Estimation of Chronic Stress by Measuring Sympathetic Sedation Time
Masahiro Inazawa, Yuki Ban
a
, Naoki Tateyama
b
and Shin’ichi Warisawa
c
Graduate School of Frontier Science, The University of Tokyo, Kashiwa, Chiba, Japan
Keywords:
Chronic Stress, Sympathetic Activity, Corticotropin-releasing Hormone, Cortisol.
Abstract:
Intermittent exposure to stressors disrupts the negative feedback mechanism of cortisol toward corticotropin-
releasing hormones. In this study, this condition is referred to as chronic stress. Chronic stress causes a variety
of recurring, long-term, incurable illnesses, such as major depression. Therefore, it is important to understand
chronic stress on a daily basis. We propose a chronic stress estimation method using sympathetic sedation
time measurements as a non-invasive, short-time, and highly accurate method. This method determines the
degree of chronic stress according to the length of time until the sympathetic activity subsides after stressor
loading. To verify the feasibility of the proposed method, we conducted an experiment comparing the sympa-
thetic sedation times among a healthy group, middle group, and chronic stress group classified by the Quick
Inventory of Depressive Symptomatology. We calculated sympathetic sedation time from the trend of change
in RRV at calm after stressor loading due to a two-back task. As a result of the experiment, which consisted
of nine participants, the sympathetic sedation time in the chronic stress group was longer than in the healthy
and middle groups, supporting the feasibility of this method.
1 INTRODUCTION
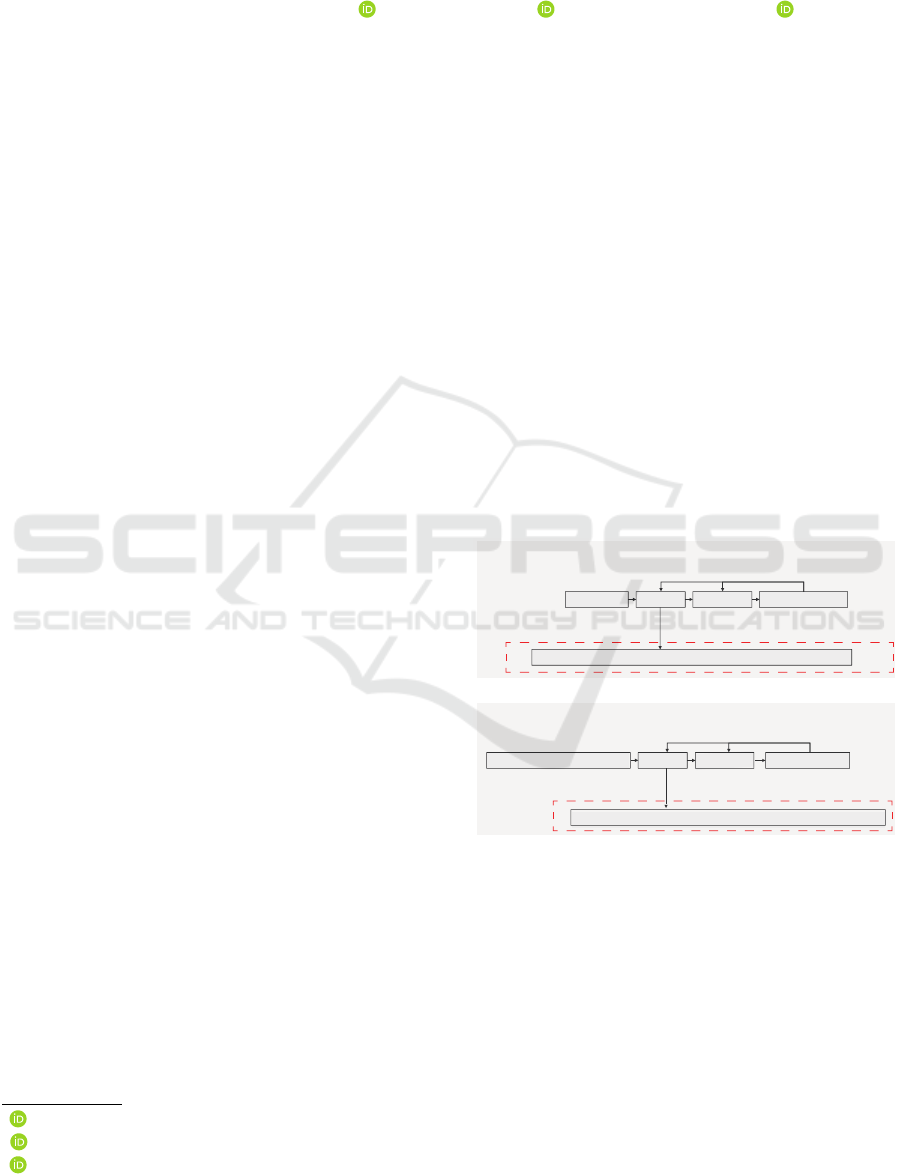
Normally, when a person is exposed to a stressor,
corticotropin-releasing hormone (CRH) is secreted
from the hypothalamus. As a result, adrenocorti-
cotropic hormone (ACTH) is secreted from the ante-
rior pituitary gland, which promotes the secretion of
cortisol from the adrenal cortex. Even though cortisol
causes various stress responses, including hippocam-
pal atrophy, it has a negative feedback mechanism for
CRH and ACTH, and this feedback will eventually
cause the stress response to disappear. However, in-
termittent exposure to stressors deteriorates the neg-
ative feedback function of cortisol (Fink, 2010; Con-
toreggi, 2015) (Fig.1). In this study, we define a con-
dition in which the negative feedback function of cor-
tisol deteriorates as chronic stress.
Chronic stress can cause a variety of illnesses.
Chronically high levels of cortisol can cause hip-
pocampal atrophy and impaired memory (Vachon-
Presseau et al., 2013). In addition, it causes chronic
high blood pressure and blood sugar levels, which
can lead to diabetes. Chronically high levels of
CRH due to the deterioration of the negative feedback
a
https://orcid.org/0000-0001-7349-6383
b
https://orcid.org/0000-0001-6747-5295
c
https://orcid.org/0000-0001-9815-6801
6WUHVVUHVSRQVHGXULQJKHDOWK
6WUHVVRU
&5+
$&7+
6\PSDWKHWLFQHUYHV DFWLYDWHG
߲
GHDFWLYDWHG
&RUWLVRO
QHJDWLYHIHHGEDFN
6WUHVVUHVSRQVHGXULQJFKURQLFVWUHVV
&RQWLQXRXVVWUHVVRU &5+ $&7+ &RUWLVRO
'HWHULRUDWLRQRIQHJDWLYHIHHGEDFN
6\PSDWKHWLFQHUYHV
FRQWLQXRXVO\DFWLYDWHG
+\SRWKHVLV
+\SRWKHVLV
Figure 1: Stress response in healthy and chronic stress
states.
mechanism of cortisol may promote fear conditioning
by the amygdala and predispose individuals to post-
traumatic stress disorder (Hashimoto et al., 2017).
High CRH levels can also lead to high dopamine lev-
els and impair cognitive function (Fink, 2010). If
these diseases persist for a long period of time, they
can lead to major depression. These diseases, in-
cluding major depression, are prone to recurrence and
have a long treatment period (Clinic, 2020). More-
over, high levels of cortisol exposure damages the
hippocampal and prefrontal cortex nerves, and may
292
Inazawa, M., Ban, Y., Tateyama, N. and Warisawa, S.
Estimation of Chronic Stress by Measuring Sympathetic Sedation Time.
DOI: 10.5220/0010326502920298
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 4: BIOSIGNALS, pages 292-298
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
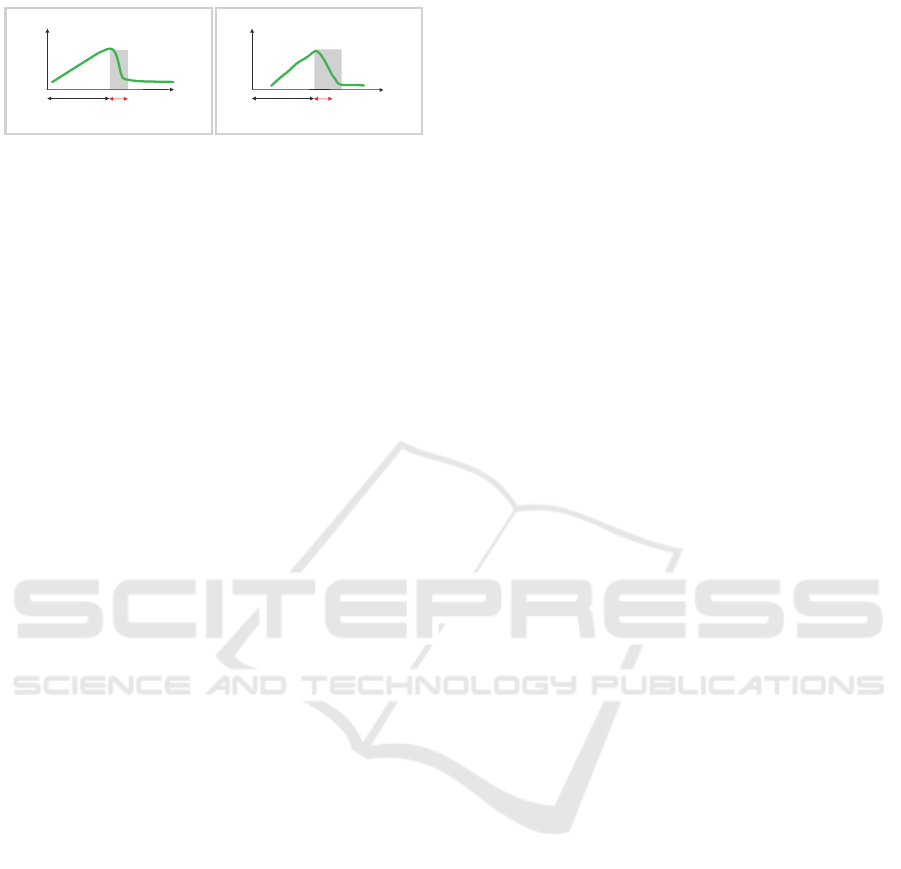
7LPH
6WUHVV
VWLPXOXV
6\PSDWKHWLF
6HGDWLRQ7LPH
3HRSOHLQKHDOWK 3HRSOHLQFKURQLFVWUHVV
6WUHVV
VWLPXOXV
6\PSDWKHWLF
6HGDWLRQ7LPH
7LPH
Figure 2: Estimation of chronic stress by measuring sympa-
thetic sedation time after stressor.
not cause neurogenesis, depending on age and sever-
ity of the depression (Contoreggi, 2015; Van Wingen
et al., 2012). Chronic stress conditions can cause var-
ious diseases that are prone to recurrence, have a long
treatment period, and may not be completely cured.
Therefore, we believe it is important to understand the
chronic stress state on a daily basis to prevent these
diseases.
The prior methods of chronic stress estimation can
be broadly classified into two types. One method
measures changes in behavior and cognition caused
by chronic stress, and the other measures biological
information related to the negative feedback mecha-
nism of cortisol.
As a method for measuring changes in behavior
and cognition, questionnaires, such as PSS-14, and
the estimation of a chronic stress state from a change
tendency of the pressure distribution on the seat sur-
face of a chair are used (Katsunori, 2006; Cohen et al.,
1983; Kuroha et al., 2019). In addition, chronic stress
can be estimated by lifestyle change such as sleep-
ing time collected from smartphones (Opoku Asare
et al., 2019; Dogan et al., 2017; Rohani et al., 2018;
Wang et al., 2018). PSS-14 is a questionnaire that
consisting of 14 question items on a five-level Likert
scale. It is assumed that the response is made by re-
membering the events within one month or one week,
and the chronic stress state can be estimated in a short
time. Since the answers in the questionnaire are sub-
jective evaluations, there are difficulties of inaccurate
answers if the participant does not answer seriously
or if they are not aware of their own stress. In the
method using the change in seat pressure, the chronic
stress state can be easily estimated non-invasively by
sitting on a chair for a long duration using a cush-
ion equipped with a pressure sensor. In the method
using smartphones, chronic stress can be easily esti-
mated from information such as screen startup time
and sleep time collected from them. However, the es-
timation accuracy is low in these methods because the
biological reaction under chronic stress is not directly
measured.
Two of the methods for estimating biological
information with regards to the negative feedback
mechanism of cortisol are the DEX/CRH test and
salivary cortisol concentration measurement (Heuser
et al., 1994; Hellhammer et al., 2009). In the
DEX/CRH test, dexamethasone (DEX) is adminis-
tered before bedtime, and the blood cortisol concen-
tration when CRH is administered in the next morn-
ing is measured. Dexamethasone is a long-acting ar-
tificial cortisol. Healthy individuals have low cortisol
levels even after CRH administration due to the neg-
ative feedback mechanism. On the contrary, under
chronic stress, high cortisol is measured after CRH
administration, thus, the chronic stress state can be
measured. The challenge with this method is that it
is invasive, requiring at least three injections of DEX
and CRH administration, and cortisol collection. An-
other difficulty is that the load test time is up to several
hours. Estimating by salivary cortisol concentration,
another measurement method, can measure chronic
hypercortisol status non-invasively. The challenges
of this method is that the salivary hypercortisol sta-
tus is caused not only by a decrease in the negative
feedback mechanism of cortisol, but also by the di-
urnal variation of cortisol and acute stressors, thus,
strict control is required for measurements. Another
difficulty is that it takes several hours to analyze the
salivary cortisol concentration.
Overall, the behavioral and cognitive changes due
to chronic stress can be assessed non-invasively and
quickly, but the accuracy is low. On the contrary, the
method for measuring biological information with re-
gards to the negative feedback mechanism of cortisol
is highly accurate because it directly measures the bi-
ological reaction in a chronic stress state, but the mea-
surement is long-term or invasive. Therefore, the pur-
pose of this study is to design a non-invasive, fast, and
highly accurate chronic stress measurement method
and to evaluate its accuracy.
2 CHRONIC STRESS
ESTIMATION METHOD BY
MEASURING SYNPATHETIC
SEDATION TIME
In this study, we propose chronic stress estimation
by sympathetic sedation time measurements as a non-
invasive, fast, and highly accurate chronic stress mea-
surement method. A stressor is applied to an user,
the time during which the sympathetic nerve activity
subsides is measured, and the chronic stress state is
estimated from this time duration. We believe that the
sympathetic nerve activity sedation time is longer un-
der chronic stress, as detailed below (Fig.2).
The negative feedback function of cortisol is dete-
Estimation of Chronic Stress by Measuring Sympathetic Sedation Time
293
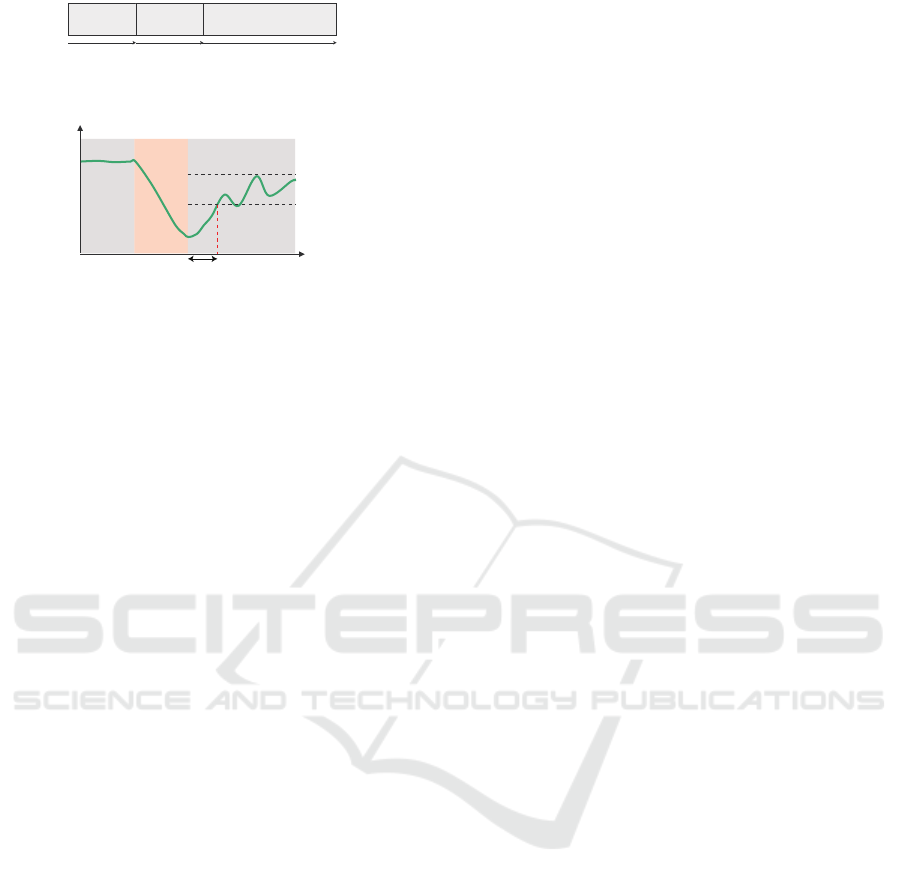
7LPH
&DOP
6WUHVVRU
ORDGHG
&DOP
PLQ PLQ PLQ
Figure 3: Proposal method procedure.
559PD[LPXPYDOXH
RIWKH
559PD[LPXPYDOXH
6\PSDWKHWLF6HGDWLRQ7LPH
7LPH>PLQ@
&DOP
559>V
@
&DOPEDFN
Figure 4: How to calculate sympathetic sedation time.
riorated under chronic stress. As a result, those with
chronic stress are considered to have high CRH for
a long time after stressor loading. CRH may pro-
mote sympathetic nerve activity. Habib et al. found
that male rhesus monkeys orally administered with
the CRH antagonist antaramine have reduced sympa-
thetic nerve activity when confronted with other male
individuals compared to placebo individuals (Habib
et al., 2000). Therefore, we believe that under a
chronic stress state, the sympathetic nerve activity ac-
tivated by the stressor is difficult to sedate because
CRH does not decrease after stressor loading(Fig.1).
Therefore, we believe that the chronic stress state
can be estimated by loading the stressor on the user
and measuring the time for the subsequent sympa-
thetic nerve activity to subside. This method is ex-
pected to be highly accurate as it measures biological
reactions related to the negative feedback mechanism.
In addition, since it is a sympathetic nerve measure-
ment, it can be measured non-invasively by a sensor,
such as an electrocardiogram. The measurement time
is approximately 30 min. This time is shorter than
that of the DEX/CRH test and saliva cortisol concen-
tration measurements, which is an advantage.
We describe the details of the proposed method.
First, the user remains calm for 5 min to soothe sym-
pathetic activity. During this time, respiratory con-
trol is performed to enhance the effect of calming.
The respiratory cycle is set to 12 times/min as a suf-
ficiently slow cycle to sedate the sympathetic nerves.
Next, user is loaded the stressor for 5 min. Then, the
user remains calm for 10 min. During this period,
the sympathetic nerve activity is measured, and sym-
pathetic sedation time is calculated. As sympathetic
nerve activity changes depending on the respiratory
cycle, it may be necessary to suppress the variation in
sympathetic sedation time due to the respiratory cy-
cle by controlling breathing during calming after the
stressor. Therefore, the effect of sympathetic sedation
time due to respiratory control during calming after
the stressor was examined. The flow of the proposed
method is described in Fig.3.
In this study, the required function of the stres-
sor used in the proposed method was defined as caus-
ing a stress response that causes cortisol secretion. In
addition, as a constraint condition, the load can be
loaded within a short time of several minutes. We
selected the two-back task as the stressor that satisfies
these functions. Even though there are many varia-
tions of this task, we selected one in which recordings
of numbers from one to five are played through head-
phones every second and a participant has to press the
space key only when the number matches that read
two prior.
In this study, we assumed that the sympathetic
nerve was sedated when the R-R interval variabil-
ity (RRV) at calm after the two-back task exceeded
60% of the maximum value of RRV at calm. The
time taken until the sympathetic nerve was sedated
for the first time after the two-back was defined as
the sympathetic nerve sedation time (Fig.4). RRV is
the variance of the interval between R waves during
1 min for electrocardiography and is a parasympa-
thetic index. Since the sympathetic and parasympa-
thetic nerves have an antagonistic effect, the sympa-
thetic nerve appears to be sedated by the increase of
RRV.
3 EXPERIMENT
3.1 Overview
To verify the possibility of estimating chronic stress
by measuring the sympathetic sedation time, we re-
cruited experimental participants and conducted an
experiment. Assuming that the higher the degree of
depression, the higher the degree of chronic stress,
the participants were divided into three groups using
the Quick Inventory of Depressive Symptomatology
(QIDS), which is a diagnostic criterion for depres-
sion (Rush et al., 2003). The QIDS scores ranged
from zero to five points in the healthy group, six to
10 in the middle group, and at least 11 points in the
chronic stress group. The sympathetic nerve sedation
time of each group was compared. In addition, as de-
scribed in the previous chapter, the sympathetic nerve
sedation time may change depending on the respira-
tory control, thus, the effect of the respiratory control
at calm after the two-back task shown in Fig.3 was
examined. This experiment is approved by the Ethics
Review Board.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
294

4,'6
6\PSDWKHWLFVHGDWLRQWLPH
PHDVXUHPHQWH[SHULPHQW
7LPH
ZLWKLQGD\V ZLWKLQGD\V
Figure 5: Experiment flow.
*D]HSRLQW
%DUXVHGIRUUHVSLUDWRU\FRQWURO
Figure 6: Point of gaze at calm.
3.2 Experiment Flow
First, the participants filled in the QIDS within one
week prior to the sympathetic sedation time measure-
ment experiment. Then, the participants were sub-
jected to a sympathetic sedation time measurement
experiment three times on different days. The three
sympathetic sedation time measurement experiments
differed only in the respiratory control conditions at
calm after the two-back task. The first and last ex-
periments for each participant were scheduled within
seven days so that the chronic stress status of the par-
ticipants did not change significantly between exper-
iments. Since the sympathetic nerve sedation time
changes depending on the diurnal variation of corti-
sol, the sympathetic nerve sedation time measurement
experiment was performed from 15:00 to 17:00 when
the diurnal variation of cortisol was small. In addi-
tion, to suppress fluctuations in the diurnal variation
of cortisol, alcohol and caffeine intake, strenuous ex-
ercise, and staying up late the day before the experi-
ment were prohibited. The flow of the experiment is
shown in Fig.5.
In the sympathetic sedation time measurement ex-
periment, the process described in the previous chap-
ter was performed using a GUI.
1. Calm1: Participants were instructed to look at
a cross gaze point for 5 min and rest to calm
the sympathetic nerves (Fig.6). At that time,
respiratory control was performed at a cycle of
12 times/min to enhance the effect of sympa-
thetic nerve sedation. Respiratory control was
performed according to the expansion and con-
traction cycle of the blue bar shown in Fig.6.
2. 2-Back Task (Stressor Loaded): Participants
were instructed to perform the two-back task for 5
min while looking at the same gazing point as in
7LPH
+ROG 3UHVVVSDFHNH\+ROG+ROG3DUWLFLSDQWܳVDFWLRQ
PDWFK
Figure 7: 2 Back task.
Calm1. This task involves the playing of a read-
ing of any number from one to five through head-
phones every second, and participants press the
space key when the number matches two numbers
prior (Fig.7). Unlike Calm1, respiration was not
controlled. This is because we believe that res-
piratory control reduces the ability to concentrate
on the two-back task and reduces stress load.
3. Calm2: To measure the sympathetic sedation
time, participants were instructed to look at the
gaze point for 10 min and calm. Three condi-
tions were prepared for respiratory control as fol-
lows: no respiratory control, fast respiratory con-
trol (20 times/min), and slow respiratory control
(12 times/min). Under the no respiratory con-
trol condition, the blue breathing control bar did
not appear, participants were instructed to breathe
naturally. Under fast or slow respiratory control
conditions, participants were instructed to breathe
according to the expansion and contraction cycle
of the blue bar. Before the experiment, partici-
pants were told under which respiratory control
conditions the experiment would be conducted.
The order of these conditions was counterbal-
anced among the participants.
This process was performed by looking at the PC
screens installed in the compartments separated by
partitions. During this process, the electrocardiogram
was measured and the R-R Interval (RRI) was calcu-
lated. Electrocardiogram was measured at 1000 Hz
using biosignalsPlux. R waves were detected by first
passing the electrocardiogram through a Butterworth
filter from 0.05 to 26 Hz and then using the biosppy li-
brary(Carreiras et al., 15). Furthermore, the RRI vari-
ability (RRV) was calculated for each minute window
and used as a parasympathetic nerve activity index.
The time when RRV after the two-back task exceeded
the threshold for the first time was defined as sympa-
thetic sedation time. The threshold was set to 60%
of the maximum value of RRV after the two-back
task (Fig.4).
Estimation of Chronic Stress by Measuring Sympathetic Sedation Time
295
559>PV
@
7LPH>PLQ@
7LPH>PLQ@
&DOP
&DOPEDFN
&DOP &DOP
EDFN
Figure 8: RRV time series of participants. The left side
refers to the healthy group. The right side refers to the
chronic stress group.
6\PSDWKHWLF
6HGDWLRQ7LPH>PLQ@
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
1RUHVSLUDWRU\
FRQWURO
)DVWUHVSLUDWRU\
FRQWURO
6ORZUHVSLUDWRU\
FRQWURO
Figure 9: Sympathetic sedation time for each respiratory
condition. The left side is under no respiratory control. The
center is under fast respiratory control. The right is under
slow respiratory control.
4 RESULTS
We recruited nine participants to this study. The
QIDS classification revealed three healthy individuals
(one male and two females in their 20s), three middle
groups (three males in their 20s), and three chronic
stress groups (three males in their 20s). The mean
and standard errors of the QIDS scores were 3.33 ±
0.98 in the healthy group, 6.00 ± 0.00 in the middle
group, and 13.66 ± 1.78 in the chronic stress group.
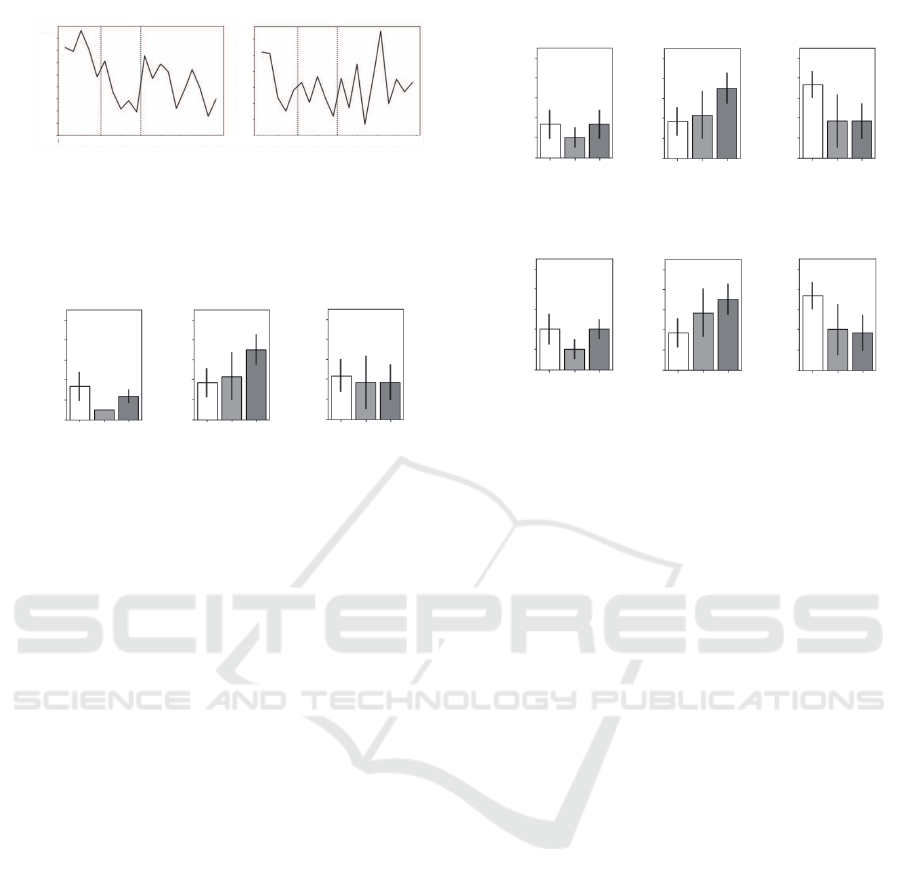
Fig.8 shows the RRV data of a participant in a
healthy group under no respiratory control and that
of another participant in the chronic stress group un-
der no respiratory control. In the healthy participant
(left), RRV increased immediately after the two-back
task, that is, the sympathetic nerves were sedated im-
mediately. On the contrary, in the chronic stress par-
ticipant (right), RRV increased approximately 5 min
after two-back (experimental time 15 min), that is, it
took some time to sedate the sympathetic nerves.
Next, we calculated the mean and standard error
of the sympathetic sedation time described in Chapter
3 for each respiratory control condition and partici-
pant group. The results are shown in Fig.9. In ad-
dition, the threshold values of sympathetic nerve se-
dation used when calculating the sympathetic nerve
sedation time were 70% and 80% of the RRV maxi-
mum value, shown in Fig.10.
6\PSDWKHWLF
6HGDWLRQ7LPH>PLQ@
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
1RUHVSLUDWRU\
FRQWURO
)DVWUHVSLUDWRU\
FRQWURO
6ORZUHVSLUDWRU\
FRQWURO
6\PSDWKHWLF
6HGDWLRQ7LPH>PLQ@
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
+HDOWK\
&KURQLF
VWUHVV
0LG
GOH
1RUHVSLUDWRU\
FRQWURO
)DVWUHVSLUDWRU\
FRQWURO
6ORZUHVSLUDWRU\
FRQWURO
Figure 10: Upper row: Results when the threshold for
sympathetic sedation is 70% of the maximum RRV value.
Lower: 80%.
5 DISCUSSION
5.1 Respiratory Control
As shown in Fig.9,under no and slow respiratory con-
trol,the hypotheses of shorter sympathetic sedation
time in the healthy group and longer sympathetic se-
dation time in the chronic stress group were not met.
However, under fast respiratory control conditions,
the hypothetical tendency was confirmed. A similar
tendency was confirmed when the threshold for sym-
pathetic sedation was set to 70% or 80% of the max-
imum RRV value (Fig.10).The hypothesis being met
only under fast respiratory control was considered to
be because this respiratory control at calm is also a
stressor, and the sympathetic nerve sedation time is
longer than that of no respiratory control in both the
healthy and chronic stress groups. However, in the
chronic stress group, the negative feedback function
of cortisol was reduced, and the effect of prolonging
the sympathetic sedation time by fast respiratory con-
trol was greater than that of other groups. The chronic
stress state may be estimated from the sympathetic se-
dation time by performing fast respiratory control at
calm after two-back. To make the feasibility verifi-
cation of the proposed method more reliable, the fol-
lowing factors could be improved.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
296

5.2 Sympathetic Sedation Time
In this verification, the sympathetic sedation time was
calculated using only the RRV of the electrocardio-
gram as an index, but RRV is affected by the respira-
tory cycle as well as the sympathetic nerves. The re-
sults must be verified from various angles using other
sympathetic nerve indexes, such as pupil diameter,
electro dermal activity, etc. In addition, the sym-
pathetic nerves were sedated when RRV after two-
back exceeded 60% of the maximum value for the
first time, but the threshold value of 60% is necessary
for comparison with various threshold values of other
sympathetic nerve indexes.
5.3 Stressor
In this study, the two-back task was selected as a stres-
sor that secretes cortisol, but this task leads to a low
amount of cortisol secretion (Henckens et al., 2011).
Since the proposed method is intended to measure the
decrease in the negative feedback mechanism of cor-
tisol, the stressor needs to cause the secretion of corti-
sol. For example, the Trier Social Stress Test (TSST)
results in the secretion of cortisol, but the process is
very long and the second presentation shows acclima-
tization and significantly reduced cortisol secretion
(Yao et al., 2016; Dhabhar et al., 1997; W
¨
ust et al.,
2005; Schommer et al., 2003). We need to design a
cortisol-secreting stressor that can be presented in a
short period of time and with little familiarity.
6 CONCLUSION
In this study, we propose chronic stress estimation
through sympathetic sedation time measurements as
a non-invasive, fast, and highly accurate method. In
this method, a stressor is applied to an user, the time
until the sympathetic nerve activity subsides is mea-
sured, and the chronic stress state is estimated from
the length of time.
We recruited nine participants to this study. As
a result, we confirmed that the sympathetic sedation
time tended to be longer in the chronic stress group
than in the healthy group by performing fast respira-
tory control after stressor loading. Moreover, we be-
lieve that the following improvements could make the
demonstration of feasibility more reliable.
1. Multifaceted verification of whether the sympa-
thetic nerve has sedated.
2. Optimal stressor design for sympathetic sedation
time measurement.
After demonstrating the feasibility of chronic
stress estimation by measuring sympathetic sedation
time through the above improvements, we aim to de-
sign a wearable device that integrates a sensor for
measuring sympathetic sedation time as well as a
stressor presentation device. We believe that with the
development of such devices, anyone will be able to
identify their chronic stress state on a daily basis and
prevent mental illnesses, such as major depression.
REFERENCES
Carreiras, C., Alves, A. P., et al. (2015–). BioSPPy: Biosig-
nal processing in Python. [Online; accessed 2018-8-
2].
Clinic, M. (2020). Mitsuoka clinic. https://www.mitsuoka-
clinic.com/depression2.htm.
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A
global measure of perceived stress. Journal of health
and social behavior, pages 385–396.
Contoreggi, C.(2015). Corticotropin releasing hormone and
imaging, rethinking the stress axis. Nuclear medicine
and biology, 42(4):323–339.
Dhabhar, F. S., McEwen, B. S., and Spencer, R. L.(1997).
Adaptation to prolonged or repeated stress–
comparison between rat strains showing intrinsic
differences in reactivity to acute stress. Neuroen-
docrinology, 65(5):360–368.
Dogan, E., Sander, C., Wagner, X., Hegerl, U., and Kohls,
E. (2017). Smartphone-based monitoring of objective
and subjective data in affective disorders: Where are
we and where are we going? systematic review. Jour-
nal of Medical Internet Research, 19.
Fink, G.(2010). Encyclopedia of STRESS, 2nd Ed.
Maruzen.
Habib, K. E., Weld, K. P., Rice, K. C., Pushkas, J., Cham-
poux, M., Listwak, S., Webster, E. L., Atkinson, A. J.,
Schulkin, J., Contoreggi, C., et al.(2000). Oral ad-
ministration of a corticotropin-releasing hormone re-
ceptor antagonist significantly attenuates behavioral,
neuroendocrine, and autonomic responses to stress in
primates. Proceedings of the National Academy of
Sciences, 97(11):6079–6084.
Hashimoto, T. et al.(2017). Expression analyses of stress-
related factors in the brain of the single prolonged
stress rats. Human developmental research CODER
annual Report, 31:105–113.
Hellhammer, D. H., W
¨
ust, S., and Kudielka, B. M.(2009).
Salivary cortisol as a biomarker in stress research.
Psychoneuroendocrinology, 34(2):163–171.
Henckens, M. J., van Wingen, G. A., Jo
¨
els, M., and
Fern
´
andez, G.(2011). Time-dependent corticosteroid
modulation of prefrontal working memory process-
ing. Proceedings of the National Academy of Sciences,
108(14):5801–5806.
Heuser, I., Yassouridis, A., and Holsboer, F. (1994). The
combined dexamethasone/crh test: a refined labora-
Estimation of Chronic Stress by Measuring Sympathetic Sedation Time
297

tory test for psychiatric disorders. Journal of psychi-
atric research, 28(4):341–356.
Katsunori, S. (2006). Reliability and validity of the japanese
version of the perceived stress scale. Journal of Health
Psychology Research, 19(2):44–53.
Kuroha, M., Ban, Y., Fukui, R., and Warisawa, S. (2019).
Chronic stress level estimation focused on motion pat-
tern changes acquired from seat pressure distribution.
In 2019 International Conf. on Cyberworlds (CW),
pages 135–142.
Opoku Asare, K., Visuri, A., and Ferreira, D. S. (2019).
Towards early detection of depression through smart-
phone sensing. In Adjunct Proceedings of the 2019
ACM International Joint Conf. on Pervasive and
Ubiquitous Computing and Proceedings of the 2019
ACM International Symposium on Wearable Comput-
ers, pages 1158–1161.
Rohani, D. A., Faurholt-Jepsen, M., Kessing, L. V., and
Bardram, J. E. (2018). Correlations between objec-
tive behavioral features collected from mobile and
wearable devices and depressive mood symptoms in
patients with affective disorders: systematic review.
JMIR mHealth and uHealth, 6(8):e165.
Rush, A. J., Trivedi, M. H., Ibrahim, H. M., Carmody, T. J.,
Arnow, B., Klein, D. N., Markowitz, J. C., Ninan,
P. T., Kornstein, S., Manber, R., et al.(2003). The 16-
item quick inventory of depressive symptomatology
(qids), clinician rating (qids-c), and self-report (qids-
sr): a psychometric evaluation in patients with chronic
major depression. Biological psychiatry, 54(5):573–
583.
Schommer, N. C., Hellhammer, D. H., and Kirschbaum,
C. (2003). Dissociation between reactivity of
the hypothalamus-pituitary-adrenal axis and the
sympathetic-adrenal-medullary system to repeated
psychosocial stress. Psychosomatic medicine,
65(3):450–460.
Vachon-Presseau, E., Roy, M., Martel, M.-O., Caron, E.,
Marin, M.-F., Chen, J., Albouy, G., Plante, I., Sul-
livan, M. J., Lupien, S. J., et al. (2013). The stress
model of chronic pain: evidence from basal cortisol
and hippocampal structure and function in humans.
Brain, 136(3):815–827.
Van Wingen, G., Geuze, E., Caan, M., Kozicz, T.,
Olabarriaga, S., Denys, D., Vermetten, E., and
Fern
´
andez, G.(2012). Persistent and reversible con-
sequences of combat stress on the mesofrontal cir-
cuit and cognition. Proceedings of the National
Academy of Sciences of the United States of America,
109(38):15508–15513.
Wang, R., Wang, W., DaSilva, A., Huckins, J. F., Kelley,
W. M., Heatherton, T. F., and Campbell, A. T. (2018).
Tracking depression dynamics in college students us-
ing mobile phone and wearable sensing. Proceed-
ings of the ACM on Interactive, Mobile, Wearable and
Ubiquitous Technologies, 2(1):1–26.
W
¨
ust, S., Federenko, I. S., van Rossum, E. F., Koper, J. W.,
and Hellhammer, D. H.(2005). Habituation of corti-
sol responses to repeated psychosocial stress—further
characterization and impact of genetic factors. Psy-
choneuroendocrinology, 30(2):199–211.
Yao, Z., Zhang, L., Jiang, C., Zhang, K., and Wu, J. (2016).
Stronger cortisol response to acute psychosocial stress
is correlated with larger decrease in temporal sensitiv-
ity. PeerJ, 4:e2061.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
298
