
The Impact of the Wound Shape on Wound Healing Dynamics: Is it
Time to Revisit Wound Healing Measures?
Gennadi Saiko
1,2 a
1
Swift Medical Inc., 1 Richmond St. W., Toronto, Canada
2
Ryerson University, Toronto, Canada
Keywords: Wound, Wound Healing. Epithelisation, Wound Measurements, Planimetry.
Abstract: Introduction: Wound healing is a multifaceted process, which can be impacted by many endogenous (e.g.,
compromised immune system, limited blood supply) or exogenous (e.g., dressing, presence of infection)
factors. An essential step in wound management is to track wound healing progress. It typically includes
tracking the wound size (length, width, and area). The wound area is the most often used indicator in wound
management. In particular, wound closure is the single parameter used by the FDA to measure wound
therapeutics' efficiency. Here, we present some arguments on why the wound area alone is insufficient to
predict wound healing progress. Methods: We have developed an analytical approach to characterize an
epithelization process based on the wound's area and perimeter. Results: We have obtained the explicit results
for wound healing trajectory for several wound shapes: round (2D), elongated cut (1D), and rectangular. The
results can be extended to complex shapes. Conclusions: From geometrical considerations, the wound healing
time is determined by the shortest dimension (the width) of the wound. However, within that time, the wound
healing trajectory can be different. Our calculations show that the shape of the wound may have significant
implications on a wound healing trajectory. For example, in the middle of the wound healing process (t/T=0.5),
the 1D wound model predicts 50% closure, while the 2D model predicts 75% closure (25% remaining). These
considerations can be helpful while analyzing clinical data or designing clinical or pre-clinical experiments.
1 INTRODUCTION
Wound healing is a multifaceted process, which can
be impacted by many endogenous (e.g., compromised
immune system, limited blood supply) or exogenous
(e.g., dressing, presence of infection) factors.
Successful acute wound healing depends on
orderly progression through four phases: hemostasis,
inflammation, proliferation, and remodeling or
maturation. During hemostasis and the early
inflammatory phase, platelets and inflammatory cells
migrate into the wound bed. During the inflammatory
phase, neutrophils enter the wound (Diegelmann,
2004), followed by macrophages that are responsible
for neutrophil and damaged matrix removal
(Meszaros, 2000). During the proliferative phase, the
migration of fibroblasts and keratinocytes into the
wound occurs. Keratinocytes cause re-epithelization
of the wound. Fibroblasts produce collagen and other
extracellular matrix (ECM) proteins necessary for
granulation tissue formation. During the final phase
a
https://orcid.org/0000-0002-5697-7609
of wound remodeling (which takes months and
years), collagen deposition continues, and collagen
III is gradually replaced with collagen I (Xue, 2015).
The emphasis of the current work is the
proliferation phase. The sign of the successful
proliferation is the re-epithelization of the tissue,
which occurs due to keratinocytes' migration.
An important aspect of wound management is to
track wound healing progress. Geometrical wound
measurements (length, width, and depth) are essential
tools in wound care armamentarium. In particular,
wound closure is the single parameter used by the
FDA to measure wound therapeutics' efficiency.
The geometrical wound measurements typically
are being performed manually, using a ruler. There
are two primary methods used for wound
measurements (Swezey, 2014):
- “Greatest length and width method: In this
method, the greatest length and the greatest width of
the wound are measured across the wound's diameter,
from wound edge to the opposite wound edge.
182
Saiko, G.
The Impact of the Wound Shape on Wound Healing Dynamics: Is it Time to Revisit Wound Healing Measures?.
DOI: 10.5220/0010337601820187
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 2: BIOIMAGING, pages 182-187
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

- Clock method: In this method, the face of a clock
is used to guide measurement. The 12:00 reference
position is towards the head of the body, and
measurements are obtained from 12:00 to 6:00
(length) and from 9:00 to 3:00 (width).” Notice that
the width can be larger than the length in this case.
However, only the length, L, and width, W, can
be determined using these methods. The surface area
of the wound, S in this case, can be estimated as
S=LxW, which is a very rough approximation that
does not take into account the shape of the wound.
This type of calculation has been shown to
overestimate the wound area by 10% to 44%
(Goldman, 2002), with accuracy decreasing as wound
size increases (Majeske, 1992). Thus, tracking the
wound healing progress in time manually will be
relatively imprecise. The accuracy of wound
measurements can be increased with digital
photography. In this case, the precise wound area can
be calculated in addition to the more accurate length
and width measurements. Note that it can also be
done with wound tracing on a sterile sheet or film
(Langemo, 1998). However, it is a very labor-
intensive process. In particular, digital technology
may lead to a 10x increase in the accuracy of wound
measurements. However, the initial implementation
of such techniques using DSL cameras got limited
clinical traction, primarily due to the significant extra
time required to take pictures using specialized
equipment. Thus, this process did not fit well in a
busy clinical workflow. With the advent of
smartphones, wound management was
revolutionized. The ability to capture an image and
annotate the wound using the same tool significantly
improved the overall wound management workflow.
Many wound healing measures are proposed (a
brief overview can be found in (Cukjati, 2001)).
However, most commonly, a measure of the change
in wound area is used and is expressed either as a raw
number (cm
2
) or as a percentage of the initial wound
area. The wound area, S, is an important clinical
indicator of the wound status and can be used to
predict wound healing progress and clinical
outcomes. In particular, S is a part of several wound
indices (e.g., PUSH score for pressure injuries).
However, it is known that the wound healing rate
expressed as absolute area healed per day tends to
exaggerate larger wounds' healing rates. Similarly,
the healing rate expressed as a percentage of initial
area healed per day tends to exaggerate smaller
wounds' healing rates (Cukjati, 2001). Thus, more
objective methods need to be adopted into clinical
use.
Here we present some arguments why wound area
alone is not sufficient to predict wound healing
progress. The shape of the wound (e.g., round,
complex, or elongated) also play an essential role in
wound healing dynamics. In particular, we argue that
wound healing for quasi-2D (round shape) wounds
will have significantly different dynamics from quasi-
1D wounds (e.g., cuts). Thus, objective wound
healing measures need to account for the wound
shape.
2 METHODS
Let's consider a wound with an arbitrary shape. It can
be characterized by the area S and the perimeter P. If
the epithelization starts from the wound margin, and
keratinocytes propagate on the distance dx, then in the
first approximation, we can write
𝑑𝑆 𝑃𝑑𝑥
(1)
Now, let's consider this process in more detail. In
particular, we will be interested in how the shape of
the wound affects wound healing dynamics.
2.1 Lattice Model
To elucidate the details, we will consider a lattice
model of the wound. The epithelized tissue bounds
the wound. During each time interval
t, the wound
wall propagates inwards by
x=v
t, where v is the
wound closure speed. For practical reasons, the time
interval
t can be set to one day.
If we consider the wound of an arbitrary shape,
then the wall propagation dynamics will be different
in different parts of the curve. For the square lattice,
there are three cases to consider: a convex angle
(Figure 1), a concave (or reflex) angle (Figure 2), and
a flat segment (Figure 3).
Figure 1: Healing dynamics in the case of a convex
segment.
The Impact of the Wound Shape on Wound Healing Dynamics: Is it Time to Revisit Wound Healing Measures?
183
Figure 2: Healing dynamics in the case of a concave
segment.
Suppose we calculate the perimeter at each step of the
wall propagation. In that case, one can see that the
perimeter (and according to Eq.1, the rate of wound
closure) increases at reflex segments (Figure 2),
decreases at convex segments (Figure 1), and stays
constant for the flat segments (Figure 3).
Figure 3: Healing dynamics in the case of two parallel walls.
Thus, intuitively, the wound healing rate behaves
very differently at various points of the closed curve
(wound margin).
2.2 Wound Closure Rate
Let's discuss what the wound closure speed, v is. The
naïve idea would be to link this speed with the
migration rate of keratinocytes. According to Tao et
al. (Tao, 2007), the keratinocytes migration rate is 10-
20 m/h in vitro. However, to have a viable epithelial
layer, it needs to be supplied with oxygen and
nutrients. In particular, to have adequate oxygen
supply, the oxygen diffusion length should not exceed
200m. Thus, the tissue underneath a new epithelium
needs to be vascularized, which may include
angiogenesis and collagen matrix deposition by
fibroblasts. Without an appropriate collagen scaffold,
the vascular network will collapse.
Consequently, we can consider the re-
epithelization process as three processes run in
parallel: 1) keratinocytes migration, 2)
revascularization via angiogenesis beneath a newly
epithelized surface, and 3) fibroblast migration and
collagen deposition. Based on this model, we can
estimate the wound closing speed, v. Based on the
maximal oxygen diffusion length (L
d
=200m), we
can expect that the epithelial layer cannot overtake
the new vasculature by much more than L
d
. Thus, we
can expect that the wound closing rate, v, will be
equal to the smallest of three values: keratinocytes
migration rate, angiogenesis rate, and fibroblast
migration rate.
Epithelization happens through two primary
mechanisms: crawling of cells on the substratum and
constriction of a supracellular actomyosin cable at the
edge of the gap in a purse-string-like mechanism
(Klarlund, 2012). In the presence of ECM, crawling
is a predominant mechanism. However, in the general
case, wound closure is an interplay of these two
mechanisms (Ravasio, 2015). In (Ravasio, 2015), it
was also found that the shape of the wound affects the
epithelization rate. These two mechanisms act in the
same direction near a convex surface; thus, the
closure rate is higher (up to 75 m/h for MDCK
(Madin-Darby canine kidney) cells). Near a concave
surface, these two mechanisms act in opposite
directions. Thus the closure speed is lower (close to 0
m/h for MDCK cells).
Angiogenesis is a multistage process by itself.
According to Felmeden et al. (Felmeden, 2003),
angiogenesis consists of 7 distinct phases (endothelial
cell and pericyte activation, degradation of the
basement membrane, migration of endothelial cells
(e.g., sprouting), proliferation of endothelial cells,
differentiation of endothelial cells, and reconstitution
of basement membrane). The primary factors, which
drive the angiogenesis and the morphology of the
newly developed vascular network are proliferation
rate (PR) and migration rate (MR) of endothelial
cells. The overall growth of vasculature is a result of
both proliferation and migration. They were
researched intensively in (Norton, 2016) numerically,
where the authors found that to get normally
vascularized tissue, these parameters must be close to
PR=0.025 1/h and MR=10-16 m/h. These values
agree with experimental observations that doubling
times for microvascular endothelial cells range from
about 12 hours to 4 days (Anagnostou, 1990). Based
on these values, we can conclude that assuming a
sufficient supply of endothelial cells, the endothelial
cell migration rate will limit the vascularization rate.
Subsequently, the wound healing rate, v, will be
limited by a smaller value (vascularisation rate in this
case), which is 10-16 m/h=240-380m/d. Note that
it is hard to expect that the vascularization rate will be
affected by the wound curvature.
This estimation is very close to experimental data
on humans and animal models. For example, the
wound closure rate can be estimated as 0.25mm/d in
the rat model (Rong, 2019).
BIOIMAGING 2021 - 8th International Conference on Bioimaging
184
3 RESULTS
3.1 2D Case
Let's consider the 2D case: a round wound with the
radius r. In this case, the perimeter 𝑃2𝜋𝑟
2𝜋
/
𝑆
/
. If we substitute this expression in Eq.1
and notice that dx=vdt, we can write
𝑑𝑆 2𝜋
/
𝑆
/
𝑣𝑑𝑡
(2)
We can divide both sides of the equation by 2𝑆
/
and integrate them
𝑆
√
𝑆 𝜋
/
𝑣𝑡
(3)
Here, S
0
is the wound area at the moment t=0.
From Eq.3, we can find the explicit expression for the
wound area, S
𝑆
𝑆
√
𝜋𝑣𝑡
(4)
The wound healing time will be 𝑇
𝑆
0
/𝜋
/2𝑣
𝑊/2𝑣
3.2 1D Case
Let's consider the 1D case: a long rectangular cut,
where the length of the cut L is much bigger than its
width, W. In this case, the perimeter P=2L+2W
≅
2L.
The wound area is S=LW. From this expression, one
may deduce that P=2S/W. However, W is not
constant. It decreases over time. Thus, this expression
is not useful for calculations. Instead, we may notice
that the perimeter P is approximately constant during
wound healing. Therefore, we can write P=2L instead
(note that P~S
0
). If we substitute this expression in
Eq.1 and notice that dx=vdt, we can write
𝑑𝑆 2𝐿𝑣𝑑𝑡
(5)
If we integrate both parts and reshuffle terms, we
will get
𝑆 𝑆
2𝐿𝑣𝑡
(6)
The wound healing time will be 𝑇
𝑆
0
/2𝐿𝑣
𝑊/2𝑣
3.3 The Generalization to Simple
Shapes
For a rectangular wound, we can find an exact
solution to the wound closure problem. If we consider
the rectangle with the length L and width W (𝐿𝑊)
and the wall is moving with the speed v, then the
unclosed area of the wound at time t will be
𝑆
𝐿2𝑣𝑡
𝑊2𝑣𝑡
𝑊
𝐿/𝑊 2𝑣𝑡/𝑊
12𝑣𝑡/𝑊
(7)
for 0𝑡𝑊/2𝑣.
If we introduce the wound healing time 𝑇𝑊/2𝑣
and normalized time 𝜏𝑡/𝑇 then we can obtain an
expression for the normalized surface area s:
𝑠
𝑆
𝑆
1𝜏𝑊/𝐿
1𝜏
(8)
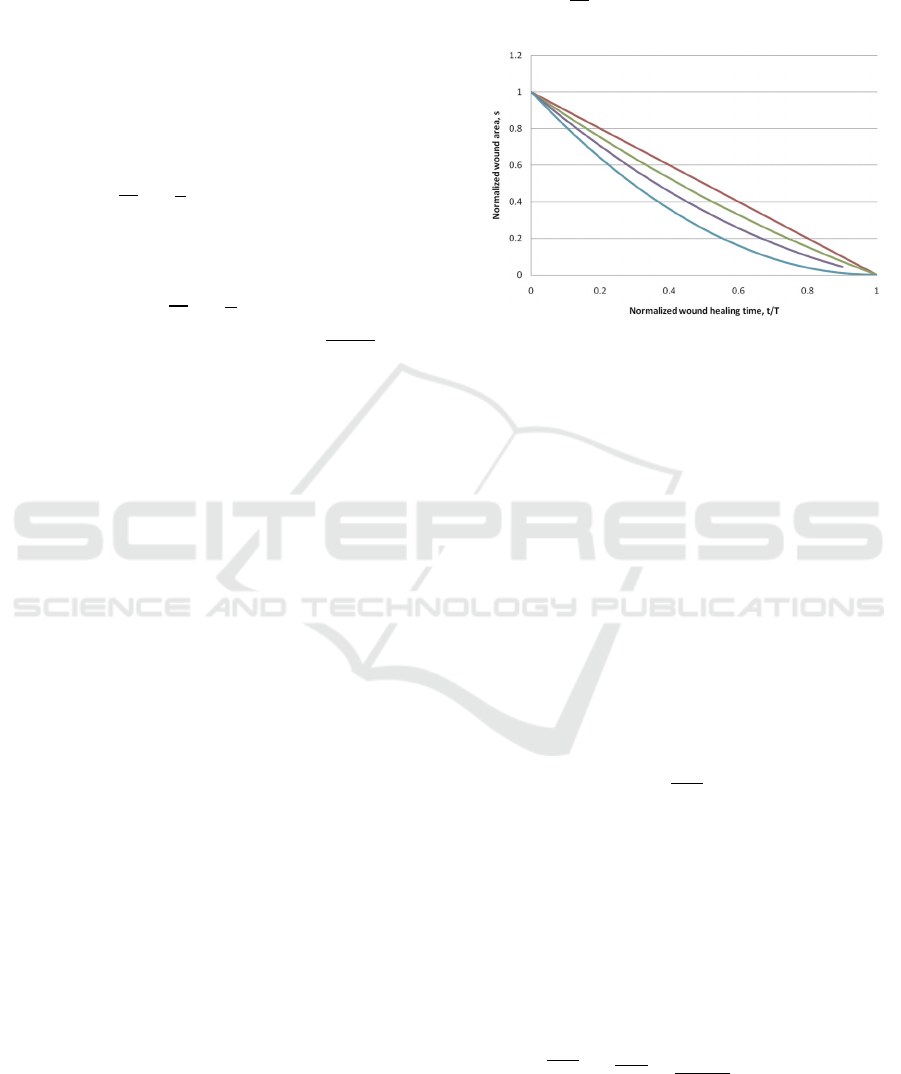
Figure 4: Wound healing trajectories for a rectangular
wound for various values of W/L ratios: 0 (red curve), 0.3
(green curve), 0.6 (purple curve), and 1 (blue curve).
In Figure 4, one can see several wound healing
scenarios for various W/L ratios.
The case W/L->0 corresponds to the 1D case
considered above. If W/L=1, then it is a square
wound, which is very similar to the round wound
considered in the 2D section.
3.4 The Generalization to Complex
Shapes
2D and 1D cases can be generalized differently if we
consider the wound margin as a fractal curve with a
dimension d
f
, where 1𝑑
2. One can see that the
perimeter P can be expressed through wound area S
as
𝑃𝑎𝑆
(9)
where a is a constant. In particular, this expression
holds in the case of d=2 and d=1. This expression can
be used to calculate d
f
for a specific curve. We need
to visualize the curve at several pixel sizes, calculate
P and S for each of them, and plot these values against
each other (P vs. S). Then, the parameter a and the
fractal dimension d
f
can be found numerically using
curve fitting.
If we substitute Eq.9 in Eq.1, divide both parts on
𝑆
/
and integrate them, we will get
𝑆
𝑆
3𝑑
2
𝑎𝑣𝑡
(10)
Thus, we can obtain an explicit expression for the
area S at any given time t
The Impact of the Wound Shape on Wound Healing Dynamics: Is it Time to Revisit Wound Healing Measures?
185
𝑆𝑆
3𝑑
2
𝑎𝑣𝑡
(11)
This expression holds for d=2 (see Eq.4) and d=1
(see Eq.6). Similarly, we can get an expression for the
normalized area s:
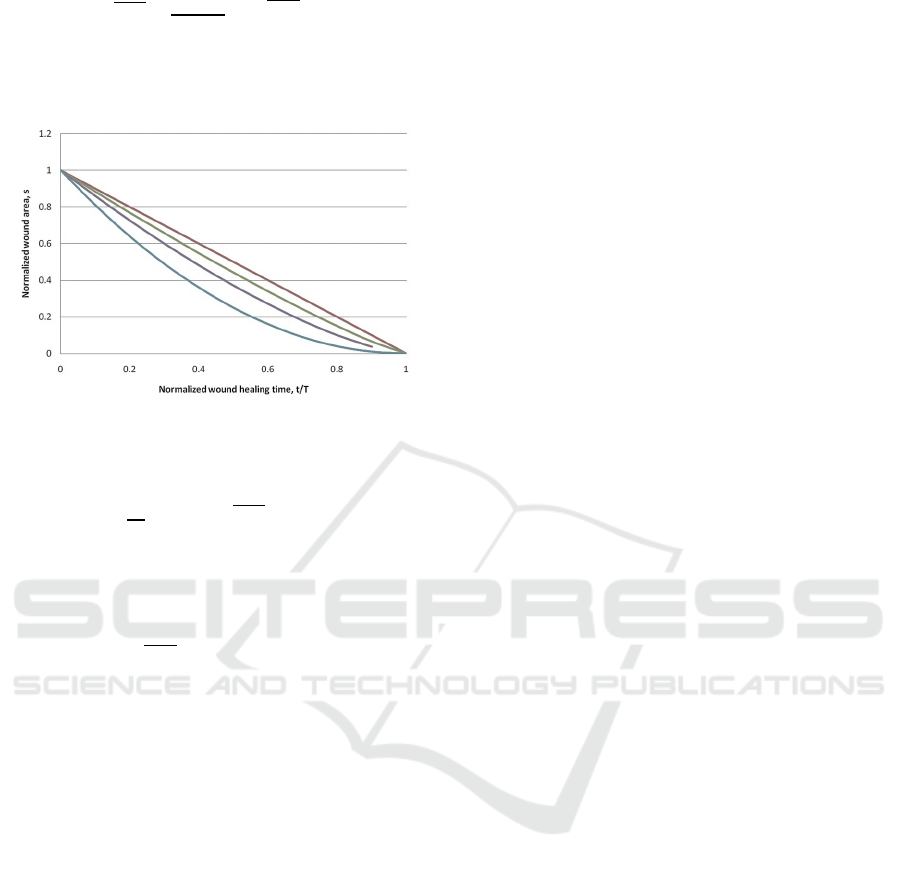
Figure 5: Wound healing scenarios for various fractal
dimensions d
f
: 1 (red curve), 1.3 (green curve), 1.6 (purple
curve), and 2 (blue curve).
𝑠
𝑆
𝑆
1𝜏
(12)
In Figure 5, one can see several wound healing
scenarios for various fractal dimensions d
f
. Note that
the wound healing time, in this case, will be
𝑇2𝑆
/𝑎𝑣3 𝑑
(13)
4 DISCUSSION
From simple geometrical considerations, one can see
that the wound healing time is determined by the
shortest dimension (the width) of the wound. That is
why the surgical closure of the wound (wherever
possible) is the best strategy. However, within that
time, the wound healing trajectory can be different.
Our simple calculations show that the wound's shape
may have significant implications on a wound healing
trajectory. For example, in the middle of the wound
healing process (t/T=0.5), the 1D wound model
predicts 50% closure, while the 2D model predicts
75% closure (25% remaining).
We have proposed two approaches to account for
the wound shape: a) based on the W/L ratio and b)
based on the fractal dimension. Both methods do not
require any clinical workflow changes if the wound
were measured using digital photography.
The rectangular wound model can be easily
extended to any elliptical shape. In this case, the
wound trajectory (Eq.8) will be the same. The fractal
model can be extended to more complex shapes,
which include concave segments. Thus, the models
are complementary, and the combination of these two
models covers the broad range of wound shapes.
We also found that the wound perimeter is a fairly
important factor in the wound healing process. For
example, the perimeter can be linked with the wound
area through a fractal dimension of the shape. Note
that the "fractal dimension" term is used quite loosely
here. We don't expect wound shape similarity
extended through multiple scales.
The proposed approach also helps identify which
wound healing rate used in clinics is the most
informative. As we mentioned in the introduction, the
current wound healing tracking methods based on
wound area S are imprecise. Partially it can be
explained by the fact that they do not account for the
wound shape. The more relevant metric could be a
linear healing rate D proposed in (Gilman, 1990),
which can be calculated as D=∆S/P from the change
in the area
S and mean perimeter P. It can be seen
that D, which was originally termed as “the advance
of the wound margin toward the wound centre,” is an
experimental measure for the wound closure rate v.
In particular, a study on 49 wounds found (Gorin,
1996) that the linear healing rate does not correlate
with the initial wound area, perimeter, and W/L ratio.
Thus the linear healing rate is independent of the
wound shape. These results were confirmed in
(Cukjati, 2001) on 300 wounds. Therefore, these
results can be considered as experimental
confirmation of the validity of the proposed model.
To validate these theoretical predictions further,
experimental verification is required, particularly for
complex shapes.
There are certain limitations to the proposed
model. Firstly, it was derived under the assumption
that wound epithelization occurs for all wound
perimeter points. From a physiological point of view,
it means that wound healing is not impaired. While it
can be the case for an acute wound, it is not apparent
for chronic wounds. For example, the
revascularization of the ischemic wound can be
impossible without revascularization surgery.
Secondly, the wound healing rate may depend on the
wound thickness. In the case of superficial wounds,
the vascular network may stay almost intact. Thus
only re-epithelization is required. Therefore, the rate
of wound closure is limited by keratinocytes
migration only (up to 20 m/h) and can be higher than
for partial-thickness wounds (10-16 m/h). For the
full-thickness wounds, which require collagen
deposition, the wound closure rate will be even
slower.
BIOIMAGING 2021 - 8th International Conference on Bioimaging
186

The linear healing rate was assessed in several
studies. Pecoraro et al. (Pecoraro, 1991) found 0.064
mm/day on diabetic foot patients. Margolis et al.
(Margolis, 1993) found 0.093 mm/day on venous
ulcers. Gorin et al. (Gorin, 1996) found a similar
result of 0.11 mm/day on venous ulcers. Cukjati et al.
(Cukjati, 2001) found 0.068 mm/day for the wound
of unknown etiology. All these values are 2-4 times
lower than the angiogenesis-limited healing rate.
Thus, one can expect that these rates are limited to
slower collagen-deposition processes or presence
areas with impaired healing.
These considerations can be helpful while
analyzing clinical data or designing clinical or pre-
clinical experiments.
5 CONCLUSIONS
Wound shape has significant implications on a wound
healing trajectory, which is not taken into account by
metrics currently used for wound healing progress
tracking. Wound area (closure) and wound area
(closure) as a percentage of the initial wound area are
important clinical endpoints. However, they do not
account for wound shape and do not allow an accurate
comparison of different wounds and treatment
methods. With the ubiquity of smartphones and
digital wound measurements, it is time to start
developing more accurate wound healing metrics.
The smallest size of the wound (width) and a linear
wound healing rate can be the basis for such metrics.
REFERENCES
Diegelmann, R.F., Evans, M.C., 2004, Wound Healing: An
Overview of Acute, Fibrotic and Delayed Healing.
Frontiers in Bioscience, 9: 283-289. doi:10.2741/1184
Meszaros, A.J., Reichner, J.S., Albina, J.E., 2000,
Macrophage-induced neutrophil apoptosis. J Immunol.
165(1):435-41. doi: 10.4049/jimmunol.165.1.435.
Xue, M, Jackson, C.J., 2015, Extracellular Matrix
Reorganization During Wound Healing and Its Impact
on Abnormal Scarring. Adv Wound Care. 4(3):119-136.
doi:10.1089/wound.2013.0485
Swezey, L., 2014, Methods and Strategies for Accurate
Wound Measurement. https://www.woundsource.com/
blog/5-techniques-accurate-wound-measurements
Goldman, R.J, Salcido, R., 2002, More than one way to
measure a wound: an overview of tools and techniques.
Adv Skin Wound Care. 15(5):236-43.
Majeske, C., 1992, Reliability of wound surface area
measurements. Phys Ther. 72(2):138-41.
Langemo, D.K, Melland, H., Hanson, D., et al., 1998, Two-
dimensional wound measurement: comparison of 4
techniques. Adv Wound Care 11(7): 337-43.
Cukjati, D., Reberšek, S., Miklavčič, D., 2001, A reliable
method of determining wound healing rate. Med. Biol.
Eng. Comput. 39: 263–271. doi: 10.1007/BF02344811
Tao, H, Berno, A.J, Cox, D.R, et al., 2007, In Vitro Human
Keratinocyte Migration Rates Are Associated with
SNPs in the KRT1 Interval. PLoS ONE 2(8): e697. doi:
10.1371/journal.pone.0000697
Klarlund, J. K., 2012, Dual modes of motility at the leading
edge of migrating epithelial cell sheets. Proc. Natl
Acad. Sci. USA 109: 15799–15804.
Ravasio, A., Cheddadi, I., Chen, T. et al., 2015, Gap
geometry dictates epithelial closure efficiency. Nat
Commun 6:7683.doi: 10.1038/ncomms8683
Felmeden, D.C, Blann, A.D, Lip, G.Y.H, 2003,
Angiogenesis: basic pathophysiology and implications
for disease, European Heart Journal, 24(1): 586–603,
doi: 0.1016/S0195-668X(02)00635-8
Norton, K., Popel, A., 2016, Effects of endothelial cell
proliferation and migration rates in a computational
model of sprouting angiogenesis. Sci Rep 6, 36992. doi:
10.1038/srep36992
Anagnostou, A., Lee, E. S., Kessimian, N., et al. 1990,
Erythropoietin has a mitogenic and positive
chemotactic effect on endothelial cells. Proceedings of
the National Academy of Sciences of the United States
of America 87: 5978–5982.
Rong, X., Chu, W., Zhang, H., et al. 2019, Antler stem cell-
conditioned medium stimulates regenerative wound
healing in rats. Stem Cell Res Ther 10: 326.
doi:10.1186/s13287-019-1457-9
Gilman, T., 1990, Parameter for measurement of wound
closure. Wounds; 2:95-101.
Gorin, D.R, Cordts, P.R, LaMorte, W.W, et al. 1996, The
influence of wound geometry on the measurement of
wound healing rates in clinical trials. J Vasc Surg.
23(3):524-8. doi: 10.1016/s0741-5214(96)80021-8.
Pecoraro, R.E, Ahroni, J.H, Boyko, E.J, et al., 1991,
Chronology and determinants of tissue repair in
diabetic lower extremity ulcers. Diabetes;40:1305-13.
Margolis, D.J., Gross, E.A., Wood, C.R., et al. 1993,
Planimetric rate of healing in venous ulcers of the leg
treated with pressure bandage and hydrocolloid
dressing. J Am Acad Dermatol; 28:418-21.
The Impact of the Wound Shape on Wound Healing Dynamics: Is it Time to Revisit Wound Healing Measures?
187
