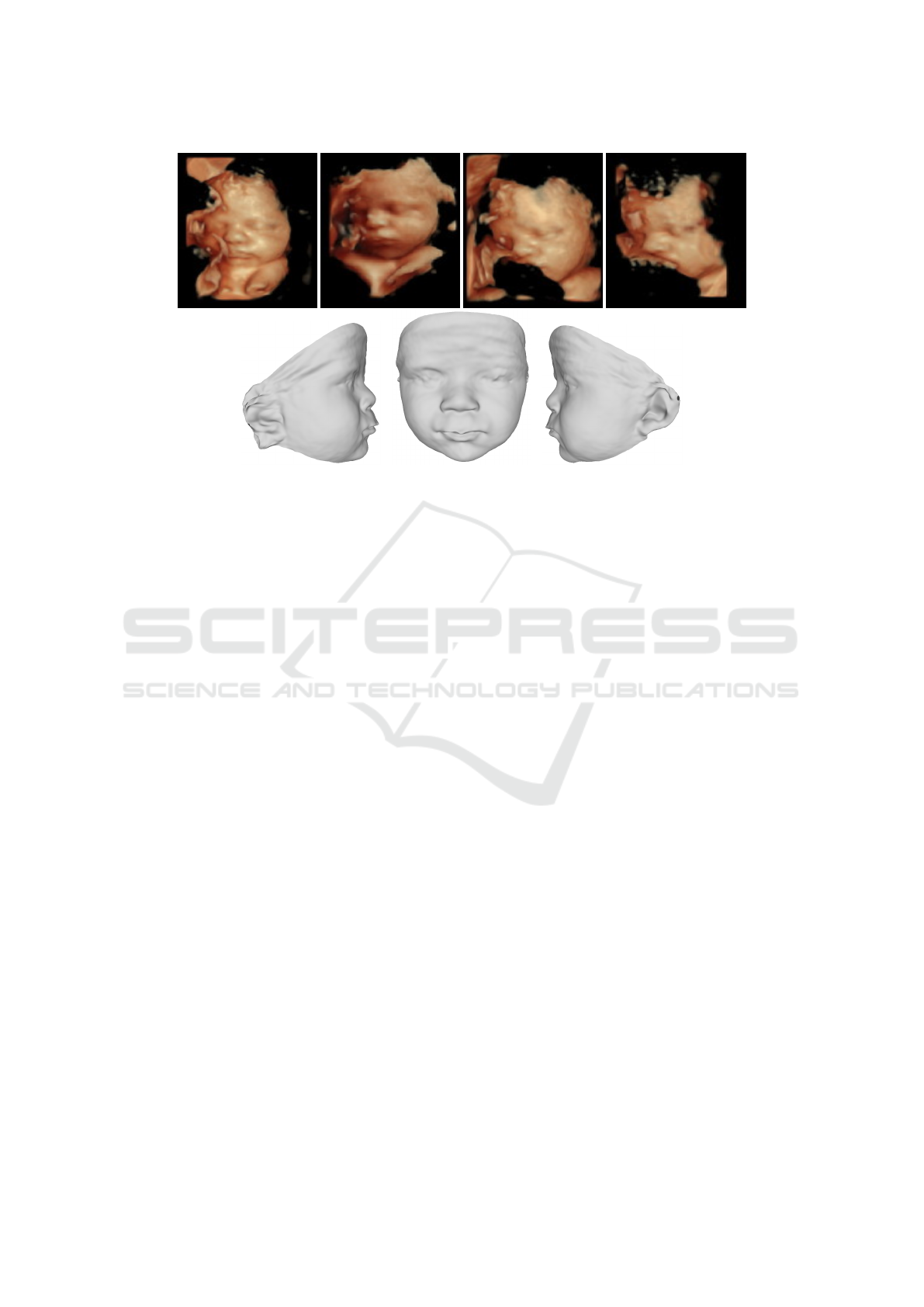
Figure 7: US reconstruction from multiples views for case 3. Top: US images. Bottom: single reconstruction shown from
different views.
tion error (see Figure 9), whereas the forehead region
-where no landmarks and few edges are available-
shows the largest reconstruction error. It is expected
that after applying some age correction mechanism,
the resemblance will be even stronger.
5 DISCUSSION
In this study, our aim is to obtain accurate fetal face
reconstructions from 3DUS images in the prenatal
stage in order to assess craniofacial morphology as
early as possible. In spite of the growing interest in
the early assessment of craniofacial morphology, the
3D renderings provided by the standard clinical view
software have some limitations, e.g., two images from
different views seems to be two different babies, or
the facial features cannot be distinguished due to the
smoothing or the large amount of noise. For this rea-
son, clinicians usually have to manually remove the
noise to assess the baby’s morphology. Manual seg-
mentation to remove the noise in the US is very time
consuming, even for expert clinicians. The presented
algorithm can help them to visualize the fetal face
with more quality and without performing any manual
segmentation. Therefore, the subjectivity introduced
by manual segmentation is avoided.
Our algorithm overcomes the limitation of noisy
data of the fetal face reconstruction algorithms pro-
posed so far, as no restrictions to the US data are ap-
plied. In (Bonacina et al., 2016) and (Dall’Asta et al.,
2017) studies, a strict inclusion criteria was applied
to the US data, as their procedure is really suscep-
tible to the quality of the input data. As a conse-
quence, all the cases with missing facial parts, large
amounts of noise, unsatisfactory definition of the fa-
cial borders or obstructed vision caused by the uterine
wall/intervening limbs/cord loops, were excluded for
those studies, limiting the applicability of their algo-
rithms. In contrast, our approach exploits the facial
geometry statistics encoded in the BabyFM, which
allows us to reconstruct the 3D fetal face even with
large amounts of noise, occlusions or missing parts.
Also, it allows reconstructing a unique facial geome-
try from multiple US scans, so that the specific details
available from each different view can be adequately
combined to produce an accurate reconstruction of the
whole facial geometry.
The reliability and accuracy of the reconstructions
depend on the quality of the US and the fetal pose
and expression. Higher levels of noise and/or oc-
clusions inevitably result in less accurate reconstruc-
tions. Nevertheless, our algorithm demonstrates that
reconstructions of the same individual from different
US scans are very similar and the faces differ in small
details. A large quantitative analysis was not possi-
ble, but the proposed 2D-3D fitting tests performed
for those cases with ground-truth, show promising re-
sults that could be used as proxy ground-truth for the
fetal reconstructions. Qualitative assessment of the
results were largely convincing, highlighting the po-
tential of the proposed technique to aid prenatal diag-
nosis.
The immediate next steps in our work will be
to address a larger quantitative validation of the
reconstruction algorithm, especially in terms of com-
parisons between the reconstructions before and after
3D Fetal Face Reconstruction from Ultrasound Imaging
621

Figure 8: 3D reconstruction from 2D images. Top: 2D images (only the frontal view is shown). Middle: Error reconstruction
(using the frontal, right, and left views) to the ground truth scans. Bottom: 3D reconstruction with texture.
Figure 9: Comparison between prenatal and postnatal reconstructions for case 3. From left to right: frontal 2D image, Us
reconstruction, 3D from 2D reconstruction, and error between reconstructions.
birth. The US scans analyzed so far, were acquired
between weeks 20 and 30 of gestation and the post-
natal images were taken once the baby was already a
few weeks old. Thus, the time difference between the
US and the photos used to get the 3D reconstructions
was approximately about 20 weeks. This difference
might have a clear impact in the face morphology of
the baby. For this reason, we are also investigating an
age correction mechanism to be applied to the mesh
extracted from the 3D fetal faces to see if we can de-
scribe a realistic age progression of the face of
the baby.
After performing the quantitative analysis inves-
tigating whether the estimated morphology is signif-
icantly different between patients with intrauterine
grow restriction and controls is also an interesting line
of continuation of this work.
VISAPP 2021 - 16th International Conference on Computer Vision Theory and Applications
622

6 CONCLUSIONS
To conclude, in this paper we proposed a fetal face re-
construction algorithm from 3DUS images. The ap-
proach differs from the existing ones proposed in the
literature, as it is based on the fitting of a deformable
BabyFM to 3DUS to remove the noise and to recover
the whole baby facial morphology. It was demon-
strated that our algorithm is able to reconstruct the
whole facial morphology of the babies under differ-
ent conditions (large amounts of noise, missing parts
or multiple US scans), obtaining promising results.
In the future, the presented technique could aid in
the prenatal assessment and in-utero diagnosis of syn-
dromes and diseases in which facial dysmorphology
is an indicator of early craniofacial abnormalities.
ACKNOWLEDGEMENTS
This work is partly supported by the Spanish Ministry
of Economy and Competitiveness under project grant
TIN2017-90124-P, and the Maria de Maeztu Units of
Excellence Programme (MDM-2015-0502).
REFERENCES
Albu, A., Horhoianu, I., Dumitrascu, M., and Horhoianu,
V. (2014). Growth assessment in diagnosis of fetal
growth restriction. review. J Med Life, 7(2):150–154.
Andresen, C., Matias, A., and Merz, E. (2012). Fetal Face:
The Whole Picture. Ultraschall in Med, 33:431–440.
Bas, A., Smith, W., Bolkart, T., and Wuhrer, S. (2016). Fit-
ting a 3D Morphable Model to Edges: A Compari-
son Between Hard and Soft Correspondences. Asian
Conference on Computer Vision Workshop on Facial
Informatics, Taipei (Taiwan), pages 377–391.
Bonacina, L., Froio, A., Conti, D., Marcolin, F., and
Vezzetti, E. (2016). Automatic 3D foetal face model
extraction from ultrasonography through histogram
processing. Journal of Medical Ultrasound, 24:124–
149.
Dall’Asta, A., Schievano, S., and Bruse, J. (2017). Quan-
titative analysis of fetal facial morphology using 3D
ultrasound and statistical shape modeling: a feasibil-
ity study. Am J Obstet Gynecol, 217(76):1–8.
EvansAnne, K. N., HingMichael, V., and Cunningham, L.
(2018). 100 - Craniofacial Malformations. Avery’s
Diseases of the Newborn, 10:1417–1437.
Learned-Miller, E., Lu, Q., Paisley, A., Trainer, P., Blanz,
V., Dedden, K., and Miller, R. (2006). Detecting
acromegaly: screening for disease with a morphable
model. In International Conference on Medical Im-
age Computing and Computer-Assisted Intervention
(MICCAI), page 495–503.
Menezes, G. A., J
´
unior, E. A., Lopes, J., S. Belmonte, G. T.,
and Werner, H. (2016). Prenatal diagnosis and phys-
icalmodel reconstruction of agnathia–otocephaly with
limb deformities (absent ulna, fibula and digits) fol-
lowing maternalexposure to oxymetazoline in the first
trimester. Journal of Obstetrics and Gynaecology Re-
search, 42(8):1016–1020.
Merz, E. and Abramowicz, J. (2012). 3D/4D ultrasound in
prenatal diagnosis: is it time for routine use? Clin
Obstet Gynecol, 55(1):336–351.
Merz, E. and Welter, C. (2005). 2D and 3D Ultrasound in
the evaluation of normal and abnormal fetal anatomy
in the second and third trimesters in a level III center.
Ultraschall Med., 1:1–16.
Morales, A., Porras, A. R., Tu, L., Linguraru, M. G.,
Piella, G., and Sukno, F. M. (2020). Spectral Corre-
spondence Framework for Building a 3D Baby Face
Model. In 15th IEEE International Conference on
Automatic Face and Gesture Recognition, pages 507–
514.
Mossey, P. A. and Catilla, E. E. (2001). Global registry
and database on craniofacial anomalies. WHO Reg-
istry Meeting on Craniofacial Anomalies.
on Government Affairs, A. C. (2020). Craniofacial Anoma-
lies. AAOMS.
S
,
orop Florea, M., Dragus
,
in, R.-C., Marinas
,
, C., S
,
orop, V.-
B., P
˘
atru, C. L., Zoril
˘
a, L. G., Neamt
,
u, C., Vedut
,
a,
A., Iliescu, D. G., and Cernea, N. (2018). Congenital
Abnormalities of the Fetal Face. IntechOpen.
Peleg, D., Kennedy, C. M., and Hunter, S. K. (1998). In-
trauterine growth restriction: Identification and man-
agement. Am Fam Physician, 58(2):453–460.
Pooh, R. and Kurjak, A. (2011). 3D/4D sonography moved
prenatal diagnosis of fetal anomalies from the second
to the first trimester of pregnancy. J Matern Fetal
Neonatal Med, 25(5):433–455.
Speranza, D., Citro, D., Padula, F., Motyl, B., Marcolin, F.,
Cal
`
ı, M., and Martorelli, M. (2017). Additive Man-
ufacturing Techniques for the Reconstruction of 3D
Fetal Faces. Applied Bionics and Biomechanics.
Tu, L., Porras, A., Morales, A., Perez, D., Piella, G., Su-
kno, F., and Linguraru, M. (2019). Three-Dimensional
Face Reconstruction from Uncalibrated Photographs:
Application to Early Detection of Genetic Syndromes.
In: Uncertainty for Safe Utilization of Machine
Learning in Medical Imaging and Clinical Image-
Based Procedures. Lecture Notes in Computer Sci-
ence, 11840:182–189.
Tu, L., Porras, A. R., Boyle, A., and Linguraru, M. G.
(2018). Analysis of 3D Facial Dysmorphology in
Genetic Syndromes from Unconstrained 2D pho-
tographs. In International Conference on Medical Im-
age Computing and Computer - Assisted Intervention
(MICCAI), page 347–355.
Werner, H., Lopes, J., Tonni, G., and J
´
unior, E. A.
(2015). Physicalmodel from 3D ultrasound and
magnetic resonance imaging scan data reconstruc-
tion of lumbosacral myelomeningocelein a fetus with
chiari ii malformation. Child’s Nervous System,
31(4):511–513.
3D Fetal Face Reconstruction from Ultrasound Imaging
623

Werner, H., Santos, J., Belmonte, S., Ribeiro, G., Daltro, P.,
Gasparetto, E., and Marchiori, E. (2016). Applicabil-
ity of three-dimensional imaging techniques in fetal
medicine. Radiol Bras, 49(5):281–287.
Werner, H., Santos, J. R. L. D., and Fontes, R. (2010). Addi-
tive manufacturing models of fetuses built from three-
dimensional ultrasound, magnetic resonance imaging
and computed tomography scan data. Ultrasound in
Obstetrics & Gynecology, 36(3):355–361.
VISAPP 2021 - 16th International Conference on Computer Vision Theory and Applications
624
